Key Points
Adsorption of VWF type 2B mutants to platelets induces thrombocytopenia in VWD type 2B mice.
VWF/platelet complexes are phagocytosed by macrophages in liver and spleen.
Abstract
Von Willebrand disease (VWD) type 2B is characterized by mutations causing enhanced binding of von Willebrand factor (VWF) to platelets. Bleeding tendency is associated with heterogeneous clinical manifestations, including moderate to severe thrombocytopenia. The underlying mechanism of the thrombocytopenia has remained unclear. Here, a mouse model of VWD type 2B was used to investigate pathways contributing to thrombocytopenia. Immunohistochemical analysis of blood smears revealed that mutant VWF was exclusively detected on platelets of thrombocytopenic VWD type 2B mice, suggesting that thrombocytopenic VWD type 2B mice were elevated two- to threefold upon chemical macrophage depletion. Colocalization of platelets with CD68-positive Kupffer cells and CD168-positive marginal macrophages in liver and spleen, respectively, confirmed the involvement of macrophages in the removal of VWF/platelet complexes. Significantly more platelets were found in liver and spleen of VWD type 2B mice compared with control mice. Finally, platelet survival was significantly shorter in VWD type 2B mice compared with control mice, providing a rationale for lower platelet counts in VWD type 2B mice. In conclusion, our data indicate that VWF type 2B binds to platelets and that this is a signal for clearance by macrophages, which could contribute to the thrombocytopenia in patients with VWD type 2B.
Introduction
Von Willebrand factor (VWF) is particularly known for its role in hemostasis, where it functions as a chaperone protein for coagulation factor VIII and is essential for the recruitment of platelets to the growing thrombus under conditions of high shear stress.1 Its importance for the hemostatic system is illustrated by the bleeding tendency that is associated with the functional deficiency of VWF, a disorder known as von Willebrand disease (VWD).
VWD can originate from a quantitative deficiency (type 1 and type 3 for mild and severe deficiencies, respectively) and or qualitative abnormalities (type 2).2 VWD type 2 is classified according to the functional defect, and of particular relevance to this study is VWD type 2B, which is caused by gain-of-function mutations that increase the affinity of VWF for platelets.2 These gain-of-function mutations are clustered in exon 28 of the VWF gene encoding the VWF A1 domain, which harbors the binding site for its platelet receptor glycoprotein Ibα (GpIbα). The increased affinity for platelets is often detected via enhanced ristocetin-induced platelet aggregation.3 Besides their bleeding tendency, VWD type 2B patients frequently manifest a decrease of high-molecular-weight (HMW) VWF multimers in their plasma and a moderate to severe thrombocytopenia.4 Blood smear analysis further revealed that the thrombocytopenia is associated with the presence of giant platelets and spontaneous platelet aggregates.4 Interestingly, a large study involving 67 VWD type 2B patients revealed a marked mutation-dependent variety in the degree of thrombocytopenia and the clinical severity of the disease.4 Importantly, a strong correlation between the extent of thrombocytopenia and the bleeding score of VWD type 2B patients was observed.
Despite the progress in the diagnosis of VWF type 2B, there are still a number of unknowns in our understanding of the disease. For instance, the molecular mechanism underlying the thrombocytopenia has not yet been identified. Several mechanisms can be considered in this regard. First, Nurden et al have reported that megakaryocytopoiesis is disturbed in VWD type 2B, due to continuous interactions between VWF and GpIbα at the surface of the megakaryocyte.5-7 This impaired megakaryocytopoiesis could not only explain the occurrence of giant platelets, but also contribute to a lower platelet count in VWD type 2B patients. A second possibility was proposed 3 decades ago, in which the thrombocytopenia is explained by an accelerated clearance of VWF/platelet complexes.8,9 This possibility is still being referred to in the literature,10,11 although no experimental evidence in support of this possibility has been provided so far. It should also be noted that both of the previously discussed possibilities are not mutually exclusive.
Recently, we and others have developed a murine model for VWD type 2B, in which the clinical phenotype of human patients is faithfully reproduced.12,13 VWD type 2B mice are characterized by a severe bleeding phenotype, thrombocytopenia, circulating aggregates, giant platelets, and an increased clearance of the VWF type 2B mutant protein. Interestingly, the loss of HMW-VWF multimers was observed in 50% of the mice.12 Importantly, the severity of the thrombocytopenia was dependent on the mutation tested, with the p.V1316M mutation being more severe than the p.R1306Q mutations, similar to the observations in human patients.4,12,13
In the present study, we have used this mouse model to show that VWD type 2B platelets have a shorter circulatory half-life than wild-type (wt) platelets, which could contribute to the lower platelet counts in VWD type 2B mice. Further analysis revealed that VWF type 2B is present at the surface of platelets of thrombocytopenic VWD type 2B mice, and that these VWF/platelet complexes are taken up efficiently by macrophages in liver and spleen. We thus provide direct evidence that part of the thrombocytopenia in VWD type 2B can be explained by an increased clearance of VWF/platelet complexes by macrophages in liver and spleen.
Materials and methods
An extensive description of materials and methods can be found in the supplemental Methods (see the Blood website), a brief summary of which is given subsequently.
Mice
VWF-deficient mice on a C57BL/6 background were used throughout the study.14 Both male and females were used, and body weight varied between 20 and 25 g. All experimental procedures were performed in accordance with European legislation concerning the use of laboratory animals and approved by the local animal care and ethical committee (CEEA 26) under the authorization 2012-039.
Perfusion experiments
Perfusions experiments were performed using phorbol 12-myristate 13-acetate (PMA)–differentiated THP-1 macrophages that were grown on glass coverslips. All perfusions were executed using a shear stress of 5 dynes/cm2. Following perfusion with VWF (5 μg/mL) in the absence or presence of botrocetin (1.6 U/mL), cells were fixed and stained for VWF as described in the supplemental Methods.
Scanning electron microscopy
Following incubation of THP-1 macrophages with VWF-coated microspheres, cells were fixed using glutaraldehyde and osmium tetroxide, dehydrated using successive ethanol incubations, and finally coated with gold alloy. Images were acquired using a Hitachi S-2500 scanning electron microscope.
Plasmids and hydrodynamic injection
Plasmids encoding full-length wt murine VWF (wt-mVWF) or VWD type 2B mutants p.V1316M (p.V1316M-mVWF) and p.R1306Q (p.R1306Q-mVWF) have been described previously and were used to induce in vivo expression following hydrodynamic gene transfer.12 All experiments were performed or initiated 4 days after hydrodynamic gene transfer. For each mouse included in the study, VWF antigen (VWF:Ag) and platelet counts were determined at day 4 after hydrodynamic injection, and only mice with the antigen being within the 200% to 1200% range were included in the study. VWF:Ag levels were quantified as described,15 and antigen levels were found to be 607 ± 34% (mean ± standard error of the mean [SEM]; n = 77), 627 ± 28% (n = 90), and 597 ± 50% (n = 37) for wt-mVWF, p.V1316M-mVWF, and p.R1306Q-mVWF, respectively. In some cases, experiments were performed over several days (eg, clearance experiments and effects of liposome-clodronate). In accordance with our previous reports,12,16 antigen levels remained stable throughout the duration of the experiments. For platelet counts, blood was collected on EDTA, and counts were performed with an Because antigen levels were above the normal range (200% to 1200%), we checked how antigen levels affected platelet counts. For both wt-mVWF and p.R1306Q-mVWF, no correlation between antigen levels and platelet counts was observed (r2 = 0.022 [P = .20] and r2 = 0.04 [P = .23], respectively; supplemental Figure 1). For p.V1316M-mVWF, a poor but statistically significant correlation was observed (r2 = 0.06, P = .02). It should be noted that platelet counts in mice within the lower antigen range (200% to 300% VWF:Ag) were already thrombocytopenic (212 ± 29 [mean ± SEM]) and just slightly higher than platelet counts in mice within the higher antigen range (1100% to 1200% VWF:Ag; platelet counts: 134 ± 25).
Quantitative analysis of platelet biodistribution
Washed platelets were fluorescently labeled with CMTMR [5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine]-Orange and infused in the tail vein of wt-mVWF or mutant mVWF-expressing mice. Two hours after injection, liver and spleen were isolated and used for cryosection preparation. Accumulation of platelets in liver and spleen sections was determined using NanoZoomer 2.0-HT equipment, which allows quantitative whole-slide assessment (see the supplemental Methods for details).
Qualitative analysis of platelet biodistribution
For quantitative analysis of platelet biodistribution, liver and spleen cryosections were stained for specific subpopulations of macrophages. Immunofluorescence microsopic analysis was then performed as described in detail in the supplemental Methods to detect colocalization between platelets and macrophages.
Platelet survival and flow cytometry
Platelet survival was essentially performed as described previously.17 More detailed information is provided in the supplemental Methods.
Results
VWF activation facilitates its uptake by macrophages
Previously, we have reported that macrophages bind and endocytose VWF,18 and recently, it was found that activation of VWF via exposure to shear stress enhances this process.19,20 VWF may also be forced in its active conformation via VWD type 2B mutations or via modulators such as botrocetin. Therefore, we first addressed the possibility that botrocetin enhances uptake of VWF by macrophages. To this end, VWF was perfused over PMA-stimulated THP-1 cells at low shear stress (5 dynes/cm2) for 15 minutes in the absence or presence of botrocetin. Cells were then washed to remove extracellular VWF, fixed, and stained for VWF via immunofluorescence. The number of VWF-positive cells was similar in the absence or presence of botrocetin (16/29 and 17/27, respectively). However, it appeared that more VWF was taken up per cell by macrophages in the presence of botrocetin compared with in its absence (Figure 1A-H). Quantitative analysis using ImageJ confirmed that botrocetin enhanced VWF endocytosis by sevenfold (Figure 1I). Apparently, VWF is endocytosed by macrophages more efficiently when it is in its active, platelet-binding conformation.
VWF active conformation enhances endocytosis by macrophage. (A-H) wt-rVWF (5 μg/mL) was perfused over PMA-stimulated THP-1 macrophages in the absence (A-D) or presence (E-H) of botrocetin (1.6 U/mL). Perfusion was performed for 15 minutes at 5 dynes/cm2. Nuclei were stained with 4,6-diamidino-2-phenylindole (blue), actin was stained with AlexaFluor 488–conjugated phalloidin (green), and VWF was detected with primary rabbit anti-human VWF antibody followed by tetramethyl rhodamine iso-thiocyanate–conjugated goat anti-rat IgG antibody (red). Original magnification is ×100. (I) VWF internalization was quantified with ImageJ as the percentage of surface covered by red spots (VWF staining) per number of cells per field. Data represent mean ± SEM. *P < .05.
VWF active conformation enhances endocytosis by macrophage. (A-H) wt-rVWF (5 μg/mL) was perfused over PMA-stimulated THP-1 macrophages in the absence (A-D) or presence (E-H) of botrocetin (1.6 U/mL). Perfusion was performed for 15 minutes at 5 dynes/cm2. Nuclei were stained with 4,6-diamidino-2-phenylindole (blue), actin was stained with AlexaFluor 488–conjugated phalloidin (green), and VWF was detected with primary rabbit anti-human VWF antibody followed by tetramethyl rhodamine iso-thiocyanate–conjugated goat anti-rat IgG antibody (red). Original magnification is ×100. (I) VWF internalization was quantified with ImageJ as the percentage of surface covered by red spots (VWF staining) per number of cells per field. Data represent mean ± SEM. *P < .05.
VWD type 2B mutations enhance VWF internalization by macrophages
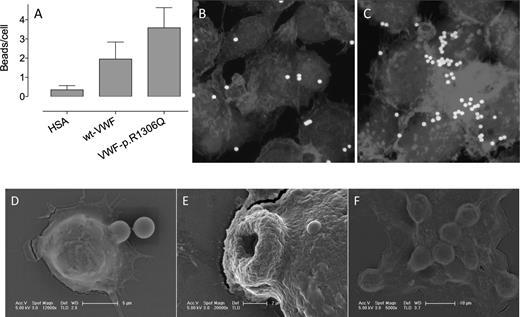
Next, we tested how the active VWD type 2B configuration affects uptake of VWF by macrophages. Fluorescent beads were coated with human albumin (HSA), wt recombinant VWF (rVWF), or the p.R1306Q-rVWF type 2B mutant and were subsequently incubated with THP-1 macrophages. Cells were then washed to remove extracellular microspheres. More wt-rVWF–coated than HSA-coated beads were phagocytosed (2.0 ± 0.9 vs 0.4 ± 0.2 beads per cell, P < .05; Figure 2A), further validating that macrophages bind and internalize VWF. Interestingly, the uptake was increased even further for p.R1306Q-rVWF–coated beads (3.6 ± 1.1 beads per cell, P < .05 compared with wt-rVWF–coated beads; Figure 2A-C). The process of internalization of p.R1306Q-rVWF–coated beads was analyzed in more detail via scanning electron microscopy. Several stages could be distinguished: the beginning of the formation of ruffles around a coated bead (stage 1; Figure 2D), engulfment in an advanced stadium (stage 2; Figure 2E), and complete internalization of multiple beads (stage 3; Figure 2F). These results indicate that VWD type 2B mutations promote the uptake of VWF by macrophages.
Uptake of p.R1306Q-coated beads by macrophages. Fluoresbrite-YG microspheres (1 × 104 beads per well) coated with HSA, wt-rVWF, or p.R1306Q-rVWF (25 μg/L × 104 beads) were incubated with PMA-stimulated THP-1 cells adhered to glass coverslips for 1 hour at 37°C. Subsequently, cells were thoroughly washed to remove nondigested beads. The number of phagocytosed beads was quantified via microscopic analysis and is expressed as beads per cell (A). Data represent the mean ± standard deviation of 2 independent experiments, in which at least 3 microscopic fields were analyzed. (B-C) Representative images of wt-rVWF–coated and p.R1306Q-coated beads phagocytosed by THP-1 macrophages. (D-F) Scanning electron images of macrophages phagocytosing microspheres coated with p.R1306Q-rVWF. (D) The first moment of capturing a VWF-coated bead. (E) A 3-μm hole through which a bead has been phagocytosed. (F) A macrophage that has phagocytosed multiple beads.
Uptake of p.R1306Q-coated beads by macrophages. Fluoresbrite-YG microspheres (1 × 104 beads per well) coated with HSA, wt-rVWF, or p.R1306Q-rVWF (25 μg/L × 104 beads) were incubated with PMA-stimulated THP-1 cells adhered to glass coverslips for 1 hour at 37°C. Subsequently, cells were thoroughly washed to remove nondigested beads. The number of phagocytosed beads was quantified via microscopic analysis and is expressed as beads per cell (A). Data represent the mean ± standard deviation of 2 independent experiments, in which at least 3 microscopic fields were analyzed. (B-C) Representative images of wt-rVWF–coated and p.R1306Q-coated beads phagocytosed by THP-1 macrophages. (D-F) Scanning electron images of macrophages phagocytosing microspheres coated with p.R1306Q-rVWF. (D) The first moment of capturing a VWF-coated bead. (E) A 3-μm hole through which a bead has been phagocytosed. (F) A macrophage that has phagocytosed multiple beads.
VWF-covered platelets circulate in vivo in VWD type 2B mice
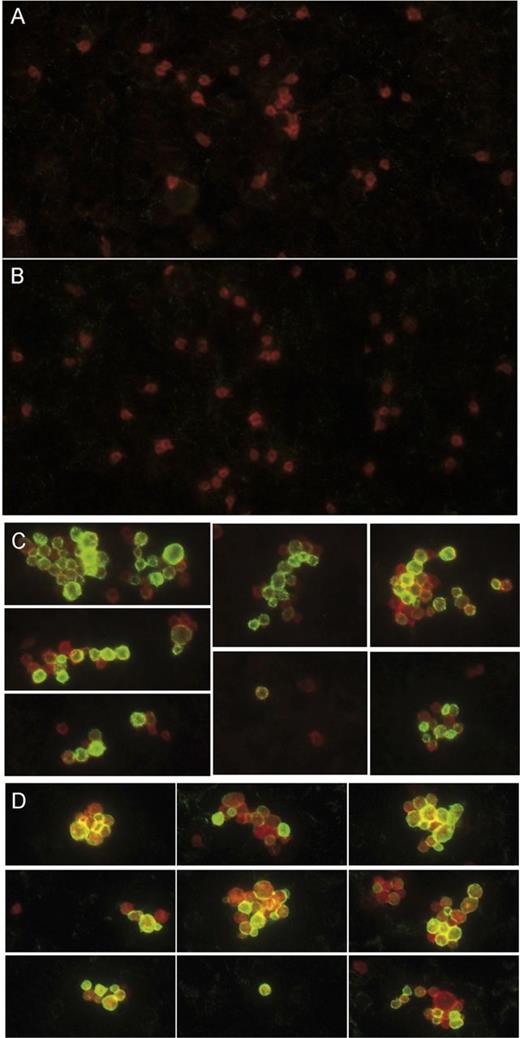
The observation that VWD type 2B mutations promote phagocytosis of microspheres by macrophages may be extended to platelets likely to absorb VWD type 2B mutants to their surface. Indeed, electron microscopic analysis has previously revealed the presence of VWF at the surface of platelets derived from VWD type 2B patients,21 showing that VWF-coated platelets may be present in the circulation of VWD type 2B patients. We therefore tested whether VWF is also present at the surface of platelets in VWD type 2B mice and if this correlates with the presence or absence of thrombocytopenia. Different sets of mice were considered: mice expressing (1) wt-mVWF, which have normal platelet counts (652 ± 196 × 103 platelets per μL; n = 18); (2) p.V1316M-mVWF, which have severe thrombocytopenia (177 ± 113 × 103 platelets per μL; n = 26); or (3) p.R1306Q, which have either mild thrombocytopenia (485 ± 93 × 103 platelets per μL; n = 37) or severe thrombocytopenia (221 ± 65 × 103 platelets per μL; n = 14). Blood smears were prepared with EDTA-anticoagulated blood, and immunofluorescence staining was performed for VWF together with the platelet surface marker CD41. In nonthrombocytopenic mice or mildly thrombocytopenic mice, we observed numerous single CD41-positive platelets. However, no VWF could be detected at the surface of these platelets (Figure 3A-B). As expected, blood smears of thrombocytopenic mice contained fewer platelets, which were present as single platelets or assembled in platelet aggregates. Interestingly, numerous platelets (both isolated and assembled in aggregates) stained strongly positive for VWF, irrespective of whether thrombocytopenic mice were expressing mutant p.V1316M-mVWF or p.R1306Q-mVWF (Figure 3C-D). These data indicate that VWF mutants carrying type 2B mutations may adsorb onto circulating platelets and that platelet binding seems to be associated with the occurrence of thrombocytopenia.
VWF/platelet complexes circulate in the blood of VWD type 2B mice. Blood smears prepared with blood collected from mice expressing either wt-mVWF (A), p.R1306Q-mVWF (B-C), or p.V1316M-mVWF (D). Mice had normal or slightly low platelet counts (A-B) or were thrombocytopenic (C-D). Platelets were detected by staining for the CD41 surface marker (red), and VWF (green) was detected with primary rabbit anti-human VWF antibody followed by a secondary AlexaFluor 488–goat anti-rabbit antibody. Original magnification is ×100. (A-B) Single platelets can be observed, and no VWF at the platelet surface could be detected. (C-D) Thrombocytopenic mice display circulating single platelets and platelet aggregates, both of which are characterized by the presence of VWF at the platelet surface.
VWF/platelet complexes circulate in the blood of VWD type 2B mice. Blood smears prepared with blood collected from mice expressing either wt-mVWF (A), p.R1306Q-mVWF (B-C), or p.V1316M-mVWF (D). Mice had normal or slightly low platelet counts (A-B) or were thrombocytopenic (C-D). Platelets were detected by staining for the CD41 surface marker (red), and VWF (green) was detected with primary rabbit anti-human VWF antibody followed by a secondary AlexaFluor 488–goat anti-rabbit antibody. Original magnification is ×100. (A-B) Single platelets can be observed, and no VWF at the platelet surface could be detected. (C-D) Thrombocytopenic mice display circulating single platelets and platelet aggregates, both of which are characterized by the presence of VWF at the platelet surface.
Macrophage depletion relieves thrombocytopenia in VWD type 2B mice
Having established that (1) microspheres coated with a VWD type 2B mutant are taken up efficiently by macrophages, (2) such mutants adsorb to the surface of circulating platelets, and (3) adsorption to platelets seems to be associated with thrombocytopenia, we further studied the role of macrophages in vivo in the removal of VWF/platelet complexes from the circulation. Mice expressing wt-mVWF, p.R1306Q-mVWF, or p.V1316M-mVWF were treated with a single-dose infusion of the macrophage-destructing agent liposome-clodronate or the control liposome–phosphate-buffered saline (PBS).22 Blood was collected 1 hour before and 24 and 48 hours after liposome treatment and analyzed for platelet count and VWF:Ag levels. VWF:Ag was used as a measure for the efficacy of macrophage depletion.18 Administration of control liposome-PBS did not affect VWF levels in mVWF-expressing mice (Figure 4A). In contrast, VWF levels were increased three- to fivefold after liposome-clodronate treatment in mice expressing either wt-mVWF or mutant mVWF (Figure 4A), indicating that macrophage depletion was successful in these mice. As for platelet counts, these remained unchanged in wt-mVWF mice after injection of either the control liposome-PBS or liposome-clodronate, suggesting that macrophages play a minor role in the clearance of normal platelets in mice (Figure 4B). As for VWD type 2B mice, severe thrombocytopenia remained stable after control liposome-PBS treatment (Figure 4C-D). In contrast, the infusion of liposome-clodronate in these mice resulted in a two- to threefold increase in platelet counts (from 180 ± 24 × 103 platelets per μL to 405 ± 34 × 103 platelets per μL after 48 hours, P = .0015, for p.V1316M-mVWF; from 326 ± 88 × 103 platelets per μL to 608 ± 21 × 103 platelets per μL after 48 hours, P = .0028, for p.R1306Q-mVWF), thereby relieving the severe thrombocytopenic state (Figure 4C-D). These results point to a dominant role of macrophages in the uptake of VWF/platelet complexes that circulate in VWD type 2B.
Liposome-clodronate macrophage depletion relieves VWD type 2B–associated thrombocytopenia. (A) VWF:Ag levels were measured in mice before and 24 hours after treatment with liposome-PBS or liposome-clodronate. VWF:Ag levels after treatment were compared with those before treatment. Antigen levels before treatment were arbitrarily set at 100%. (B-D) Platelet counts were determined in mice expressing wt-mVWF (B), p.V1316M-mVWF (C), or p.R1306Q-mVWF (D) before and 24 and 48 hours after injection with liposome-PBS or liposome-clodronate. Data represent mean ± SEM of 3 injected mice/treatment.
Liposome-clodronate macrophage depletion relieves VWD type 2B–associated thrombocytopenia. (A) VWF:Ag levels were measured in mice before and 24 hours after treatment with liposome-PBS or liposome-clodronate. VWF:Ag levels after treatment were compared with those before treatment. Antigen levels before treatment were arbitrarily set at 100%. (B-D) Platelet counts were determined in mice expressing wt-mVWF (B), p.V1316M-mVWF (C), or p.R1306Q-mVWF (D) before and 24 and 48 hours after injection with liposome-PBS or liposome-clodronate. Data represent mean ± SEM of 3 injected mice/treatment.
Platelets are targeted to macrophages in liver and spleen of VWD type 2B mice
Next, we determined whether VWF/platelet complexes are indeed targeted to macrophages in liver and/or spleen of VWD type 2B mice. To this end, platelets isolated from p.V1316M-mVWF–expressing mice were isolated, stained with fluorescent CMTMR cell tracker, and reinfused in mice expressing p.V1316M-mVWF. Two hours after platelet infusion, liver and spleen were harvested and snap-frozen, and organ cryosections were prepared. To identify the cellular destination of VWF/platelet complexes in liver and spleen of these mice, cryosections were stained for organ-specific macrophage subpopulations via immunofluorescence. In liver, the vast majority (78 ± 9%) of platelets colocalized with resident Kupffer cells, identified via a monoclonal anti-mouse CD68 antibody (Figure 5A). A more dispersed picture was obtained for the spleen. We observed some platelets colocalizing with F4/80-positive macrophages in the red pulp, and with macrophage receptor with collagenous structure (MARCO)–positive macrophages in the marginal zone (not shown). However, most of the platelets present in the spleen (68 ± 6%) colocalized with CD169-positive marginal metallophilic macrophages (Figure 5B).
Platelets colocalize with macrophages in liver and spleen of VWD type 2B mice. Platelets were isolated from wt-mVWF–expressing or p.V1316M-mVWF–expressing mice, stained with CMTMR cell tracker and reinfused (8 × 107 platelets per mouse) in wt-mVWF–expressing or p.V1316M-mVWF–expressing mice, respectively. Two hours after platelet infusion, liver and spleen were isolated to prepare cryosections. Macrophage subpopulations were identified via immunofluorescence: CD68-positive resident macrophages (Kupffer cells) in liver (A) and CD169-positive marginal metallophilic macrophages in spleen (B). Macrophages are in red, CMTMR-fluorescent platelets are in green, and nuclei are in blue. Original magnification is ×20.
Platelets colocalize with macrophages in liver and spleen of VWD type 2B mice. Platelets were isolated from wt-mVWF–expressing or p.V1316M-mVWF–expressing mice, stained with CMTMR cell tracker and reinfused (8 × 107 platelets per mouse) in wt-mVWF–expressing or p.V1316M-mVWF–expressing mice, respectively. Two hours after platelet infusion, liver and spleen were isolated to prepare cryosections. Macrophage subpopulations were identified via immunofluorescence: CD68-positive resident macrophages (Kupffer cells) in liver (A) and CD169-positive marginal metallophilic macrophages in spleen (B). Macrophages are in red, CMTMR-fluorescent platelets are in green, and nuclei are in blue. Original magnification is ×20.
Increased uptake of platelets in spleen and liver of VWD type 2B mice
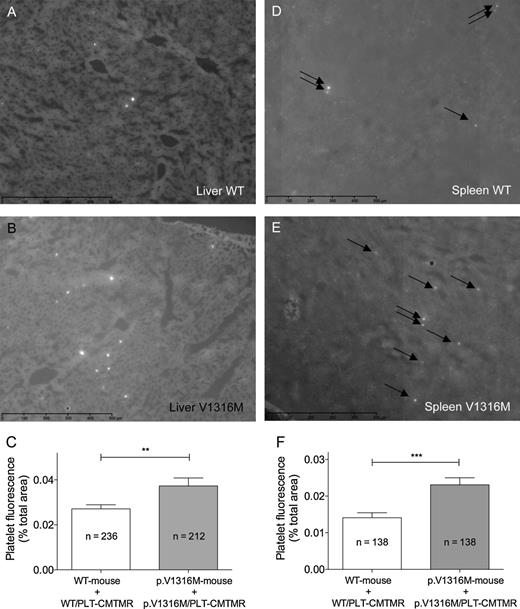
To compare the quantitative distribution of platelets in wt-mVWF–expressing and p.V1316M-mVWF–expressing mice, organs were isolated 2 hours after injection of fluorescent platelets. Subsequently, liver and spleen nonconsecutive whole cryosection slides (n = 6 and 9 for liver and spleen, respectively) were scanned using NanoZoomer equipment, and fluorescence was subsequently quantified with ImageJ software. Representative images of liver and spleen sections of wt-mVWF–expressing and p.V1316M-mVWF–expressing mice are shown in Figure 6. Quantitative analysis revealed that both liver and spleen of wt-mVWF–expressing mice contained significantly fewer fluorescent spots, and thus platelets, compared with organs taken from p.V1316M-mVWF–expressing mice (P = .0098 and P = .0002 for liver and spleen, respectively; Figure 6C,F). Thus, our results indicate that there is an accumulation of VWF/platelet complexes in liver and spleen of VWD type 2B mice and that macrophages are implicated in this process.
Quantification of wt-platelet or V1316M-platelet recovery in liver and spleen of wt-expressing and p.V1316M-mVWF–expressing mice. Liver (A-B) and spleen (D-E) cryosections were prepared as described in the legend of Figure 5 and scanned with a NanoZoomer. Images comprising whole sections were generated via virtual microscopy technology using NDPview software. Quantification (C,F) was performed using ImageJ software and is expressed as the percentage of surface covered by fluorescent spots (corresponding to fluorescent platelets) per field. Data represent mean ± SEM. The number of fields analyzed is indicated. **P = .0098; ***P = .0002. Representative images for cryosections of liver (A-B) and spleen (D-E) obtained from wt-mVWF–expressing mice (A-D) or p.V1316M-mVWF–expressing mice (B-E) are shown. Arrows point to fluorescent spots.
Quantification of wt-platelet or V1316M-platelet recovery in liver and spleen of wt-expressing and p.V1316M-mVWF–expressing mice. Liver (A-B) and spleen (D-E) cryosections were prepared as described in the legend of Figure 5 and scanned with a NanoZoomer. Images comprising whole sections were generated via virtual microscopy technology using NDPview software. Quantification (C,F) was performed using ImageJ software and is expressed as the percentage of surface covered by fluorescent spots (corresponding to fluorescent platelets) per field. Data represent mean ± SEM. The number of fields analyzed is indicated. **P = .0098; ***P = .0002. Representative images for cryosections of liver (A-B) and spleen (D-E) obtained from wt-mVWF–expressing mice (A-D) or p.V1316M-mVWF–expressing mice (B-E) are shown. Arrows point to fluorescent spots.
Platelet clearance is accelerated in VWD type 2B
The finding that VWF/platelet complexes in VWD type 2B mice are taken up efficiently by macrophages in liver and spleen may suggest that the platelets have a shorter circulatory life span in these mice compared with normal mice. We therefore decided to measure platelet survival in mice expressing wt-mVWF or mutant mVWF. Four days after the onset of VWF expression, mice were intravenously infused with N-hydroxysuccinimide-biotin allowing biotinylation of >90% of the platelets.17 It has previously been demonstrated that this approach does not interfere with platelet function or life span in vivo.17 The disappearance of biotinylated platelets was followed daily as the percentage of residual biotinylated platelets relative to the total CD41-positive platelet population. A more rapid disappearance of platelets in p.V1316M-mVWF–expressing or p.R1306Q-mVWF–expressing mice compared with wt-mVWF–expressing mice was observed (Figure 7). For instance, residual platelet numbers 48 hours after injection were 29.6 ± 8.4% (p.V1316M) and 34.9 ± 8.8% (p.R1306Q) vs 47.3 ± 7.1% in VWD type 2B and wt-mVWF–expressing mice, respectively (P < .001 and P = .02). Interestingly, the p.R1306Q seems to have an intermediate clearance rate between p.V1316M-mVWF and wt-mVWF: similar platelet counts as wt-mVWF were observed after 24 hours; although similar to p.V1316M-mVWF, no platelets could be detected after 72 hours. Of note, a similar disappearance rate of platelets was found for wt-mVWF–expressing mice and for control wt-C57B6 mice (49.5 ± 6.2% residual platelets after 48 hours; not shown). The observation that platelet life span is reduced in VWD type 2B mice suggests that the presence of mutated VWF at the platelet surface might accelerate their clearance.
Platelet life span is reduced in VWD type 2B mice with respect to wt-mVWF–expressing mice. Mice expressing wt-mVWF (A-B, closed circles), p.V1316M-mVWF (A, open circles), or p.R1306Q-mVWF (B, open squares) were infused with N-hydroxysuccinimide-biotin, which allows platelet biotinylation. Residual biotinylated platelets were quantified by flow cytometry. Data are expressed as the percentage of biotinylated platelets relative to the total CD41-positive platelet population with t = 0 being arbitrarily set at 100%. Data represent mean ± standard deviation. N = 3 to 13 mice.
Platelet life span is reduced in VWD type 2B mice with respect to wt-mVWF–expressing mice. Mice expressing wt-mVWF (A-B, closed circles), p.V1316M-mVWF (A, open circles), or p.R1306Q-mVWF (B, open squares) were infused with N-hydroxysuccinimide-biotin, which allows platelet biotinylation. Residual biotinylated platelets were quantified by flow cytometry. Data are expressed as the percentage of biotinylated platelets relative to the total CD41-positive platelet population with t = 0 being arbitrarily set at 100%. Data represent mean ± standard deviation. N = 3 to 13 mice.
Discussion
VWD type 2B is a paradoxical example where the assembly of platelets into aggregates is associated with bleeding rather than thrombosis. Interestingly, the bleeding tendency in VWD type 2B patients correlates strongly with their platelet count, as became apparent in a cohort study involving 67 patients.4 The bleeding risk is indeed fivefold higher in patients having platelets counts below 140 × 103 platelets per μL compared with those with 140 × 103 platelets per μL or more.4 The degree of thrombocytopenia is also of relevance for the clinical management of the patients in that it may affect the choice of treatment. Desmopressin is usually contraindicated for use in VWD type 2B patients, but it has been used safely in several patients with a mildly reduced platelet count.11,23-26 In contrast, replacement therapy is preferred for VWD type 2B patients that manifest severe thrombocytopenia, eventually in combination with platelet transfusion. Alternatively, agents blocking VWF-platelet interactions may be used to correct desmopresin-induced thrombocytopenia.27
The molecular basis of the thrombocytopenia in VWD type 2B has remained obscure and may originate from a disturbed megakaryocytopoiesis.5-7 However, it has originally been proposed that the thrombocytopenia would result from the removal of VWF/platelet aggregates from the circulation.8-10 By using a mouse model for VWD type 2B, we provide direct evidence that the formation of VWF/platelet aggregates is indeed associated with an enhanced uptake of such complexes in macrophages in liver and spleen (Figures 5 and 6). In addition, the life span of platelets in VWD type 2B mice is significantly shorter compared with the life span of platelets in control mice (Figure 7). Together, these mechanisms may contribute to the VWD type 2B–related thrombocytopenia observed in these mice.
Evidence for the involvement of macrophages in the clearance of the VWF/platelet complexes became apparent from different experimental approaches. First, we tested the effect of chemical macrophage depletion. The efficacy of macrophage depletion was assessed via the measurement of VWF:Ag levels, which were increased following liposome-clodronate infusions but not after control liposome infusion (Figure 4). These data are in agreement with our previous observation in which GdCl3-mediated depletion of macrophages resulted in increased endogenous VWF:Ag levels.18 Macrophage depletion did not affect platelet counts in mice expressing wt-mVWF within the 48-hour observation period (Figure 4). This observation is in line with a previous study that reported no effect on platelet numbers in control mice within the first 48 hours after liposome-clodronate injection.28 Interestingly, we observed a remarkable two- to threefold increase in platelet counts in thrombocytopenic mice expressing p.V1316M-mVWF: from 157 ± 62 × 103 platelets per μL to 420 ± 30 × 103 platelets per μL after 24 hours and 405 ± 60 × 103 platelets per μL after 48 hours (Figure 4). Thus, macrophage depletion relieves the thrombocytopenic state in VWD type 2B mice. Similarly, in a mouse model for immune thrombocytopenia, a comparable increase in platelet counts following macrophage depletion was described by Alves-Rosa and colleagues.28
More direct evidence for the role of macrophages in the scavenging of VWF/platelet complexes came from immunohistochemical analysis of liver and spleen sections of mice injected with fluorescently labeled platelets. In liver, nearly 80% of fluorescent platelets colocalized with CD68-positive macrophages (ie, Kupffer cells) (Figure 5). In spleen, the majority of platelets (68%) colocalized with CD169-positive marginal metallophilic macrophages (Figure 5), although some platelets were found in association with F4/80-positive macrophages in the red pulp, and with MARCO-positive macrophages in the marginal zone. This selective targeting of VWF/platelets complexes to CD169-positive marginal metallophilic macrophages rather than F4/80-positive or MARCO-positive macrophages is unexpected, and additional studies are needed to decipher the mechanism behind this selective targeting. CD169-positive marginal macrophages are known for their role in neutrophil homeostasis, clearance of apoptotic cells, and the suppression of immune responses toward these apoptotic cells.29,30 In contrast to CD169-positive marginal macrophages, the role of liver Kupffer cells in the clearance of platelets is less surprising. It has been shown previously that these cells mediate the uptake of platelets in immune thrombocytopenia.31 In addition, liver Kupffer cells also mediate the uptake of short-term refrigerated platelets.32
The mechanism that explains the enhanced phagocytosis of platelets by macrophages in VWD type 2B mice remains to be elucidated. Our observation that thrombocytopenia only occurs in those mice where circulating platelets are covered by VWF mutants (Figure 3) seems to indicate that increased phagocytosis is limited to VWF/platelet complexes. It has been assumed that, in particular, HMW multimers adsorb to the platelet surface because it is those multimers that are lacking in VWD type 2B. However, we have reported that HMW multimers are selectively lacking in VWD type 2B mice that express ADAMTS13, whereas the full multimer range is present in VWD type 2B mice that are deficient for this VWF-cleaving protease.12 This strongly suggests that the lack of HMW multimers is mainly due to ADAMTS13-mediated degradation rather than adsorption on platelets. In contrast, adsorption of either large or small multimers onto platelets is more likely to be the signal that provokes uptake of VWF/platelet complexes by macrophages.
The notion that both VWF and platelets are potential ligands for macrophages complicates the elucidation of the exact phagocytosis mechanism. Previously, we and others have shown that shear stress enhances the LRP1-dependent uptake of VWF by macrophages.18-20 In the present study, we found that conversion of VWF in its active, platelet-binding conformation (either by botrocetin or via a gain-of-function VWD type 2B mutation) also promotes the uptake of VWF by macrophages (Figures 1 and 2). It is possible therefore that platelets are a by-catch when macrophages bind and endocytose VWF type 2B mutants that are at the platelet surface. Alternatively, binding of mutant VWF to the platelet GpIbα receptor may modulate platelets in such a manner that the platelet is more easily phagocytosed by macrophages. For instance, VWF binding may mimic exposure of platelets to cold and induce clustering of the VWF receptor GpIbα, a process known to promote platelet uptake by macrophages.33 Additional studies are needed to distinguish between the various possibilities.
In conclusion, we have now identified at least 1 of the mechanisms underlying thrombocytopenia in VWD type 2B. Although long suspected, the increased removal of VWF/platelet aggregates from the circulation had never been formally demonstrated. It is becoming clear that in severe VWD type 2B, such as in patients with the p.V1316M mutations, other mechanisms are also involved, in particular with relation to megakaryocytopoiesis. Our results with mice expressing the p.R1306Q mutation are also of particular interest because they suggest that the situation can be fluctuating: under specific circumstances, the mutant can start accumulating on platelets, a situation associated with more severe thrombocytopenia. In a clinical setting, such potential variations should be closely monitored, for instance during pregnancy. Our findings may also be of relevance for other pathological conditions where the presence of active VWF is associated with thrombocytopenia, such as thrombotic thrombocytopenic purpura or malaria.34,35 Indeed, both disorders are associated with an increased uptake of platelets by macrophages,36,37 which could be due to the adsorption of active VWF to the platelet surface under these conditions. It is tempting to speculate that this process may be a mechanism to minimize the presence of circulating VWF/platelet complexes, which have the potential to occlude the microvasculature.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Nico van Rooijen of the Foundation Clodronate Liposomes (Haarlem, The Netherlands) for generously providing the liposome-clodronate, Dr Olivier Trassard for help with microscopic analysis, and Hamamatsu (Massy, France) for making the NanoZoomer 2.0-HT available for analysis.
This work was supported by the Fondation pour la Recherche Médicale (FRM-SPF20101220866) (C.C. and P.J.L.), the Agence Nationale de la Recherche (ANR-11-BSV1-010-01) (C.V.D.), the Dutch Heart Foundation (NHS 2006T5301) (B.d.L.), and the Sanquin Blood Supply Foundation (B.d.L.).
Authorship
Contribution: C.C., V.D., and Y.-P.W. performed experiments; C.C., V.D., Y.-P.W., A.K., B.d.L., and P.J.L. analyzed the data; P.G.d.G., O.D.C., C.V.D., B.d.L., and P.J.L. were responsible for conception, design, and supervision of the study; C.C. and P.J.L. wrote the manuscript; and all authors corrected draft versions and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter J. Lenting, INSERM U770, 80 rue du General Leclerc, 94276 Le Kremlin-Bicêtre, France; e-mail: peter.lenting@inserm.fr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal