To the editor:
Hairy cell leukemia (HCL) shows distinct clinicopathologic, immunophenotypic, and gene expression features.1,–3 We previously identified the BRAF-V600E mutation as the disease-defining genetic event in HCL.4 This mutation is present in virtually all cases of HCL but rarely in other B-cell lymphomas, remains stable over time (being consistently detectable at relapse), and leads to the constitutive activation of the mitogen–activated protein kinase (MAPK) pathway4 that is potentially druggable with BRAF-V600E inhibitors. These findings have been confirmed in 2 studies recently published in this journal.5,6
Functional assays in HCL have been hampered by the scarcity of leukemic cells available for analysis because of frequent pancytopenia and/or punctio sicca at marrow aspiration. Moreover, no animal models of HCL are available.3 To overcome these problems, cell lines have been established from HCL patients7,,–10 and used for functional studies. However, there is no definitive evidence that they are of authentic HCL origin.
To clarify this issue, we searched for BRAF-V600E (the genetic hallmark of HCL) in the human HCL cell lines BONNA-12, ESKOL, HAIR-M, and HC-1 obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ). None of them carried BRAF-V600E (Figure 1). However, HAIR-M carried a clonal heterozygous T-to-A transversion at position 1782 of the BRAF coding sequence (not shown), leading to the replacement of aspartic acid with glutammic acid (D594E). This variant has been previously reported in only 1 case (a primary cutaneous melanoma11 ) of > 19 000 BRAF-mutated cancer samples listed in COSMIC-v56 (Catalog of Somatic Mutations In Cancer). Although this missense variant (D594E) is not described as a germ line polymorphism in single-nucleotide polymorphism (SNP) databases (dbSNP135 and 1000-genomes), its somatic origin in that melanoma patient was not evaluated.11 Thus the functional consequences of BRAF-D594E in the HAIR-M cell line remain unclear. Taken together, these results cast doubts on the HCL origin of the BONNA-12, ESKOL, HAIR-M, and HC-1 cell lines.
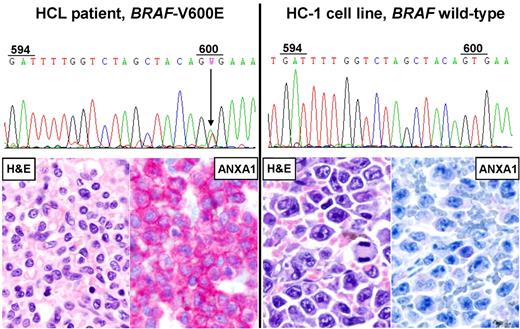
Human cell lines of putative HCL origin lack the BRAF-V600E mutation and key phenotypic features of HCL. Direct DNA Sanger sequencing of BRAF-exon154 in the HC-1 cell line (right chromatogram) shows the absence of the T-to-A point mutation at codon 600 leading to the V600E amino acid replacement, which is instead present heterozygously in primary leukemic cells MACS-purified from the peripheral blood of an HCL patient (left chromatogram). Both HC-1 cells and patient's leukemic cells display a wild-type codon 594 (GAT), as opposed to HAIR-M cells harboring a clonal heterozygous T-to-A point mutation at this codon (not shown) that leads to the D594E amino acid replacement. HC-1 cells xenografted in an NSG mouse show diffuse infiltration of the spleen by large B-cell lymphoma-like cells (right H&E staining) that are negative for annexin-1 (ANXA1; right ANXA1 immunostaining1 ). Conversely, the splenectomy specimen of the HCL patient is infiltrated by small mature-looking lymphoid cells with wide pale cytoplasm (left H&E staining) strongly expressing annexin-1 (left ANXA1-staining). All micrographs were collected using an Olympus B61 microscope (equipped with an Olympus UPlanApo 40×/0.8 NA objective and with an Olympus E330-ADU1.2x camera) and were acquired and processed using Olympus cell∧B imaging software.
Human cell lines of putative HCL origin lack the BRAF-V600E mutation and key phenotypic features of HCL. Direct DNA Sanger sequencing of BRAF-exon154 in the HC-1 cell line (right chromatogram) shows the absence of the T-to-A point mutation at codon 600 leading to the V600E amino acid replacement, which is instead present heterozygously in primary leukemic cells MACS-purified from the peripheral blood of an HCL patient (left chromatogram). Both HC-1 cells and patient's leukemic cells display a wild-type codon 594 (GAT), as opposed to HAIR-M cells harboring a clonal heterozygous T-to-A point mutation at this codon (not shown) that leads to the D594E amino acid replacement. HC-1 cells xenografted in an NSG mouse show diffuse infiltration of the spleen by large B-cell lymphoma-like cells (right H&E staining) that are negative for annexin-1 (ANXA1; right ANXA1 immunostaining1 ). Conversely, the splenectomy specimen of the HCL patient is infiltrated by small mature-looking lymphoid cells with wide pale cytoplasm (left H&E staining) strongly expressing annexin-1 (left ANXA1-staining). All micrographs were collected using an Olympus B61 microscope (equipped with an Olympus UPlanApo 40×/0.8 NA objective and with an Olympus E330-ADU1.2x camera) and were acquired and processed using Olympus cell∧B imaging software.
Doubts were also reinforced by the morphologic appearance of these cell lines (similar to lymphoblastoid cells), their EBV-positivity (except for HAIR-M),7,,–10 and their lack of the typical HCL immunophenotype (coexpression of annexin-1/CD25/CD11c/CD103; not shown). Similarly, ESKOL and HC-1 cells xenotransplanted in severely immunodeficient NSG mice showed the morphology of diffuse large B-cell lymphoma and were annexin-1 negative (Figure 1).
Because these cell lines do not represent a reliable HCL model either in vitro or in vivo, we sought to engraft in NSG mice MACS-purified (> 90% pure) patients' hairy cells. No engraftment was observed in a mouse that was injected intravenously with 3.8 million HCL cells and died 1 year later. Engraftment was not achieved even in another mouse that died 10 months after injection of 15 million leukemic cells from a different HCL patient harboring a hemizygous/homozygous BRAF-V600E mutation and presenting with markedly high hairy cell count (WBC 39000/mmc). Conversely, as few as 1.2 million ESKOL cells and 0.98 million HC-1 cells were enough to kill the animals as early as 3 weeks after intravenous injection. These results suggest that primary HCL cells appear difficult to engraft even in NSG mice.
Altogether our findings call for the development of conditional knock-in mice expressing BRAF-V600E in specific mature B-cell subsets as appropriate models for studying HCL.
Authorship
Acknowledgments: This work was supported by AIRC (Associazione Italiana per la Ricerca sul Cancro), Hairy Cell Leukemia Research Foundation, AULL (Associazione Umbra Leucemie e Linfomi), and MIUR (Ministero dell'Istruzione, Università e Ricerca; Program “Futuro in Ricerca 2010”).
Contribution: E.T. designed the study, analyzed data, and wrote the manuscript; A.P., B.B., V.P., F.S., M.P.M. and A.T. performed research and analyzed data; H.G.D. contributed vital reagents; and B.F. led the project, supervised the study, and wrote the manuscript.
Conflict-of-interest disclosure: B.F. and E.T. applied for a patent on the clinical use of the BRAF-V600E mutant in HCL. The remaining authors declare no competing financial interests.
Correspondence: Brunangelo Falini, Institute of Hematology, Ospedale S. Maria della Misericordia, 06132 Perugia, Italy; e-mail: faliniem@unipg.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal