Key Points
16-16 dimethyl-PGE2 treatment enhances long-term HSC repopulation without lineage bias or transformation.
Treatment of HSC with 16-16 dimethyl-PGE2 does not alter long-term competitiveness.
Abstract
Hematopoietic stem cell (HSC) transplantation is a lifesaving therapy for malignant and nonmalignant hematologic diseases and metabolic disorders. Although successful, hematopoietic transplantation can be hindered by inadequate stem cell number or poor engrafting efficiency. To overcome these deficits, we and others have previously reported the HSC-enhancing ability of a short-term exposure of prostaglandin E2 (PGE2); this strategy has now progressed to phase 1 clinical trials in double cord blood transplantation. To further analyze the short- and long-term effects of HSC exposure to PGE2, we followed the repopulation kinetics of PGE2-treated hematopoietic grafts through 5 serial transplantations and compared inherent long-term competitiveness in a HSC head-to-head secondary transplantation model. Treatment with PGE2 did not result in a long-term increase in HSC competitiveness, lineage bias, or enhanced proliferative potential, demonstrating that pulse exposure to PGE2 results in transient increases in HSC homing and engraftment potential.
Introduction
Since the mid-1970s, a hematopoietic regulatory role for prostaglandin E2 (PGE2) has been described, demonstrating both inhibitory and stimulatory effects dependent on the cell type studied and exposure kinetics (reviewed elsewhere).1-4 Using a zebrafish embryo chemical screen, a long-acting agonist of PGE2, 16-16 dimethyl-PGE2 (dmPGE2), was shown to increase hematopoiesis, whereas inhibitors of PGE2 biosynthesis decreased hematopoiesis.5 Using short-term ex vivo pulse exposure of bone marrow cells, similar to exposure strategies previously reported,6,7 North and colleagues elegantly demonstrated that murine bone marrow transplantation was enhanced by dmPGE2.5 We later demonstrated that this enhanced hematopoietic stem cell (HSC) engraftment resulted from an increase in CXCR4 on hematopoietic stem and progenitor cells and enhanced homing to the marrow; it also increased expression of Survivin, with reduced HSC apoptosis and increased HSC division.8 In addition, we showed that enhanced HSC engraftment was maintained in secondary transplantation.8 Enhanced HSC production and long-term repopulation by PGE2 was also shown to be mediated through enhanced Wnt/β-catenin signaling.9
Based on these preclinical findings, a phase 1 clinical trial evaluating safety and efficacy of ex vivo dmPGE2 pulse treatment of umbilical cord blood cells was initiated, the results of which are published in this edition of Blood.10 One prime question raised by treatment of HSCs with dmPGE2, however, is whether its effects on HSCs are short lived or whether treatment alters long-term potential. Here, we report on extended analysis of dmPGE2-treated hematopoietic grafts, following multilineage repopulation of hematopoiesis through 5 serial transplantations and comparison of the engraftment potential of vehicle- and dmPGE2-treated HSCs on a cell-to-cell basis. We demonstrate that dmPGE2 treatment does not alter long-term HSC competitiveness, lineage bias, or proliferative potential.
Methods
Mice
C57Bl/6 (CD45.2) mice were purchased from Jackson Laboratories (Bar Harbor, ME). B6.SJL-PtrcAPep3B/BoyJ (BOYJ) (CD45.1) and C57Bl/6 × BOYJ F1-hybrid mice (CD45.1/CD45.2) were bred in-house. All mice in transplant studies received doxycycline feed for 30 days posttransplant. The Animal Care and Use Committee of the Indiana University School of Medicine approved all protocols.
Competitive transplantation
Competitive transplantation was performed as previously described,8 and the primary and secondary transplant data are reflective of these previously published results. For serial transplants, 2 × 106 whole bone marrow (WBM) cells from previously transplanted CD45.1/CD45.2 F1-hybrid mice were injected into lethally irradiated CD45.1/CD45.2 F1-hybrid mice in noncompetitive fashion. Secondary, tertiary, quaternary, and quinary transplants were performed in a similar manner, with the tertiary transplant performed 24 weeks after the secondary transplant, and the quaternary and quinary transplants performed 12 weeks after the prior transplant. Chimerism and multilineage flow analysis was performed as previously described and representative flow plots of multilineage gating were shown.8
Long-term competitiveness assay
WBM cells from CD45.1 and CD45.2 donors were isolated and treated with vehicle or dmPGE2 for 2 hours on ice. One cohort of lethally irradiated F1-hybrid CD45.1/CD45.2 mice were transplanted with 5 × 105 vehicle-treated CD45.1 cells and 5 × 105 dmPGE2-treated CD45.2 cells. A separate cohort of irradiated F1-hybrid CD45.1/CD45.2 mice were transplanted in a similar fashion with strain and treatment groups reversed. After 12 weeks, peripheral blood chimerism was evaluated and bone marrow was acquired and stained for CD45.1/CD45.2 and SLAM SKL (lineage negative, c-kit+, Sca-1+, CD150+, CD48−) markers. CD45.1+ and CD45.2+ SLAM SKL cells were individually isolated by fluorescence-activated cell sorting, and a second group of irradiated F1-hybrid CD45.1/CD45.2 mice were transplanted with 2.5 × 102 CD45.1+ SLAM SKL cells, 2.5 × 102 CD45.2+ SLAM SKL cells, and 2.0 × 105 CD45.1/CD45.2 F1-hybrid WBM cells as competitors. Contribution to chimerism of CD45.1 and CD45.2 SLAM SKL cells was evaluated 12 weeks later.
Results and discussion
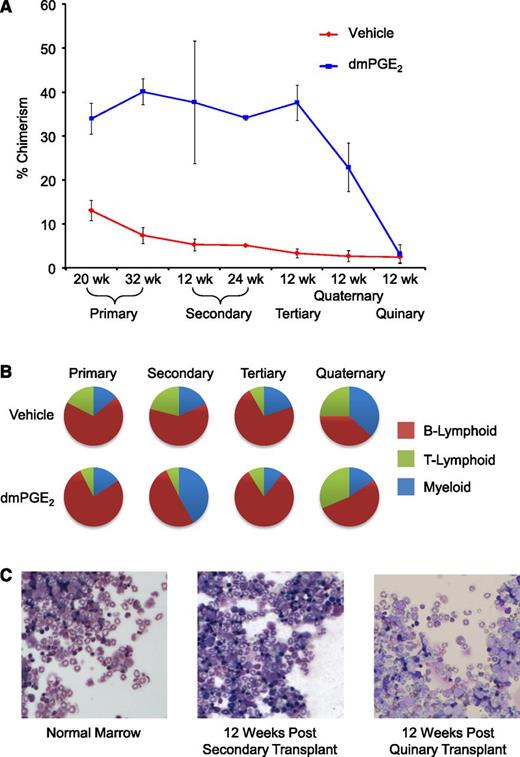
In a previous report, we used a competitive head-to-head transplant model that permitted comparison of engraftment and competitiveness of vehicle- and dmPGE2-treated HSCs within the same animal.8 In the prior report, we validated enhanced engraftment after dmPGE2 treatment and showed that this enhancement persisted through secondary transplantation without additional treatment of cells before retransplant (Figure 1A).8 We continued serial transplantations at 12-week intervals, monitoring chimerism and multilineage reconstitution through 5 serial transplantations. Grafts previously treated with dmPGE2 maintained higher levels of chimerism through quaternary serial transplantation, with HSC exhaustion occurring by the quinary transplant, which is consistent with normal HSC exhaustion in a similar experimental model.11 Bone marrow grafts from vehicle or dmPGE2 treatment groups contributed to full multilineage reconstitution throughout the first 4 serial transplantations (Figure 1B). Although it was commonly assumed that a single HSC compartment gives rise to all hematopoietic lineages equivalently, transplant studies have demonstrated the presence of HSCs biased toward lymphoid or myeloid differentiation.12-14 In secondary transplants, we observed a myeloid bias in mice transplanted with dmPGE2-treated HSCs, suggesting a possible selective effect on myeloid-biased HSCs. However, a consistent lineage bias was not observed in subsequent serial transplants (Figure 1B).
dmPGE2 pulsed grafts maintain repopulating ability through serial transplantations. (A) Increased chimerism of dmPGE2-treated cells vs vehicle is shown for primary transplant at 20 weeks (time of secondary transplant) and in a subcohort at 32 weeks (time of 12-week analysis of secondary transplant); for secondary transplant at 12 weeks and 24 weeks; and for tertiary, quaternary, and quinary at 12 weeks. Data for 20-week primary transplant were from 2 pooled experiments, n = 5 mice per group, per experiment, each assayed individually. Data for secondary, tertiary, quaternary, and quinary transplants were from n = 5 mice per group, each assayed individually. Data are expressed as mean ± SEM; *P < .05. (B) Relative contribution to lineages of myeloid and B- and T-lymphoid. Multilineage analysis for primary transplant (32 weeks) and at 12 weeks posttransplant in serially transplanted secondary, tertiary, and quaternary mice. n = 5-10 mice per group, each assayed individually. (C) Representative Wright’s Giemsa-stained cytospins from normal bone marrow, marrow from secondary bone marrow transplant 12 weeks after transplantation, and marrow from quinary transplanted mice 12 weeks after transplantation. Cytospins were photographed at ×200 (×20 objective) with a Leica DM2500 Microscope outfitted with Q-Imaging micropublisher camera (W. Nushbaum Inc., McHenry, IL). Bone marrow cytospins show normal cellularity. Both myeloid and precursors are present and show normal maturation as well as megakaryocytes. There was no evidence of myeloid hyperplasia, no noticeable increase in myeloid or erythroid blasts, and no obvious increase in immature granulocytic, monocytic cells, or evidence of lymphoblastic transformation.
dmPGE2 pulsed grafts maintain repopulating ability through serial transplantations. (A) Increased chimerism of dmPGE2-treated cells vs vehicle is shown for primary transplant at 20 weeks (time of secondary transplant) and in a subcohort at 32 weeks (time of 12-week analysis of secondary transplant); for secondary transplant at 12 weeks and 24 weeks; and for tertiary, quaternary, and quinary at 12 weeks. Data for 20-week primary transplant were from 2 pooled experiments, n = 5 mice per group, per experiment, each assayed individually. Data for secondary, tertiary, quaternary, and quinary transplants were from n = 5 mice per group, each assayed individually. Data are expressed as mean ± SEM; *P < .05. (B) Relative contribution to lineages of myeloid and B- and T-lymphoid. Multilineage analysis for primary transplant (32 weeks) and at 12 weeks posttransplant in serially transplanted secondary, tertiary, and quaternary mice. n = 5-10 mice per group, each assayed individually. (C) Representative Wright’s Giemsa-stained cytospins from normal bone marrow, marrow from secondary bone marrow transplant 12 weeks after transplantation, and marrow from quinary transplanted mice 12 weeks after transplantation. Cytospins were photographed at ×200 (×20 objective) with a Leica DM2500 Microscope outfitted with Q-Imaging micropublisher camera (W. Nushbaum Inc., McHenry, IL). Bone marrow cytospins show normal cellularity. Both myeloid and precursors are present and show normal maturation as well as megakaryocytes. There was no evidence of myeloid hyperplasia, no noticeable increase in myeloid or erythroid blasts, and no obvious increase in immature granulocytic, monocytic cells, or evidence of lymphoblastic transformation.
PGE2 signaling is a known mediator of cancer15,16 and intersects with multiple signaling pathways downstream of 4 G protein–coupled receptors. Wnt/β-catenin signaling was recently shown to be required for the development of leukemia stem cells in acute myelogenous leukemia17 ; this pathway lies downstream of PGE2 receptors. Throughout the quinary transplants, peripheral blood white blood cell differential counts remained within normal range in all mice at the 12-week time points. Moreover, histological observation indicated that there was no obvious alteration in the bone marrow cellular composition between quinary transplanted mice that had received grafts originally treated with dmPGE2 before primary transplant vs bone marrow from control-graft transplanted mice (Figure 1C). These results suggest that dmPGE2 pulse treatment does not lead to a preleukemic or myeloproliferative state.
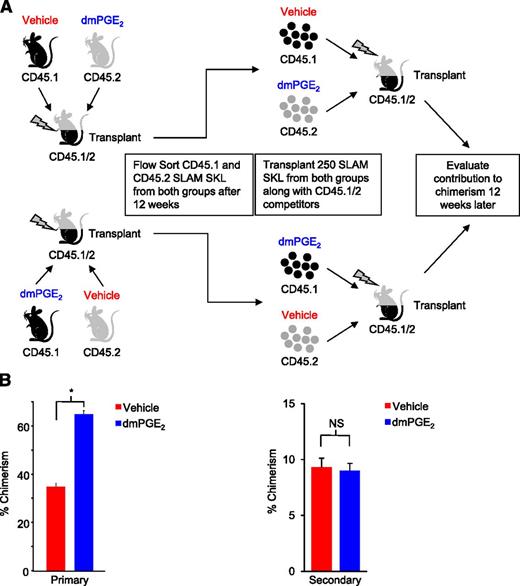
Increased hematopoietic output by dmPGE2-treated grafts through serial transplants could simply be the result of higher numbers of HSCs homed and engrafted in the primary graft, proportionally occupying available niche space and self-renewing. Alternatively, enhanced hematopoietic output could result from epigenetic changes in HSC elicited by dmPGE2 treatment,18,19 leading to long-term enhanced competitiveness, preferential dmPGE2 stimulation of a subset of HSCs with enhanced inherent competitiveness,20 or an unknown mechanism eliciting long-term competitive advantage. We reasoned that if serial transplant results are simply due to an increase in homed and engrafted HSCs in the primary graft, then “equalizing” the HSC content from dmPGE2- and vehicle-treated grafts in a subsequent secondary transplant should result in equal repopulation. To test this hypothesis, dmPGE2- and vehicle-treated congenic bone marrow cells were transplanted head to head into lethally irradiated F1-hybrid mice, and chimerism was evaluated 12 weeks later (Figure 2A). As seen in earlier transplants, dmPGE2 treatment resulted in significantly increased chimerism (Figure 2B, left). Bone marrow from these primary recipient mice was subsequently harvested and fluorescence-activated cell sorted for congenic SLAM SKL cells. SLAM SKL cells derived from previously treated dmPGE2 grafts were 2.1-fold more abundant than those from vehicle-treated grafts (0.0295% SLAM SKL cells in total marrow vs 0.0138%). We subsequently transplanted equal numbers of the sorted congenic SLAM SKL cells head to head into secondary recipients along with radioprotecting F1-hybrid bone marrow competitors without additional treatment. Equalizing the HSC content in secondary grafts resulted in no differential increase in repopulating ability of previously dmPGE2-treated HSCs (Figure 2B, right). This result suggests that the enhanced engraftment of dmPGE2-treated HSCs in primary and subsequently serially transplanted recipients is a function of increased numbers of homed HSCs and their subsequent survival/self-renewal, resulting in a transient increase in competitiveness, rather than a long-term increase in inherent HSC competitiveness. Thus, short-term treatment of hematopoietic grafts before transplant is a strategy to enhance HSC engraftment without concern for long-term alteration of normal HSC function.
dmPGE2 pulsed HSCs do not have an inherent competitive advantage in secondary transplants. (A) Schematic representation of experimental design. WBM from CD45.1 mice and CD45.2 mice was treated with both vehicle and dmPGE2 and then transplanted head to head into lethally irradiated (1100 cGy, split dose) CD45.1/CD45.2 hybrid mice as shown (2.5 × 105 cells per group). Chimerism was analyzed at 12 weeks, and bone marrow from recipients was collected and stained with fluorescent antibodies for phenotypic markers, and cells were sorted for SLAM SKL. SLAM SKL cells were transplanted head to head into a second cohort of lethally irradiated CD45.1/CD45.2 mice along with 2.0 × 105 WBM CD45.1/CD45.2 competitors, and chimerism analyzed 12 weeks later. (B) Chimerism in peripheral blood is shown for 12 weeks after the primary transplant and 12 weeks after the secondary transplant (mean ± SEM). n = 10 mice per group (total of 20 mice for primary and 20 mice for secondary); *P < .001.
dmPGE2 pulsed HSCs do not have an inherent competitive advantage in secondary transplants. (A) Schematic representation of experimental design. WBM from CD45.1 mice and CD45.2 mice was treated with both vehicle and dmPGE2 and then transplanted head to head into lethally irradiated (1100 cGy, split dose) CD45.1/CD45.2 hybrid mice as shown (2.5 × 105 cells per group). Chimerism was analyzed at 12 weeks, and bone marrow from recipients was collected and stained with fluorescent antibodies for phenotypic markers, and cells were sorted for SLAM SKL. SLAM SKL cells were transplanted head to head into a second cohort of lethally irradiated CD45.1/CD45.2 mice along with 2.0 × 105 WBM CD45.1/CD45.2 competitors, and chimerism analyzed 12 weeks later. (B) Chimerism in peripheral blood is shown for 12 weeks after the primary transplant and 12 weeks after the secondary transplant (mean ± SEM). n = 10 mice per group (total of 20 mice for primary and 20 mice for secondary); *P < .001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
These studies were supported by grants from the National Institutes of Health (NIH), National Heart, Lung and Blood Institute (HL096305); NIH training grants (DK07519, HL07910, and HL087735) (J.H.); and a Center of Excellence in Hematology (P01 DK090948) for additional core support. Flow cytometry was performed in the Flow Cytometry Resource Facility of the Indiana University Simon Cancer Center (National Cancer Institute P30 CA082709).
Authorship
Contribution: J.H. designed and executed all described studies, analyzed data, and wrote the manuscript; K.S.M. performed pathologic analysis of bone marrow sections from serially transplanted mice; P.S. performed experiments and analyzed data; and L.M.P. designed and executed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: J.H. and L.M.P. have received consulting fees from Fate Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Louis M. Pelus, Department of Microbiology and Immunology, Indiana University School of Medicine, 950 West Walnut St, Indianapolis, IN 46202; e-mail: lpelus@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal