Cells can communicate directly with each other through cell-cell contact or at a distance using secreted soluble mediators. A third mode of intercellular communication may be mediated by exosomes, an emerging novel pathway with unprecedented potential. In this issue of Blood, Montecalvo and colleagues demonstrate that dendritic cells (DCs) secrete exosomes that are loaded with distinct sets of microRNA, dependent on the status of DC activation.1 Moreover, they show that DC exosomes can fuse with target cells, thereby delivering their membranous and cytosolic contents. Finally, using a clever setup, they provide proof of principle that, after being transferred by exosomes, microRNA can repress mRNAs in target cells.
Cells may release membrane vesicles into their extracellular environment either by pinching them off directly from the plasma membrane or through secretion by endocytic compartments (reviewed in Théry et al2 ). Although many in vitro studies provide evidence that such released vesicles can be transferred to acceptor cells, confirmation of in vivo function(s) is still scarce. Extracellular vesicles have been assigned several names, including microvesicles and exosomes. The term exosomes is generally coined for those vesicles that are secreted as a consequence of the fusion of multivesicular bodies with the plasma membrane (see figure).
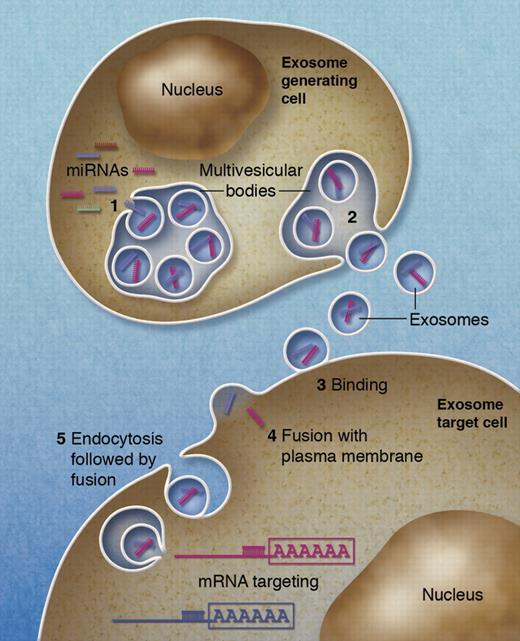
Schematic of microRNA transfer by exosomes. (1) microRNAs are selectively incorporated into the intraluminal vesicles of a multivesicular body. (2) Multivesicular bodies fuse with the plasma membrane, therewith secreting their intraluminal vesicles into the extracellular milieu. (3) Exosomes may bind to the plasma membrane of a target cell. Recruited exosomes may either fuse directly with the plasma membrane (4) or first be endocytosed and then fuse with the delimiting membrane of an endocytic compartment. (5) Both pathways result in the delivery of the exosomal microRNA to the cytosol of the target cell where it may associate with and silence corresponding mRNA. Professional illustration by Alice Y. Chen.
Schematic of microRNA transfer by exosomes. (1) microRNAs are selectively incorporated into the intraluminal vesicles of a multivesicular body. (2) Multivesicular bodies fuse with the plasma membrane, therewith secreting their intraluminal vesicles into the extracellular milieu. (3) Exosomes may bind to the plasma membrane of a target cell. Recruited exosomes may either fuse directly with the plasma membrane (4) or first be endocytosed and then fuse with the delimiting membrane of an endocytic compartment. (5) Both pathways result in the delivery of the exosomal microRNA to the cytosol of the target cell where it may associate with and silence corresponding mRNA. Professional illustration by Alice Y. Chen.
Multivesicular bodies are generated at endosomes by the inward budding of their delimiting membrane followed by the release of ∼ 100 nm vesicles into the endosomal lumen. Such intra-endosomal vesicles have a cytosolic-side inward orientation, just like cells. Many multivesicular bodies serve as a sorting station for endocytosed membrane proteins that need to be transferred to and degraded in lysosomes. Other multivesicular bodies may instead fuse with the plasma membrane, resulting in secretion of their intraluminal vesicles as exosomes. Exosomes are secreted by many if not most cell types, and are abundantly present in body fluids such as blood, ascites, urine, milk, saliva, and seminal plasma. Our laboratory demonstrated that the secretion by DCs of MHC II carrying exosomes is specifically stimulated by MHCII-peptide interacting CD4+ T cells,3 suggesting that communication through exosomes is a regulated process. Indeed the protein content of DC exosomes varies with the status of maturation of the exosome producing DCs,2 while DC exosomes can be targeted both to neighboring DCs or interacting T cells.2,3
Exosomes are proposed to have many distinct physiologic functions that may vary, depending on their cellular origin, from immune regulation, to blood coagulation, cell migration, cell differentiation, and other aspects of cell-to-cell communication. Exosomes have also been implicated in the pathogenesis of disease such as tumor development, cardiovascular disease, neurodegenerative disease and retroviral infection. This has sparked the idea that exosomes from body fluids may be useful as novel biomarkers for the detection and classification of disease, and perhaps can even be employed as therapeutic tools. This was first pioneered by Zitvogel and colleagues, who demonstrated over a decade ago that exosomes isolated from cultured DCs carried functional MHC-peptide complexes and could promote in vivo induction of antitumor immune responses in mice.4
Interest in exosomes was boosted further by a publication in 2007 from Valadi and colleagues demonstrating that exosomes from mast cells contain both mRNA and microRNA, and that at least some of these mRNAs could be translated into proteins on their transfer by exosomes to target cells.5 Since then, exosomes from many other cell types have also been demonstrated to carry RNA, as summated in the database Exocarta.org. Multivesicular bodies are functionally linked to microRNA effector complexes,6,7 perhaps indicating mechanisms for miRNA targeting to exosomes. Realization that exosomes may elicit epigenetic effects by transferring selected RNA molecules between cells has revolutionized our thinking of possible mechanisms of exosome signaling, and furthered ideas of using exosomal RNAs as biomarkers for disease.
The concept of exosome-mediated directed transfer of selected microRNA between cells is extremely attractive, although several basic elements of such a process required confirmation. Montecalvo and coworkers have now directly tested essential elements of this hypothesis by analyzing the mechanism of exosome-mediated transfer of microRNA between cells. Exosomes isolated from DC culture media enclosed > 200 microRNAs, with 5 uniquely detected in exosomes from immature DCs and 58 exclusively present in exosomes from mature DCs. These compositions differed from the microRNA content of the exosome-producing DCs, indicating selectivity for their incorporation into exosomes. Using a GFP-linked marker protein that was efficiently incorporated into exosomes, DC exosomes were demonstrated to be transferred to both bystander DCs and activated (but not naive) antigen-specific CD4+ T cells. Exosomes were confirmed to fuse with target DC membranes in two independent assays, with one indicating membrane mixing and the other illustrating mixing of the exosomal luminal content with the cytosol of the target DC. Importantly, functional transfer of two exosomal microRNAs was demonstrated by employing cells that were transfected with vectors encoding luciferase-coupled complementary targets.
Now that these in vitro requisites for transfer of exosome-shuttle microRNA have been demonstrated, a major future challenge will be to reveal physiologic relevance of this process. The molecular mechanisms that drive exosome formation and secretion have not yet been resolved. Although some Rab GTPases have been implicated, their depletion only partially interfered with exosome secretion (reviewed in Bobrie et al8 ). Dissection of the mechanism(s) that drive miRNA recruitment into exosomes may provide stronger tools to study the physiologic functions of miRNA transfer by exosomes.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal