Key Points
Aurora and IKK kinases physically and functionally interact to promote TRAIL resistance in multiple myeloma through NF-κB activation.
Pharmacological blockade of Aurora kinases abrogates TRAIL-induced Aurora-IKK kinases interactions and NF-κB activation.
Abstract
Constitutive activation of the canonical and noncanonical nuclear factor-κB (NF-κB) pathways is frequent in multiple myeloma (MM) and can compromise sensitivity to TRAIL. In this study, we demonstrate that Aurora kinases physically and functionally interact with the key regulators of canonical and noncanonical NF-κB pathways IκB kinase β (IKKβ) and IKKα to activate NF-κB in MM, and the pharmacological blockade of Aurora kinase activity induces TRAIL sensitization in MM because it abrogates TRAIL-induced activation of NF-κB. We specifically found that TRAIL induces prosurvival signaling by increasing the phosphorylation state of both Aurora and IKK kinases and their physical interactions, and the blockade of Aurora kinase activity by pan-Aurora kinase inhibitors (pan-AKIs) disrupts TRAIL-induced survival signaling by effectively reducing Aurora-IKK kinase interactions and NF-κB activation. Pan-AKIs consistently blocked TRAIL induction of the antiapoptotic NF-κB target genes A1/Bfl-1 and/or Mcl-1, both important targets for TRAIL sensitization in MM cells. In summary, these results identify a novel interaction between Aurora and IKK kinases and show that these pathways can cooperate to promote TRAIL resistance. Finally, combining pan-AKIs with TRAIL in vivo showed dramatic efficacy in a multidrug-resistant human myeloma xenograft model. These findings suggest that combining Aurora kinase inhibitors with TRAIL may have therapeutic benefit in MM.
Introduction
Multiple myeloma (MM) is a malignancy of plasma cells that accumulate in the bone marrow (BM). Although novel drugs such as bortezomib and thalidomide have extended the overall survival of MM patients, they often do not achieve lasting cures, providing an impetus to search for novel therapeutic modalities.1 TRAIL (also known as Apo2L) is a member of the tumor necrosis factor family of death receptor ligands and has significant potential for use in cancer therapy because of its potent ability to selectively kill cancer cells while sparing normal cells.2 A variety of preclinical data demonstrated that TRAIL exerts a remarkable antitumor activity both in vitro and in vivo.2,3
Despite these promising data, TRAIL resistance, both inherent and acquired, is now recognized as a common and unfortunate outcome, and phase 1/2 clinical trials have demonstrated a good toxicity profile for TRAIL but limited evidence of antitumor activity.4
Resistance to TRAIL-mediated apoptosis can occur through multiple mechanisms, including increased expression of TRAIL decoy receptors, overexpression of intracellular inhibitory proteins, and activation of mitogenic and prosurvial signals.5,6 Strategies have been developed to bypass TRAIL resistance in diverse preclinical models and are based on the combination of TRAIL and numerous conventional and investigational anticancer agents.2,3,6-11
The Aurora kinases A, B, and C are highly conserved serine/threonine kinases that play essential and distinct roles in mitosis.12 Both Aurora A and B are frequently overexpressed in human cancers and have been implicated in oncogenic transformation, including development of chromosomal instability and derangement of multiple tumor suppressor and oncoprotein regulated pathways.12,13 In this preclinical study, we demonstrate, both in vitro and in vivo, a potent and specific antimyeloma activity of the combination of pan-Aurora kinase inhibitors (pan-AKIs) with TRAIL and elucidate the multiple molecular mechanisms, most of them converging on the nuclear factor-κB (NF-κB) pathway, by which Aurora kinase inhibition increases the therapeutic effect of TRAIL in MM cells. We propose a novel mechanism linking Aurora and IKK signaling in MM and suggest that the targeted disruption of this interaction can be an effective approach to increase TRAIL sensitivity in MM.
Materials and methods
Study approval
Approval for the study was obtained from the Institutional Review Board of the Department of Clinical and Experimental Medicine, University of Parma. Approval for studies on human tissue samples was obtained from informed consent of all patients and healthy donors in accordance with the Declaration of Helsinki protocol. All procedures involving animal models were performed in accordance with national and international laws and policies. The experimental protocol was approved by the University of Parma Ethics Committee and by the Italian Ministry of Health.
Reagents
Human recombinant KillerTRAIL was purchased from Alexis, San Diego, CA. Pan-AKI MK-0457 was provided by Merck & Co. (Rahway, NJ). Pan-Aurora kinase inhibitor PHA-680632 was kindly provided by Dr Jürgen Moll from Nerviano Medical Sciences, Nerviano, Italy. For the other reagents, see supplemental Methods (available on the Blood Web site).
Cell cultures
Human Myeloma cell lines (HMCLs) RPMI 8226, OPM-2, U266, and JJN3 were purchased from DSM (Braunschweig, Germany). The multi-drug–resistant RPMI 8226/R5 cell line was established by continuous exposure of 8226 parental cells to increasing concentrations of R115777 for over 6 months.14 Primary MM cells from MM patients, primary BM stromal cells (BMSCs) from BM samples of MM patients, and peripheral blood mononuclear cells of healthy subjects were isolated and treated as described in the supplemental Methods.
Apoptosis assays, siRNA transfections, and molecular and statistical analysis
These methods were previously published15 and are described in the supplemental Methods.
Enzyme-linked immunosorbent assay
The DNA-binding activity of NF-κB subunits was quantified by the TransAm NF-κB family enzyme-linked immunosorbent assay kit according to the manufacturer’s protocols (Active Motif, Rixensart, Belgium).
Animal studies, histology, and immunohistochemistry
Five-week-old nonobese diabetic (NOD) severe combined immunodeficiency (SCID) NOD.CB17-Prkdcscid/J (NOD-SCID) mice were obtained from the The Jackson Laboratory (Bar Harbor, ME) and maintained under the same specific pathogen-free conditions. The histological and immunohistochemical studies are described in the supplemental Methods.
Results
Blockade of Aurora kinase activity enhances the cytotoxic effect of TRAIL on MM cells
We first analyzed the pharmacologic interactions between pan-AKIs (MK-0457 or PHA-680632) and TRAIL using a fixed-ratio experimental design on HMCLs displaying different degrees of sensitivity to TRAIL (Table 1). We found that the combined treatment of pan-AKIs plus TRAIL resulted in the synergistic induction of apoptosis in TRAIL-sensitive MM cells (Figure 1Ai); moreover, pretreatment with pan-AKIs significantly enhanced TRAIL-induced cell death in highly TRAIL-resistant JJN3 cells (Figure 1Aii); apoptotic cell death of the drug-treated HMCLs was evaluated by either sub-G1 or Annexin V/PI analysis (representative data in supplemental Figure 1A).
TRAIL sensitivity and NF-κB profile of HMCLs used in this study
| HMCLs . | ED50 of TRAIL, ng/mL . | NF-κB pathway . | NF-κB index . | NF-κB mutation . |
|---|---|---|---|---|
| OPM-2 | 4.7 ± 0.9 | Weak canonical | 9.03 | — |
| RPMI 8226 | 4.8 ± 0.4 | Both canonical and noncanonical | 10.44 | TRAF3 |
| U266 | 55.2 ± 6.8 | Both canonical and noncanonical | 10.41 | TRAF3 |
| RPMI 8226/R5 | 50.2 ± 7.1 | Both canonical and noncanonical | ND | ND |
| JJN3 | >1000 | Both canonical and noncanonical | 10.80 | NIK |
| HMCLs . | ED50 of TRAIL, ng/mL . | NF-κB pathway . | NF-κB index . | NF-κB mutation . |
|---|---|---|---|---|
| OPM-2 | 4.7 ± 0.9 | Weak canonical | 9.03 | — |
| RPMI 8226 | 4.8 ± 0.4 | Both canonical and noncanonical | 10.44 | TRAF3 |
| U266 | 55.2 ± 6.8 | Both canonical and noncanonical | 10.41 | TRAF3 |
| RPMI 8226/R5 | 50.2 ± 7.1 | Both canonical and noncanonical | ND | ND |
| JJN3 | >1000 | Both canonical and noncanonical | 10.80 | NIK |
HMCLs were cultured in presence of escalating doses of TRAIL (0.6-2000 ng/mL). After 24 hours, cell death was measured by annexin-V staining, and the median effective dose (ED50, dose producing 50% of cytotoxicity) was estimated using the Median Effect Equation of Chou. Values represent mean ± SD of 4 independent experiments.
The contribution of the canonical and noncanonical NF-κB pathways in HMCLs was estimated from steady-state levels of NF-κB subunits and/or effect of IKK β inhibition on nuclear localization and DNA binding activity of the NF-κB p65 and p52 subunits.19,20 The NF-κB index was calculated using the average expression of 10 NF-κB target genes19 (Genes comprising the NF-κB index in myeloma). NF-κB mutations were previously reported.19,20 —, no mutation identified; ND, nondetected; NIK, NF-κB inducing kinase.
Pan-AKIs potentiate the TRAIL-induced cell death in MM cells. (Ai) HMCLs seeded at 2.0 × 105 cells/mL were treated sequentially with escalating doses of MK-0457 (15-2000 ng/mL; 0.032-4.3 μM) or PHA-680632 (15-2000 ng/mL; 0.03-4 μM) for 3 hours and subsequently with TRAIL (0.5-50 ng/mL) alone or in combination with pan-AKIs at a fixed ratio of 25:1 for MK-0457/TRAIL and 50:1 for PHA-680632/TRAIL (in combination with pan-AKIs, TRAIL was used at 0.6, 1.2, 2.4, 4.8, 9.6, and 19.2 ng/mL). After 24 hours, cell death was measured by annexin-V binding. Combination index plots were then generated using the Calcusyn software. Combination index values <1.0 indicate synergism, 1.0 indicates additive effect, and >1.0 indicates an antagonistic effect. (Aii) JJN3 were seeded at 2.0 × 105 cells/mL in the presence of dimethyl sulfoxide (vehicle), MK-0457 (0.4 μM), or PHA-680632 (0.8 μM) for 3 hours and then incubated with TRAIL at the concentration of 300 ng/mL. After 24 hours, apoptosis was measured by annexin-V labeling. Values represent means ± SD of 4 independent experiments (*P < .01 vs either treatment alone; Dunnet test). (B) HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL at the indicated doses (ng/mL) in the presence or absence of IGF-1 (50 ng/mL), IL-6 (20 ng/mL) or BMSCs (HMCLs in direct contact to BMSC monolayer in a 5:1 ratio). After 24 hours, HMCLs were harvested and apoptotic HMCLs cells were determined by flow cytometry as CD38/CD138+ annexin-V+ cells; data represent means ± SD of quadruplicate experiments. Dexamethasone (2 μM) was used as control to monitor the protective effects of growth factors and stromal cells (*P < .05; **P < .005; Tukey-Kramer Honestly Significant Difference test). (Ci-ii) CD138-purified plasma cells from 5 patients with MM were isolated from BM and then seeded at 2.5 × 105 cells/mL. Samples from 1 to 3 were pretreated with MK-0457 (0.4 μM), and samples 4 and 5 were pretreated with PHA-680632 (0.8 μM) and then incubated with TRAIL (9.6 ng/mL) for 24 hours. Sample 4 was cultured in presence or absence of BMSCs. The cell death was measured by annexin-V staining. (i) Results are expressed as the net apoptosis induction [percentage of apoptosis in treated cells minus percentage of apoptosis in dimethylsulfoxide (vehicle-treated cells)] of all 5 primary samples tested. (ii) Histogram represents the mean percentage of apoptosis ± SD (expressed as net difference between the percentages of apoptosis in treated cells minus percentage of apoptosis in vehicle-treated cells) of the results obtained in the 5 different patient samples (*P < .05 vs either treatment alone; Dunnet test). (iii) Peripheral blood mononuclear cells from 3 healthy volunteers were treated with dimethylsulfoxide (vehicle), MK-0457 (0.4 μM), or PHA-680632 (0.8 μM) for 3 hours and then incubated with TRAIL (9.6 ng/mL). After 24 hours of treatment, cell death was then measured by annexin-V staining. (D) Transfection of Aurora A and/or Aurora B, but not the unrelated nonspecific control siRNA, led to a decrease in Aurora kinases protein expression in HMCLs without affecting the levels of the unrelated protein actin. At 48 hours after siRNA transfection, HMCLs were treated with TRAIL at the indicated doses (ng/mL) for 48 hours and the percentages of annexin-V+ apoptotic cells were then measured. Values are mean ± SD of 3 independent experiments (*P < .001 vs TRAIL nonspecific si-RNA [CONT]; Dunnet test).
Pan-AKIs potentiate the TRAIL-induced cell death in MM cells. (Ai) HMCLs seeded at 2.0 × 105 cells/mL were treated sequentially with escalating doses of MK-0457 (15-2000 ng/mL; 0.032-4.3 μM) or PHA-680632 (15-2000 ng/mL; 0.03-4 μM) for 3 hours and subsequently with TRAIL (0.5-50 ng/mL) alone or in combination with pan-AKIs at a fixed ratio of 25:1 for MK-0457/TRAIL and 50:1 for PHA-680632/TRAIL (in combination with pan-AKIs, TRAIL was used at 0.6, 1.2, 2.4, 4.8, 9.6, and 19.2 ng/mL). After 24 hours, cell death was measured by annexin-V binding. Combination index plots were then generated using the Calcusyn software. Combination index values <1.0 indicate synergism, 1.0 indicates additive effect, and >1.0 indicates an antagonistic effect. (Aii) JJN3 were seeded at 2.0 × 105 cells/mL in the presence of dimethyl sulfoxide (vehicle), MK-0457 (0.4 μM), or PHA-680632 (0.8 μM) for 3 hours and then incubated with TRAIL at the concentration of 300 ng/mL. After 24 hours, apoptosis was measured by annexin-V labeling. Values represent means ± SD of 4 independent experiments (*P < .01 vs either treatment alone; Dunnet test). (B) HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL at the indicated doses (ng/mL) in the presence or absence of IGF-1 (50 ng/mL), IL-6 (20 ng/mL) or BMSCs (HMCLs in direct contact to BMSC monolayer in a 5:1 ratio). After 24 hours, HMCLs were harvested and apoptotic HMCLs cells were determined by flow cytometry as CD38/CD138+ annexin-V+ cells; data represent means ± SD of quadruplicate experiments. Dexamethasone (2 μM) was used as control to monitor the protective effects of growth factors and stromal cells (*P < .05; **P < .005; Tukey-Kramer Honestly Significant Difference test). (Ci-ii) CD138-purified plasma cells from 5 patients with MM were isolated from BM and then seeded at 2.5 × 105 cells/mL. Samples from 1 to 3 were pretreated with MK-0457 (0.4 μM), and samples 4 and 5 were pretreated with PHA-680632 (0.8 μM) and then incubated with TRAIL (9.6 ng/mL) for 24 hours. Sample 4 was cultured in presence or absence of BMSCs. The cell death was measured by annexin-V staining. (i) Results are expressed as the net apoptosis induction [percentage of apoptosis in treated cells minus percentage of apoptosis in dimethylsulfoxide (vehicle-treated cells)] of all 5 primary samples tested. (ii) Histogram represents the mean percentage of apoptosis ± SD (expressed as net difference between the percentages of apoptosis in treated cells minus percentage of apoptosis in vehicle-treated cells) of the results obtained in the 5 different patient samples (*P < .05 vs either treatment alone; Dunnet test). (iii) Peripheral blood mononuclear cells from 3 healthy volunteers were treated with dimethylsulfoxide (vehicle), MK-0457 (0.4 μM), or PHA-680632 (0.8 μM) for 3 hours and then incubated with TRAIL (9.6 ng/mL). After 24 hours of treatment, cell death was then measured by annexin-V staining. (D) Transfection of Aurora A and/or Aurora B, but not the unrelated nonspecific control siRNA, led to a decrease in Aurora kinases protein expression in HMCLs without affecting the levels of the unrelated protein actin. At 48 hours after siRNA transfection, HMCLs were treated with TRAIL at the indicated doses (ng/mL) for 48 hours and the percentages of annexin-V+ apoptotic cells were then measured. Values are mean ± SD of 3 independent experiments (*P < .001 vs TRAIL nonspecific si-RNA [CONT]; Dunnet test).
Neither interleukin (IL)-6 (20 ng/mL) nor insuline-like growth factor 1 (IGF-1) (50 ng/mL) blocked pan-AKI/TRAIL-triggered apoptosis in HMCLs (Figure 1B). Furthermore, exogenous IL-6 conferred resistance to TRAIL-mediated apoptosis in RPMI 8226 and OPM-2, but pretreatment with pan-AKIs was able to restore TRAIL sensitivity in both HMCLs (Figure 1B).
Furthermore, consistent with previous studies, we found that adherence of MM cells to BMSCs significantly increased their resistance to TRAIL-induced cell death compared with exogenous IL-616,17 (Figure 1B). However, pan-AKIs were able to completely or almost completely reverse the BMSC-mediated TRAIL resistance in RPMI 8226/R5 and OPM-2 (Figure 1B), whereas in RPMI 8226 and U266 HMCLs that was previously reported to strongly increase the caspase-8 inhibitor FLIP when cocultured with BMSCs,16 pan-AKIs partially (but significantly) overcame stroma-mediated TRAIL resistance when TRAIL was used at fourfold higher concentrations (9.6 or 19.2 ng/mL, respectively) than those used in the absence of stroma (Figure 1B). We found that, similarly to HMCLs, the treatment with pan-AKIs significantly enhanced apoptosis of fresh, purified MM cells induced by TRAIL (P < .05; n = 5) (Figure 1Ci-ii). The characteristics of these patients are summarized in Table 2.
Clinical characteristics of the MM patients
| MM patient no. . | Age, y . | Patient stage . | BM plasma cells, % . | State of disease . |
|---|---|---|---|---|
| 1 | 80 | II | 85 | Diagnosis |
| 2 | 59 | III | 65 | Diagnosis |
| 3 | 67 | III | 60 | Diagnosis |
| 4 | 73 | III | 98 | Relapse |
| 5 | 73 | III | 60 | Relapse |
| MM patient no. . | Age, y . | Patient stage . | BM plasma cells, % . | State of disease . |
|---|---|---|---|---|
| 1 | 80 | II | 85 | Diagnosis |
| 2 | 59 | III | 65 | Diagnosis |
| 3 | 67 | III | 60 | Diagnosis |
| 4 | 73 | III | 98 | Relapse |
| 5 | 73 | III | 60 | Relapse |
Primary CD138+ MM cells were isolated from BM aspiration of MM patients at the diagnosis or relapse and were treated in vitro with MK-0457 (0.4 μM) or PHA-680632 (0.8 μM) and TRAIL (9.6 ng/mL) for 24 hours.
In contrast, no significant cytotoxicity in peripheral blood mononuclear cells from 3 healthy volunteers was observed after pan-AKI/TRAIL treatment (Figure 1Ciii).
Finally, the functional knockout of Aurora-A and -B gene expression by small interfering (si)RNAs recapitulated the ability of pan-AKIs to sensitize HMCLs to TRAIL (Figure 1D; representative data in supplemental Figure 1B), thereby confirming the significant role of these kinases in mediating TRAIL sensitization.
Taken together, these data indicate that the combination of pan-AKIs/TRAIL triggers significant antitumor activity even against MM cells in the BM milieu.
Combined exposure to pan-AKIs and TRAIL induces caspase-dependent MM cell apoptosis
To determine whether pan-AKI/TRAIL-induced cytotoxicity is mediated via activation of caspase and poly(ADP-ribose) polymerase (PARP) degradation, HMCLs were treated with pan-AKIs and/or TRAIL for 24 hours, and caspase activation and PARP fragmentation were analyzed by western blotting. Treatment of pan-AKIs strongly potentiated the TRAIL-induced cleavage/activation of caspase-8, -9, and -3 and PARP fragmentation in all HMCLs (Figure 2A). Consistent with these findings, we observed that the combined treatment induced a robust cleavage/activation of Bid, a substrate of active caspase-8, which is a key protein involved in the cross-talk between the intrinsic and extrinsic apoptotic pathways in all tested HMCLs (Figure 2A).
Coadministration of pan-AKIs and TRAIL activates the caspase cascade in MM. (A) HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) for 24 hours, after which cells were lysed and subjected to western blot analysis to monitor the expression of PARP, cleaved-PARP, cleaved caspase-8, Bid, truncated Bid (t-Bid), cleaved caspase-9, and cleaved caspase-3; blots were subsequently reprobed for actin expression to ensure equivalent loading and transfer of proteins. (B) HMCLs were cultured with pan-AKIs and TRAIL at the doses indicated in A in the presence or absence of 30 μM caspase inhibitors for 24 hours, after which the percentage of apoptotic cells was determined by the annexin-V method. Data represent means ± SD of 3 independent experiments (*P < .05; **P < .005 vs pan-AKIs/TRAIL Z-FA-FMK; Dunnet test).
Coadministration of pan-AKIs and TRAIL activates the caspase cascade in MM. (A) HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) for 24 hours, after which cells were lysed and subjected to western blot analysis to monitor the expression of PARP, cleaved-PARP, cleaved caspase-8, Bid, truncated Bid (t-Bid), cleaved caspase-9, and cleaved caspase-3; blots were subsequently reprobed for actin expression to ensure equivalent loading and transfer of proteins. (B) HMCLs were cultured with pan-AKIs and TRAIL at the doses indicated in A in the presence or absence of 30 μM caspase inhibitors for 24 hours, after which the percentage of apoptotic cells was determined by the annexin-V method. Data represent means ± SD of 3 independent experiments (*P < .05; **P < .005 vs pan-AKIs/TRAIL Z-FA-FMK; Dunnet test).
To further investigate which caspases were responsible for pan-AKI/TRAIL-induced apoptosis, we used a peptide inhibitor approach: HMCLs were cultured with pan-AKIs and/or TRAIL in the presence of caspase-8 (Z-IETD-FMK), caspase-9 (Z-LEHD-FMK), or pancaspase (Z-VAD-FMK) inhibitors. Either Z-IETD-FMK or Z-LEHD-FMK inhibitors significantly reduced pan-AKI/TRAIL-induced cytotoxicity in all tested HMCLs except for OPM-2 HMCLs, in which only Z-IETD-FMK but not Z-LEHD-FMK significantly affected pan-AKI/TRAIL-induced cell death. Moreover, the pancaspase inhibitor Z-VAD-FMK protected MM cells from pan-AKI- and/or TRAIL-induced apoptosis, confirming that caspase activity was indispensable for the 2-drug–induced cell death (Figure 2B).
Pan-AKIs disable TRAIL-directed survival pathways by targeting NF-κB
RPMI 8226/R5 cells are insensitive to a diverse group of clinically relevant antitumor agents, including Bortezomib, Melphalan, Doxorubicin, Etoposide, and their multidrug-resistant phenotype, and resistance does not correlate with K-Ras prenylation, farnesyl transferase activity, Ras mutation status, increased expression of P-glycoprotein, or elevated expression of heat shock proteins.14 Therefore, we focused our molecular analyses on RPMI 8226/R5 and its parental RPMI 8226 with the aim to identify resistance mechanisms that are clinically relevant and the potential targets of the combination pan-AKIs/TRAIL.
Because NF-κB plays an important role in MM cell survival, tumorigenesis, and drug resistance,17 and Aurora-A may play an important function in regulating the activation of NF-κB–directed gene expression,18 we investigated whether the combination of pan-AKIs /TRAIL could induce apoptosis by interfering with the NF-κB pathway in HMCLs with different NF-κB profiles19,20 (Table 1).
In agreement with previous studies demonstrating that TRAIL induces a prosurvival signal by activating NF-κB,5,6 we found that TRAIL increased nuclear localization and DNA-binding activities of p65, p50, p52, and RelB (Figure 3Ai-ii), suggesting that both canonical and noncanonical NF-κB pathways were activated by TRAIL in MM cells. Furthermore, the blockade of Aurora kinase activity reduced the basal and/or TRAIL-mediated activation of both canonical and noncanonical NF-κB in all tested HMCLs (Figure 3Ai-ii).
Pan-AKIs block TRAIL-induced canonical and noncanonical NF-κB activation in MM cells. (A) HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) and after 24 hours nuclear extracts were prepared. (i) Nuclear extracts from RPMI 8226 and RPMI 8226/R5 were immunoblotted against NF-κB p65, NF-κB1 p50, NF-κB2 p52, c-Rel, RelB, and laminin or histone H2B as nuclear loading control. Bands were subjected to densitometric scanning using the TINA 2 software, and the number below each lane represents the relative amount of the indicated proteins normalized to laminin or histone H2B expression. (ii) HMCL nuclear extracts were tested for DNA binding activity of the NF-κB p65, p50, p52, c-Rel, and RelB subunits using the TransAM NF-κB enzyme-linked immunosorbent assay kit. Results were normalized to the untreated control. Values represent mean ± SD of 3 separate experiments performed in triplicate. (B) HMCLs were cultured with pan-AKIs and/or TRAIL as previously described. Endogenous p-IKKα/β, p-NF-κB2 p100, p-IκB-α, IKKβ, and IKKα from whole cell lysates were revealed by western blot analysis. Anti-actin immunoblotting was performed as loading control. Bands were subjected to densitometric scanning and p-IKKα/β, p-NF-κB2 p100, and p-IκB-α protein expression levels were normalized to actin; the number below each lane represents the relative amount of the indicated proteins. (Ci-ii) HMCLs were electroporated with IκB-α or nontargeting siRNA (CONT), and 24 hours after siRNA transfection the cells were treated with MK-0457. After 24 hours of treatment, cytoplasmic and nuclear extracts were prepared. (i) Cytoplasmic cell lysates were immunoblotted against IκB-α and tubulin as a marker of cytoplasmic separation as well as loading control. (ii) Nuclear extracts were tested for DNA binding activity of the NF-κB p65 subunit using the TransAM NF-κB enzyme-linked immunosorbent assay kit. Results were normalized to the untreated control. Values represent mean ± SD of 3 separate experiments performed in triplicate. (iii) Transfection of IκB-α, but not the unrelated nonspecific control siRNA, led to a decrease in IκB-α protein expression in HMCLs without affecting the levels of the unrelated protein actin. At 24 hours after siRNA transfection, HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) for 24 hours, and the percentages of annexin-V+ apoptotic cells were measured. Values are mean ± SD of 3 independent experiments (*P < .01 vs pan-AKIs/TRAIL nonspecific siRNA [CONT]; Dunnet test). (D) Transfection of Aurora A or B, but not the unrelated nonspecific control siRNA, led to a decrease in Aurora kinase protein expression in RPMI 8226 and RPMI 8226/R5 without affecting the levels of the unrelated protein actin. At 48 hours after siRNA transfection, cells were lysed for immunoblot analysis to monitor the expression of Aurora A and B, p-IKKβ, p-IKKα, p-IκB-α, p-NF-κB2 p100, IKKβ, IKKα, and actin as loading control. Bands were subjected to densitometric scanning and p-IKKα/β, p-NF-κB2 p100, and p-IκB-α protein expression levels were normalized to actin. The number below each lane represents the relative amount of the indicated proteins.
Pan-AKIs block TRAIL-induced canonical and noncanonical NF-κB activation in MM cells. (A) HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) and after 24 hours nuclear extracts were prepared. (i) Nuclear extracts from RPMI 8226 and RPMI 8226/R5 were immunoblotted against NF-κB p65, NF-κB1 p50, NF-κB2 p52, c-Rel, RelB, and laminin or histone H2B as nuclear loading control. Bands were subjected to densitometric scanning using the TINA 2 software, and the number below each lane represents the relative amount of the indicated proteins normalized to laminin or histone H2B expression. (ii) HMCL nuclear extracts were tested for DNA binding activity of the NF-κB p65, p50, p52, c-Rel, and RelB subunits using the TransAM NF-κB enzyme-linked immunosorbent assay kit. Results were normalized to the untreated control. Values represent mean ± SD of 3 separate experiments performed in triplicate. (B) HMCLs were cultured with pan-AKIs and/or TRAIL as previously described. Endogenous p-IKKα/β, p-NF-κB2 p100, p-IκB-α, IKKβ, and IKKα from whole cell lysates were revealed by western blot analysis. Anti-actin immunoblotting was performed as loading control. Bands were subjected to densitometric scanning and p-IKKα/β, p-NF-κB2 p100, and p-IκB-α protein expression levels were normalized to actin; the number below each lane represents the relative amount of the indicated proteins. (Ci-ii) HMCLs were electroporated with IκB-α or nontargeting siRNA (CONT), and 24 hours after siRNA transfection the cells were treated with MK-0457. After 24 hours of treatment, cytoplasmic and nuclear extracts were prepared. (i) Cytoplasmic cell lysates were immunoblotted against IκB-α and tubulin as a marker of cytoplasmic separation as well as loading control. (ii) Nuclear extracts were tested for DNA binding activity of the NF-κB p65 subunit using the TransAM NF-κB enzyme-linked immunosorbent assay kit. Results were normalized to the untreated control. Values represent mean ± SD of 3 separate experiments performed in triplicate. (iii) Transfection of IκB-α, but not the unrelated nonspecific control siRNA, led to a decrease in IκB-α protein expression in HMCLs without affecting the levels of the unrelated protein actin. At 24 hours after siRNA transfection, HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) for 24 hours, and the percentages of annexin-V+ apoptotic cells were measured. Values are mean ± SD of 3 independent experiments (*P < .01 vs pan-AKIs/TRAIL nonspecific siRNA [CONT]; Dunnet test). (D) Transfection of Aurora A or B, but not the unrelated nonspecific control siRNA, led to a decrease in Aurora kinase protein expression in RPMI 8226 and RPMI 8226/R5 without affecting the levels of the unrelated protein actin. At 48 hours after siRNA transfection, cells were lysed for immunoblot analysis to monitor the expression of Aurora A and B, p-IKKβ, p-IKKα, p-IκB-α, p-NF-κB2 p100, IKKβ, IKKα, and actin as loading control. Bands were subjected to densitometric scanning and p-IKKα/β, p-NF-κB2 p100, and p-IκB-α protein expression levels were normalized to actin. The number below each lane represents the relative amount of the indicated proteins.
Because IκB Kinase α (IKKα) and IKKβ are key molecules that predominantly mediate noncanonical and canonical pathways in NF-κB signaling cascades, respectively, we examined whether pan-AKI and/or TRAIL treatment could affect their expression levels and/or phosphorylation/activation status.
We found that both the pan-AKIs partially, but significantly (P < .05), reduced the basal amounts of active/phosphorylated IKKα/β in all HMCLs except for OPM2, which showed low basal IKKα/β activity (Figure 3B; supplemental Figure 2) and low NF-κB index19,20 (Table 1); TRAIL strongly increased the phosphorylation/activation of IKKα/β in RPMI 8226 and OPM-2, whereas this increment was less evident in RPMI 8226/R5, U266 and JJN3 HMCLs, which have elevated constitutive IKK activity (Figure 3B; supplemental Figure 2). Moreover, pretreatment with pan-AKIs significantly reduced the amounts of active/phosphorylated IKKα/β of TRAIL-treated HMCLs (Figure 3B; supplemental Figure 2); notably, the reduced phosphorylation of IKKα/β in pan-AKI/TRAIL-treated MM cells correlated with their decreased protein expression in all HMCLs except for OPM-2 (Figure 3B). Consistent with these data, we found that basal and/or TRAIL-induced phosphorylation of NF-κB2/p100 and IκB-α, the 2 downstream direct targets of IKKα and IKKβ that are involved in the activation of noncanonical and canonical pathways, respectively, was diminished in HMCLs treated with pan-AKIs (Figure 3B), indicating that pan-AKIs act through the inhibition of TRAIL-induced NF-κB activation in MM cells. Accordingly, chemical and/or genetic disruption of IKKα/β functions or pretreatment with NF-κB inhibitor SN50 significantly enhanced TRAIL sensitivity of MM cells (supplemental Figure 3A-B).
Because pan-AKIs promote IκB-α accumulation by reducing its phosphorylation and subsequent degradation (Figure 3Ci; supplemental Figure 4),18 we examined whether its silencing affects the MM cells’ responses to pan-AKIs and/or TRAIL. Consistently, siRNA knockdown of endogenous NF-κB inhibitor IκB-α disabled pan-AKI–triggered IκB-α accumulation/p65/NF-κB inhibition (Figure 3Ci-ii) and protected MM cells from pan-AKI/TRAIL-induced apoptosis (Figure 3Ciii), therefore arguing that inhibition of NF-κB activity by pan-AKIs contributes functionally to TRAIL sensitization of MM cells.
These data suggest that NF-κB is an important constituent of cell survival pathways for MM and confers protection against TRAIL-induced apoptosis, and agents inhibiting NF-κB are potent sensitizers of TRAIL in MM.21
To determine which Aurora was required for IKKα and IKKβ phosphorylation/activation and to rule out the possibility that the pan-AKI effects were because of the inhibition of other kinases, we repressed Aurora A or B by siRNA.
Notably, the RPMI 8226 knockdown of Aurora A increased the protein level of Aurora B and the amounts of phosphorylated IKKα/β, IκB-α, and NF-κB2, whereas knockdown of Aurora B decreased the phosphorylation of IKKα/β, IκB-α, and NF-κB2 (Figure 3D). In RPMI 8226/R5, we found that the repression of either Aurora A or Aurora B resulted in decreased phosphorylation of IKKα/β, IκB-α, and NF-κB2 (Figure 3D).
Taken together, these knockdown experiments indicate that both Aurora A and Aurora B may be involved in the phosphorylation of IKKα and IKKβ in MM cells.
Aurora kinases physically and functionally interact with IKK kinases to activate NF-κB in MM
A previous study has demonstrated that Aurora A activates NF-κB via IκB-α phosphorylation,18 a downstream target of IKKβ, and we found in our experiments that the blockade of Aurora kinase activity, either with pan-AKIs or siRNA suppression, affects IKKα/β phosphorylation. Therefore, we next investigated, by co-immunoprecipitation/western blot analysis, whether there were physical interactions between Aurora and IKK kinases.
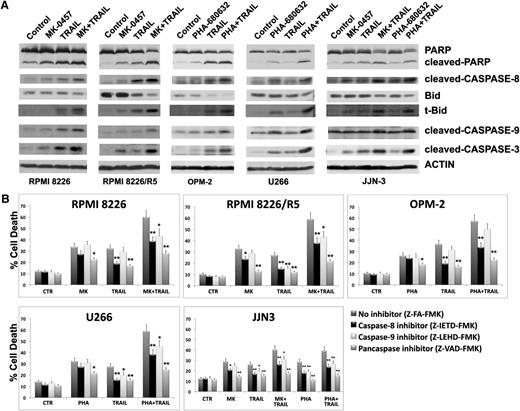
As depicted in Figure 4A, reciprocal immunoprecipitations using antibodies directed against IKKα, IKKβ, Aurora A, or Aurora B from lysates of untreated RPMI 8226/R5 showed association of Aurora A with IKKα and β and Aurora B primarily with IKKα; TRAIL significantly enhanced the association of both Aurora A and B with IKKβ and Aurora B with IKKα, but pretreatment with pan-AKIs completely abolished these TRAIL-induced increments (Figure 4A).
Aurora and IKK kinases interact in MM cells. (A) RPMI 8226/R5 were treated with PHA-680632 for 3 hours and then incubated with TRAIL (9.6 ng/mL). After 24 hours of treatment, cells were lysed in CHAPS lysis buffer and subjected to immunoprecipitation (IP) using mouse monoclonal IKKα (B-8), mouse monoclonal IKKβ (H-4), mouse monoclonal ARK-1 (35C1) (Aurora A), mouse monoclonal ARK-2 (13E8A7) (Aurora B), or control antibody (IgG) and immunoblotted (IB) with Aurora A or Aurora B or IKKα and IKKβ antibodies. Bands were subjected to densitometric scanning, and the histogram shows average quantification results ± SD of the association Aurora A/IKKα, Aurora A/IKKβ, Aurora B/IKKα, or Aurora B/IKKβ from 3 blots (*P < .01, vs untreated control cells, Tukey-Kramer test). (B) HMCLs were cultured with pan-AKIs and/or TRAIL as previously reported for 24 hours. Endogenous phospho-Aurora A (Thr288) and phospho-Aurora B (Thr232) from whole cell lysates were revealed by western blot analysis. Anti-actin immunoblotting was performed as loading control. The relative amount of phospho-Aurora A (Thr288) and phospho-Aurora B (Thr232) was determined by densitometry and normalized to that of actin. Histograms represent the mean ± SD of the ratio phospho-Aurora A (Thr288)/actin or phospho-Aurora B (Thr232)/actin normalized to the untreated control from blots of 3 separate experiments.
Aurora and IKK kinases interact in MM cells. (A) RPMI 8226/R5 were treated with PHA-680632 for 3 hours and then incubated with TRAIL (9.6 ng/mL). After 24 hours of treatment, cells were lysed in CHAPS lysis buffer and subjected to immunoprecipitation (IP) using mouse monoclonal IKKα (B-8), mouse monoclonal IKKβ (H-4), mouse monoclonal ARK-1 (35C1) (Aurora A), mouse monoclonal ARK-2 (13E8A7) (Aurora B), or control antibody (IgG) and immunoblotted (IB) with Aurora A or Aurora B or IKKα and IKKβ antibodies. Bands were subjected to densitometric scanning, and the histogram shows average quantification results ± SD of the association Aurora A/IKKα, Aurora A/IKKβ, Aurora B/IKKα, or Aurora B/IKKβ from 3 blots (*P < .01, vs untreated control cells, Tukey-Kramer test). (B) HMCLs were cultured with pan-AKIs and/or TRAIL as previously reported for 24 hours. Endogenous phospho-Aurora A (Thr288) and phospho-Aurora B (Thr232) from whole cell lysates were revealed by western blot analysis. Anti-actin immunoblotting was performed as loading control. The relative amount of phospho-Aurora A (Thr288) and phospho-Aurora B (Thr232) was determined by densitometry and normalized to that of actin. Histograms represent the mean ± SD of the ratio phospho-Aurora A (Thr288)/actin or phospho-Aurora B (Thr232)/actin normalized to the untreated control from blots of 3 separate experiments.
To determine whether the TRAIL-induced activating interactions between Aurora and IKK kinases correlated with Aurora kinase activation, we analyzed Aurora A (Thr288) and Aurora B (Thr232) phosphorylation22 by western blot and densitometric analysis. We found that TRAIL also increased the amounts of phosphorylated Aurora A and B in MM cells, and these phosphorylations were abolished in the presence of pan-AKIs (Figure 4B).
Aurora kinase activity is important for TRAIL-induced Aurora-IKK kinases interactions
Finally, to determine whether TRAIL-induced Aurora-IKK kinase interactions also depend on IKK activity, we selectively inhibited IKK activity in MM cells by using PS-1145, a specific small molecule inhibitor of IKK. Because PS-1145 is highly selective for IKKβ, with more than 1000-fold greater inhibitory activity for IKKβ than for 30 other cellular kinases, including IKKα,23 we evaluated its effects on TRAIL-induced IKKβ phosphorylation/activation and IKKβ-Aurora kinase interactions. PS-1145 markedly diminished the basal and TRAIL-mediated phosphorylation of IKKβ in MM cells (Figure 5A) but failed in reducing TRAIL-induced IKKβ-Aurora kinase interactions (Figure 5B). Conversely, pan-AKIs, which prevented TRAIL-induced phosphorylation/activation of Aurora kinases, completely abrogated TRAIL-induced Aurora-IKK kinase interactions (Figure 5B). Because we found that PS-1145 treatment alone also increased IKKβ-Aurora kinase interactions (Figure 5B), we asked whether this IKKβ inhibitor was able to modulate Aurora kinase activity; as expected, we found that PS-1145 significantly increased the activity of both Aurora A and B in MM cells (Figure 5C). Moreover, consistent with the biological significance of targeting Aurora-IKK interactions for enhancing TRAIL sensitivity in MM cells, PS-1145, which was unable to negatively affect TRAIL-induced Aurora-IKK interactions, was ineffective in sensitizing MM cells to TRAIL (Figure 5D).
Aurora kinase activity is important for Aurora-IKK interactions in myeloma cells. (A) HMCLs were cultured with dimethylsulfoxide (vehicle), IKKβ inhibitor PS-1145 (5 μM), or MK-0457 (0.4 μM) for 3 hours and then incubated with TRAIL. After 24 hours, cells were lysed and subjected to western blot analysis to monitor the expression and phosphorylation of IKKβ; anti-actin immunoblot was performed as loading control. (B) The same cell lysates were subjected to immunoprecipitation using mouse monoclonal IKKβ (H-4) or control antibody (IgG) and IB with either IKKβ or Aurora A or Aurora B antibodies. Bands were subjected to densitometric scanning, and the histogram shows average quantification results ± SD of the association Aurora A/IKKβ or Aurora B/IKKβ from 3 blots (*P < .05; **P < .01, vs untreated control cells, Tukey-Kramer test). (C) RPMI 8226 and 8226/R5 cells were cultured with dimethylsulfoxide (vehicle) or PS-1145 at 5 μM for 24 hours. Endogenous pospho-IKKβ, phospho-Aurora A (Thr288), and phospho-Aurora B (Thr232) from whole cell lysates were revealed by western blot analysis. Anti-actin immunoblotting was performed as loading control. The relative amount of phospho-Aurora A (Thr288) and phospho-Aurora B (Thr232) was determined by densitometry and normalized to that of actin. Histograms represent the mean ± SD of the ratio phospho-Aurora A (Thr288)/actin or phospho-Aurora B (Thr232)/actin normalized to the untreated control from 2 blots (*P < .01, Tukey-Kramer test). (D) HMCLs cells were cultured with dimethylsulfoxide (vehicle) or PS-1145 at 5 μM for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226, 19.2 ng/mL in RPMI 8226/R5, and 300 ng/mL in JJN3) and after 24 hours, the percentage of cell death was determined by the annexin-V method. Values are mean ± SD of 3 independent experiments. (E) Model of TRAIL action; TRAIL induces prosurvival signal in MM cells by activating both Aurora A and B kinases, leading to increased Aurora/IKK binding, IKK phosphorylation/activation, NF-κB activation, and induction of the antiapoptotic NF-κB target genes A1/Bfl-1, Mcl-1, and IAPs. Aurora kinase inhibitors disable TRAIL-directed survival pathways, because they abrogate TRAIL-induced Aurora-IKK kinase interactions and NF-κB activation.
Aurora kinase activity is important for Aurora-IKK interactions in myeloma cells. (A) HMCLs were cultured with dimethylsulfoxide (vehicle), IKKβ inhibitor PS-1145 (5 μM), or MK-0457 (0.4 μM) for 3 hours and then incubated with TRAIL. After 24 hours, cells were lysed and subjected to western blot analysis to monitor the expression and phosphorylation of IKKβ; anti-actin immunoblot was performed as loading control. (B) The same cell lysates were subjected to immunoprecipitation using mouse monoclonal IKKβ (H-4) or control antibody (IgG) and IB with either IKKβ or Aurora A or Aurora B antibodies. Bands were subjected to densitometric scanning, and the histogram shows average quantification results ± SD of the association Aurora A/IKKβ or Aurora B/IKKβ from 3 blots (*P < .05; **P < .01, vs untreated control cells, Tukey-Kramer test). (C) RPMI 8226 and 8226/R5 cells were cultured with dimethylsulfoxide (vehicle) or PS-1145 at 5 μM for 24 hours. Endogenous pospho-IKKβ, phospho-Aurora A (Thr288), and phospho-Aurora B (Thr232) from whole cell lysates were revealed by western blot analysis. Anti-actin immunoblotting was performed as loading control. The relative amount of phospho-Aurora A (Thr288) and phospho-Aurora B (Thr232) was determined by densitometry and normalized to that of actin. Histograms represent the mean ± SD of the ratio phospho-Aurora A (Thr288)/actin or phospho-Aurora B (Thr232)/actin normalized to the untreated control from 2 blots (*P < .01, Tukey-Kramer test). (D) HMCLs cells were cultured with dimethylsulfoxide (vehicle) or PS-1145 at 5 μM for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226, 19.2 ng/mL in RPMI 8226/R5, and 300 ng/mL in JJN3) and after 24 hours, the percentage of cell death was determined by the annexin-V method. Values are mean ± SD of 3 independent experiments. (E) Model of TRAIL action; TRAIL induces prosurvival signal in MM cells by activating both Aurora A and B kinases, leading to increased Aurora/IKK binding, IKK phosphorylation/activation, NF-κB activation, and induction of the antiapoptotic NF-κB target genes A1/Bfl-1, Mcl-1, and IAPs. Aurora kinase inhibitors disable TRAIL-directed survival pathways, because they abrogate TRAIL-induced Aurora-IKK kinase interactions and NF-κB activation.
In summary, these data strongly suggest that Aurora kinase activity is relevant for TRAIL-induced Aurora-IKK kinase interactions and support a model in which both Aurora A and B, activated by TRAIL, can physically and functionally interact with IKKs to activate the canonical and noncanonical NF-κB pathways. The blockade of Aurora kinase activity by pan-AKIs can effectively reduce the TRAIL-induced NF-κB transcriptional activation because of disrupted Aurora-IKK kinase interactions (Figure 5E).
Combined exposure to pan-AKIs and TRAIL negatively modulates the expression of NF-κB antiapoptotic target genes
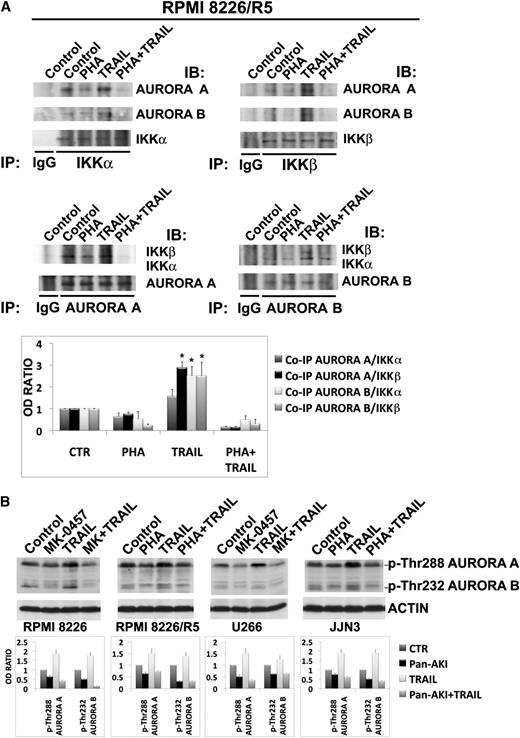
We next analyzed the effects of treatment with pan-Aurora inhibitors and/or TRAIL on the levels of A1/Bfl-1, Mcl-1, cellular inhibitor-of-apoptosis protein 1 (cIAP1), cIAP2, and X-chromosome-linked inhibitor-of-apoptosis protein (XIAP), which are well-characterized transcriptional targets of NF-κB.
Treatment with TRAIL for 24 hours increased the protein levels of A1/Bfl-1 in all tested HMCLs and Mcl-1 in RPMI 8226/R5 and, to a lesser extent, in U266 and JJN3 HMCLs (Figure 6A). Pretreatment with pan-AKIs prevented TRAIL induction of A1/Bfl-1 and Mcl-1 and significantly enhanced mitochondrial depolarization induced by TRAIL alone in all tested HMCLs Figure 6A and supplemental Figure 5.
Pan-AKIs sensitize MM cells to TRAIL by inhibiting the expression of TRAIL-induced NF-κB target genes. (A) HMCLs were treated with MK-0457 for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) for 24 hours, after which endogenous A1/Bfl-1 and Mcl-1 were revealed by immunoblotting analysis. Blots were subsequently reprobed for actin expression to ensure equivalent loading and transfer of protein. Histograms represent the mean value ± SD of the ratio of A1/Bfl-1 or Mcl-1/actin normalized to the untreated control from blots of 3 independent experiments (*P < .001; Tukey-Kramer test). (B) Transfection of A1/Bfl-1 or Mcl-1, but not the unrelated nonspecific control siRNA (CONT), led to a decrease in A1/Bfl-1 or Mcl-1 protein expression in HMCLs without affecting the levels of the unrelated protein actin (insets). At 30 minutes after siRNA transfection, the cells were treated with TRAIL at the indicated doses (ng/mL) for the indicated time. Apoptosis was measured by annexin-V staining. Values are mean ± SD of 3 independent experiments (*P < .005; **P < .001 vs non-specific control siRNA [CONT]; Dunnet test). (C) RPMI 8226/R5 cells were infected with an empty lentiviral vector or lentivirus expressing A1/Bfl-1 or Mcl-1. All lentviral expression vectors coexpressed red fluorescent protein to monitor the infection by flow cytometry. Plot represents comparable red fluorescent protein expression of RPMI 8226/R5s infected with the parental retroviral vector or those encoding for Mcl-1 or A1/Bfl1. Whole-cell lysates of uninfected or virus-infected RPMI 8226/R5 cells were prepared and analyzed by western blot to confirm the overexpression of A1/Bfl-1 or Mcl-1 (inset). Pools of RPMI 8226/R5 were expanded after infection and treated with PHA-680632 (0.8 μM) and then incubated with TRAIL at the indicated doses for 24 hours. Cell death was measured by annexin-V staining. Values are mean ± SD of 3 independent experiments (*P < .005, vs PHA/TRAIL empty vector; Dunnet test). (D) HMCLs were treated as described in A and endogenous cIAP1, cIAP2, XIAP, and actin proteins were revealed by immunoblotting analysis. Bands were subjected to densitometric scanning, and histograms represent the mean value ± SD of the ratio of cIPA1, cIAP2, or XIAP/actin normalized to the untreated control from blots of 3 independent experiments (*P < .01; **P < .001; Tukey-Kramer test).
Pan-AKIs sensitize MM cells to TRAIL by inhibiting the expression of TRAIL-induced NF-κB target genes. (A) HMCLs were treated with MK-0457 for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) for 24 hours, after which endogenous A1/Bfl-1 and Mcl-1 were revealed by immunoblotting analysis. Blots were subsequently reprobed for actin expression to ensure equivalent loading and transfer of protein. Histograms represent the mean value ± SD of the ratio of A1/Bfl-1 or Mcl-1/actin normalized to the untreated control from blots of 3 independent experiments (*P < .001; Tukey-Kramer test). (B) Transfection of A1/Bfl-1 or Mcl-1, but not the unrelated nonspecific control siRNA (CONT), led to a decrease in A1/Bfl-1 or Mcl-1 protein expression in HMCLs without affecting the levels of the unrelated protein actin (insets). At 30 minutes after siRNA transfection, the cells were treated with TRAIL at the indicated doses (ng/mL) for the indicated time. Apoptosis was measured by annexin-V staining. Values are mean ± SD of 3 independent experiments (*P < .005; **P < .001 vs non-specific control siRNA [CONT]; Dunnet test). (C) RPMI 8226/R5 cells were infected with an empty lentiviral vector or lentivirus expressing A1/Bfl-1 or Mcl-1. All lentviral expression vectors coexpressed red fluorescent protein to monitor the infection by flow cytometry. Plot represents comparable red fluorescent protein expression of RPMI 8226/R5s infected with the parental retroviral vector or those encoding for Mcl-1 or A1/Bfl1. Whole-cell lysates of uninfected or virus-infected RPMI 8226/R5 cells were prepared and analyzed by western blot to confirm the overexpression of A1/Bfl-1 or Mcl-1 (inset). Pools of RPMI 8226/R5 were expanded after infection and treated with PHA-680632 (0.8 μM) and then incubated with TRAIL at the indicated doses for 24 hours. Cell death was measured by annexin-V staining. Values are mean ± SD of 3 independent experiments (*P < .005, vs PHA/TRAIL empty vector; Dunnet test). (D) HMCLs were treated as described in A and endogenous cIAP1, cIAP2, XIAP, and actin proteins were revealed by immunoblotting analysis. Bands were subjected to densitometric scanning, and histograms represent the mean value ± SD of the ratio of cIPA1, cIAP2, or XIAP/actin normalized to the untreated control from blots of 3 independent experiments (*P < .01; **P < .001; Tukey-Kramer test).
Using RNAi strategies, we found that the knockdown of either A1/Bfl-1 or Mcl-1 significantly increased TRAIL-induced apoptosis in the majority of HMCLs analyzed (Figure 6B; representative data in supplemental Figure 6), whereas retroviral overexpression of either A1/Bfl-1 or Mcl-1 protected MM (RPMI 8226/R5) cells from TRAlL and pan-AKI/TRAIL-induced apoptosis (Figure 6C). This would indicate that both A1/Bfl-1 and Mcl-1 are important targets for TRAIL sensitization in MM cells. In RPMI 8226, OPM-2 and JJN3 HMCL Mcl-1 knockdown produced a large amount of background apoptosis (>65%) already evident 6 hours after transfection that masked the cytotoxic effect of TRAIL (data not shown), therefore confirming its pivotal role in controlling MM cell survival and apoptosis.24
Furthermore, pan-AKIs either as single agents or in combination with TRAIL did not significantly affect Bcl-xL and Bcl-2 protein expression in MM cells (supplemental Figure 7A), suggesting that these inhibitors do not act through the modulation of these antiapoptotic Bcl-2 proteins. Accordingly, NF-κB inhibitor SN50 did not reduce Bcl-xL and Bcl-2 levels in MM cells,25 and the siRNA knockdown of endogenous Bcl-xL or Bcl-2 did not significantly increase TRAIL-induced apoptosis in HMCLs (supplemental Figure 7B-C). Moreover, overexpression of either Bcl-2 or Bcl-xL in MM cells failed to protect them from TRAlL26 and pan-AKI/TRAIL-induced apoptosis (supplemental Figure 7D).
Consistent with our findings that pan-AKIs potentiate TRAIL-induced apoptosis through NF-κB inhibition, we found that the treatment with pan-AKIs significantly reduced the basal and/or TRAIL-induced protein levels of XIAP, cIAP1, and/or cIAP2, well-known NF-κB target genes that have been demonstrated to play a pivotal role in inhibiting death receptor and mitochondrial signaling27-29 (Figure 6D). Moreover, the siRNA knockdown of XIAP (in all the tested HMCLs) and cIAP1 or cIAP2 (in RPMI 8226 and JJN3, respectively) significantly increased TRAIL-induced apoptosis, thereby confirming that IAPs can be important targets for TRAIL sensitization in MM cells (supplemental Figure 8).
Pan-AKIs combined with TRAIL cause regression of multidrug-resistant tumors in vivo
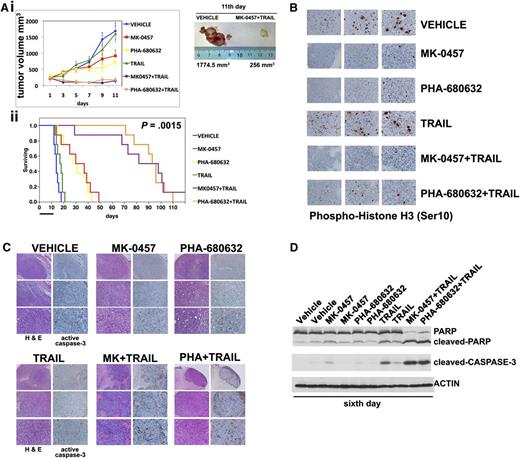
To assess the in vivo efficacy of combining TRAIL and pan-AKIs, we tested these compounds using a mouse tumor human plasmacytoma xenograft model in which the multidrug-resistant RPMI 8226/R5 cells (1.0 × 107 cells/mouse) were subcutaneously injected into NOD-SCID mice. When the tumors reached ∼250 mm3, mice bearing RPMI 8226/R5 tumors were randomized (n = 10/group) to receive vehicle or MK-0457 or PHA-680632 at 50 mg/kg or TRAIL (300 μg/mouse), MK-0457/TRAIL, or PHA-680632/TRAIL. Both pan-AKIs and TRAIL were administered by intraperitoneal injection. Mice were treated with daily doses of pan-AKIs for 11 days and 2 doses of TRAIL. Treatment of RPMI 8226/R5 MM tumor-bearing mice with pan-AKIs significantly (P < .01) reduced MM tumor growth compared with control, whereas TRAIL had a minimal effect on the growth of tumors, which increased as in control mice (Figure 7Ai). When pan-AKIs were combined with TRAIL, there was a significant (P < .001) reduction in tumor growth relative to either treatment alone (Figure 7Ai).
Pan-AKI and TRAIL combination therapy has potent antitumor activity in vivo against drug-resistant human MM xenograft model. (Ai) When tumor size reached 250 mm3, mice were randomly assigned (n = 10/group) to receive vehicle alone, MK-0457 (50 mg/kg), PHA-680632 (50 mg/kg), TRAIL (300 μg/mouse), or the combination MK-0457/TRAIL or PHA-680632/TRAIL. TRAIL was administered once on days 3 and 6. Pan-AKIs were administered once each day for 11 days. Results are tumor volume, mean ± SD mm3, plotted against time (P < .001 MK-0457/TRAIL or PHA-680632/TRAIL vs either treatment alone; Dunnet test). Inset shows tumors resected from control (vehicle) and pan-AKI/TRAIL-treated mice after 11 days of treatment (endpoint). (ii) Kaplan-Meier survival curve was evaluated from the first day of treatment until death or sacrifice using JMP version 7.0 statistical software (SAS Institute, Cary, NC). Survival was significantly prolonged in MK-0457/TRAIL-treated animals vs control (P = .0015 after Bonferroni correction). The black bar on the abscissa represents the 11-day period of treatment. After 6 days of treatment, mice from each treatment group (n = 3/group) were humanely killed, and the tumors were removed for assay. RPMI 8226/R5-derived tumors were analyzed by immunohistochemical staining for (B) phospho-Histone H3 (×10, ×20, and ×40 magnification), (C) hematoxylin and eosin (H&E), and cleaved caspase-3 (×4, ×10, and ×20 magnification). The microphotographs shown are representative of similar observations in 3 different mice receiving the same treatment. (D) Tumor tissues from mice treated for 6 days were harvested and processed, and lysates were analyzed by immunoblotting analysis for PARP, cleaved-PARP, and cleaved caspase-3. Anti-actin immunoblotting was performed as loading control.
Pan-AKI and TRAIL combination therapy has potent antitumor activity in vivo against drug-resistant human MM xenograft model. (Ai) When tumor size reached 250 mm3, mice were randomly assigned (n = 10/group) to receive vehicle alone, MK-0457 (50 mg/kg), PHA-680632 (50 mg/kg), TRAIL (300 μg/mouse), or the combination MK-0457/TRAIL or PHA-680632/TRAIL. TRAIL was administered once on days 3 and 6. Pan-AKIs were administered once each day for 11 days. Results are tumor volume, mean ± SD mm3, plotted against time (P < .001 MK-0457/TRAIL or PHA-680632/TRAIL vs either treatment alone; Dunnet test). Inset shows tumors resected from control (vehicle) and pan-AKI/TRAIL-treated mice after 11 days of treatment (endpoint). (ii) Kaplan-Meier survival curve was evaluated from the first day of treatment until death or sacrifice using JMP version 7.0 statistical software (SAS Institute, Cary, NC). Survival was significantly prolonged in MK-0457/TRAIL-treated animals vs control (P = .0015 after Bonferroni correction). The black bar on the abscissa represents the 11-day period of treatment. After 6 days of treatment, mice from each treatment group (n = 3/group) were humanely killed, and the tumors were removed for assay. RPMI 8226/R5-derived tumors were analyzed by immunohistochemical staining for (B) phospho-Histone H3 (×10, ×20, and ×40 magnification), (C) hematoxylin and eosin (H&E), and cleaved caspase-3 (×4, ×10, and ×20 magnification). The microphotographs shown are representative of similar observations in 3 different mice receiving the same treatment. (D) Tumor tissues from mice treated for 6 days were harvested and processed, and lysates were analyzed by immunoblotting analysis for PARP, cleaved-PARP, and cleaved caspase-3. Anti-actin immunoblotting was performed as loading control.
Furthermore, as depicted in Figure 7Aii, the combination of pan-AKIs/TRAIL significantly (P = .0015, after Bonferroni correction) prolonged survival compared with treatment with either drug alone (mean survival time of MK-0457/TRAIL: 90 days, 95% confidence interval [CI] = 29-102 days; PHA-680632/TRAIL: 94.5 days, 95% CI = 71-103 days; MK-0457: 33.5 days, 95% CI = 14-42 days; PHA-680632: 29.5 days, 95% CI = 15-40 days; TRAIL: 17.5 days, 95% CI = 13-19; untreated: 14 days, 95% CI = 12-16 days) and was well tolerated without significant weight loss (data not shown). We next investigated the in vivo effects of the drug combination on proliferation and apoptosis. Whole tumor-cell tissues from mice treated for 6 days (n = 3/group) were subjected to histopathologic examination and immunohistochemical staining to assess in vivo phosphorylation of histone H3 on Ser10, a proliferation marker whose phosphorylation during mitosis has been previously used to demonstrate inhibition of Aurora kinase activity,30 and cleaved caspase-3. Tumor tissues from pan-AKI treatments resulted in profound phospho-histone H3 inhibition compared with tumor tissues from vehicle control or TRAIL-treated animals (Figure 7B), thereby confirming that either MK-0457 or PHA-680632 was acting through Aurora inhibition in tumor cells and the growth retardation observed in pan-AKI–treated mice. Either pan-AKIs or TRAIL alone slightly increased caspase-3 cleavage/activation compared with tumors from control cohorts. However, the combination pan-AKIs/TRAIL dramatically increased caspase-3 cleavage/activation in tumors (Figure 7C).
Evidence for tumor reduction was demonstrated by a pan-AKI/TRAIL-induced nearly complete disappearance of tumor cell mass accompanied by substantial fibrosis (Figure 7C).
Finally, histopathological and immunohistochemical findings were additionally confirmed by parallel western blot analysis of tumor lysates that revealed a stronger activation of caspase-3 and PARP fragmentation in tumors from pan-AKI/TRAIL-treated mice compared with either treatment alone (Figure 7D).
Taken together, these findings suggest that combining pan-AKIs with TRAIL induces both cytostatic and cytotoxic responses in vivo, resulting in regression of tumors, prolongs survival in vivo and is well tolerated in vivo.
Discussion
Recombinant human TRAIL ligand and agonistic mAbs have shown remarkable promise as anticancer agents.2,21 However, TRAIL signaling also activates survival signaling that can compromise its efficacy.5,6 In the present study, we demonstrate that pan-AKIs disrupt TRAIL-induced survival signaling by effectively reducing TRAIL-mediated activation of both canonical and noncanonical NF-κB pathways and by blocking TRAIL induction of the antiapoptotic NF-κB target genes A1/Bfl-1 and/or Mcl-1 in MM cells.
NF-κB plays a crucial role in the pathogenesis of various cancers,31-33 and it has been recently shown the relationship between canonical and noncanonical NF-κB activity and genetic abnormalities in MM20,34 suggesting the biologic significance of both NF-κB pathways in MM pathogenesis.
Furthermore, canonical and noncanonical pathways show much interplay, overlap, and cross-talk.35 Previous reports have shown that specific IKKβ inhibitors have modest anti-MM activity in vitro and in vivo, because they specifically inhibit canonical NF-κB signaling but do not block total NF-κB activity.36 Yet IKKβ inhibitors may activate noncanonical pathway in MM cells, and vice versa, the downregulation of IKKα may enhance phosphorylation of IKKβ, suggesting the existence of compensatory cross-talk between canonical and noncanonical pathways.36-38
Consistent with previous findings reporting that IKKβ can antagonize Aurora-A signaling by promoting its degradation in the ubiquitin-proteasome pathway,39 we demonstrated that chemical inhibition of IKKβ increased the activation of both Aurora A and B and their physical interactions with IKKβ and were ineffective in sensitizing MM cells to TRAIL. Moreover, we found that simultaneous inhibition of both IKKβ and IKKα by specific inhibitors and/or siRNA approaches or by pan-AKIs that disrupt NF-κB signaling by abrogating TRAIL-induced Aurora-IKK kinase interactions and activation was more effective in sensitizing MM cells to TRAIL than inhibition of either kinase alone (supplemental Figure 3A-B), thus suggesting that strategies that target both canonical and noncanonical NF-κB pathways may enhance the antimyeloma effects of TRAIL.36
Furthermore, in our experiments, we demonstrated that the siRNA-mediated depletion of either Aurora A or Aurora B affected the phosphorylation/activation of both IKKα and IKKβ in MM, thus suggesting the need of targeting both Aurora A and B because the inhibition of either kinase alone may be insufficient to block NF-κB activity in MM cells.
All together, these findings reveal additional complexity in the signaling network regulating the NF-κB pathway and suggest the existence of possible reciprocal regulatory roles between IKK and Aurora kinases.
Given that mutations affecting NF-κB inducing kinase (NIK) levels (NIK, TRAF2, TRAF3, cIAP1, and cIAP2) frequently occur in HMCLs and primary MM patient samples19,20 (Table 1) and NIK accumulation activates NF-κB signaling through a NIK-mediated activation/phosphorylation of IKK,19,20 we speculate that the NIK-triggered activation of IKK may interfere with and reduce the inhibitory effects exerted by pan-AKIs on the basal IKK activity of MM cells. This could explain the partial IKK inhibition observed in the majority of pan-AKI–treated HMCLs; however, in all tested HMCLs, irrespective of their NF-κB profile and NIK levels,19,20 TRAIL enhanced the basal phosphorylation/activation state of Aurora and IKK kinases, their physical interactions, and NF-κB activation, and the pharmacological blockade of aurora kinases abrogated TRAIL-induced NF-κB survival signaling by effectively reducing Aurora-IKK kinase phosphorylation/activation and interactions, suggesting that these kinases can cooperate to promote TRAIL resistance in MM mainly through NF-κB activation. Consistently, Aurora B has been previously reported to be involved in cancer cell resistance to TRAIL.40,41
Previous studies have shown that TRAIL can activate both NF-κB and the Ras/Raf/MEK/ERK mitogen-activated protein kinase cascade signaling pathways5,6,42,43 and that Aurora A can be a downstream target of ERK.44 The results of these studies, together with our findings of TRAIL-induced activation of both Aurora and IKK kinases, define an important role for Aurora kinases in connecting mitogen-activated protein kinase signaling to NF-κB activity.
We also found that pan-AKIs blocked TRAIL induction of the anti-apoptotic NF-κB target genes A1/Bfl-1 and/or Mcl-1 and demonstrated that either A1/Bfl-1 or Mcl-1 can exert significant protection against TRAIL, thereby indicating that both these antiapoptotic molecules can be critical mediators of TRAIL resistance in MM cells. A1/Bfl-1 and Mcl-1 have been reported to interact with and antagonize the activity of proapoptotic Bcl-2 family member apoptotic proteins, including Bim and Bid.45 Bim can physically interact with both DR4 and DR5 TRAIL receptors and contribute to the activation of both extrinsic and intrinsic apoptotic pathways.15 In addition, compared with TRAIL treatment alone, cotreatment with pan-AKIs and TRAIL significantly decreased the protein levels of caspase-8 inhibitors cIAP1/228 and caspases-3, -7, and -9 inhibitor XIAP.29
For our in vitro and in vivo experiments, we used 2 potent and selective small-molecule inhibitors of Aurora A and B kinases, VX-680/MK045712,46 and the preclinical candidate PHA-680632,12,47 which has been reported to have both in vitro and in vivo similar activity against the Aurora kinases of its close analog PHA-739358, currently in phase 2 clinical trials.12 In this study, both drugs exhibited similar antitumor activities as single agents or in combination with TRAIL. Furthermore, Aurora kinase inhibitors have recently been studied as potential novel therapeutic targets in solid and hematological malignancies,12,13,48-50 and few of them are currently undergoing phase 1 and 2 clinical trials as both monotherapy and combination therapy in relapsed or refractory MM.51,52
Finally, the clinical observation that TRAIL monotherapy can be associated with limited efficacy and resistance, coupled with our present preclinical in vitro and in vivo findings demonstrating that TRAIL together with pan-AKIs triggers a more potent anti-MM effect in vitro and in vivo without increased toxicity, provide the framework for testing pan-AKIs and TRAIL combination therapy in clinical trials aimed to improve patient outcome in MM.
Our data provide evidence for a novel functional link between Aurora and IKK kinases, underscoring a critical role of these pathways in MM drug resistance, and the potential therapeutic benefit of targeting Aurora kinases as a strategy to overcome Aurora-IKK crosstalk-related resistance to TRAIL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Paola Gianelli (Department of Veterinary Sciences, University of Parma, Parma, Italy) and Davide Arienti (Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna “Bruno Ubertini,” Brescia, Italy) for technical support.
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (IG, Rif 10670, Italian Association for Cancer Research, Milan, Italy; A.B.) and Fondazione Cassa di Risparmio di Parma (Cariparma, Parma, Italy; A.B.).
Authorship
Contribution: L.M. performed cell culture experiments, apoptosis analysis, and transfection experiments and contributed to molecular biology experiments; G.L carried out the in vivo experiments; M.A. performed cell culture experiments and apoptosis analysis and contributed to transfection experiments; M.R. contributed to in vivo experiments; G.D. performed plasmid amplification and purification; N.G. provided MM primary cells; A.M.C. performed immunohistochemical analysis; A.C. supervised the immunohistochemical experiments; A.B. revised the manuscript; and P.L. designed the research, experiments, and in vivo experimental protocol, performed the molecular biology experiments, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Lunghi, Department of Clinical and Experimental Medicine, University of Parma, Via Gramsci 14, 43126 Parma, Italy; e-mail: p.lunghi@libero.it; and Antonio Bonati, Department of Clinical and Experimental Medicine, University of Parma, Via Gramsci 14, 43126 Parma, Italy; e-mail: Antonio.bonati@unipr.it.

![Figure 1. Pan-AKIs potentiate the TRAIL-induced cell death in MM cells. (Ai) HMCLs seeded at 2.0 × 105 cells/mL were treated sequentially with escalating doses of MK-0457 (15-2000 ng/mL; 0.032-4.3 μM) or PHA-680632 (15-2000 ng/mL; 0.03-4 μM) for 3 hours and subsequently with TRAIL (0.5-50 ng/mL) alone or in combination with pan-AKIs at a fixed ratio of 25:1 for MK-0457/TRAIL and 50:1 for PHA-680632/TRAIL (in combination with pan-AKIs, TRAIL was used at 0.6, 1.2, 2.4, 4.8, 9.6, and 19.2 ng/mL). After 24 hours, cell death was measured by annexin-V binding. Combination index plots were then generated using the Calcusyn software. Combination index values <1.0 indicate synergism, 1.0 indicates additive effect, and >1.0 indicates an antagonistic effect. (Aii) JJN3 were seeded at 2.0 × 105 cells/mL in the presence of dimethyl sulfoxide (vehicle), MK-0457 (0.4 μM), or PHA-680632 (0.8 μM) for 3 hours and then incubated with TRAIL at the concentration of 300 ng/mL. After 24 hours, apoptosis was measured by annexin-V labeling. Values represent means ± SD of 4 independent experiments (*P < .01 vs either treatment alone; Dunnet test). (B) HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL at the indicated doses (ng/mL) in the presence or absence of IGF-1 (50 ng/mL), IL-6 (20 ng/mL) or BMSCs (HMCLs in direct contact to BMSC monolayer in a 5:1 ratio). After 24 hours, HMCLs were harvested and apoptotic HMCLs cells were determined by flow cytometry as CD38/CD138+ annexin-V+ cells; data represent means ± SD of quadruplicate experiments. Dexamethasone (2 μM) was used as control to monitor the protective effects of growth factors and stromal cells (*P < .05; **P < .005; Tukey-Kramer Honestly Significant Difference test). (Ci-ii) CD138-purified plasma cells from 5 patients with MM were isolated from BM and then seeded at 2.5 × 105 cells/mL. Samples from 1 to 3 were pretreated with MK-0457 (0.4 μM), and samples 4 and 5 were pretreated with PHA-680632 (0.8 μM) and then incubated with TRAIL (9.6 ng/mL) for 24 hours. Sample 4 was cultured in presence or absence of BMSCs. The cell death was measured by annexin-V staining. (i) Results are expressed as the net apoptosis induction [percentage of apoptosis in treated cells minus percentage of apoptosis in dimethylsulfoxide (vehicle-treated cells)] of all 5 primary samples tested. (ii) Histogram represents the mean percentage of apoptosis ± SD (expressed as net difference between the percentages of apoptosis in treated cells minus percentage of apoptosis in vehicle-treated cells) of the results obtained in the 5 different patient samples (*P < .05 vs either treatment alone; Dunnet test). (iii) Peripheral blood mononuclear cells from 3 healthy volunteers were treated with dimethylsulfoxide (vehicle), MK-0457 (0.4 μM), or PHA-680632 (0.8 μM) for 3 hours and then incubated with TRAIL (9.6 ng/mL). After 24 hours of treatment, cell death was then measured by annexin-V staining. (D) Transfection of Aurora A and/or Aurora B, but not the unrelated nonspecific control siRNA, led to a decrease in Aurora kinases protein expression in HMCLs without affecting the levels of the unrelated protein actin. At 48 hours after siRNA transfection, HMCLs were treated with TRAIL at the indicated doses (ng/mL) for 48 hours and the percentages of annexin-V+ apoptotic cells were then measured. Values are mean ± SD of 3 independent experiments (*P < .001 vs TRAIL nonspecific si-RNA [CONT]; Dunnet test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/15/10.1182_blood-2013-02-482356/4/m_2641f1.jpeg?Expires=1766009072&Signature=0J0d7wDKfM3vvR02AK97eeVjGMQAy0SFX9MgLxCM7yuIGojXuXWBGzwdz~rGQFtytIsM-sVtOBYKS1k6faYOiDX1slUQ139P5YgsawNAvshi-f14cHCAcOS07oAbCFax5QlFw0HknXI1pRBpTPk-HDYC6V19M8sQs2wvlDXyRk379NwFmfAdbz5FL1p5aB0E9PqB0Rmg6usxfpa3iCtpe0PHfccTkzizf3NsbJGp2DqFWRW2aJUFk6vKdo0uPe5lwdhYp23snVAh6JunINaHTSSl7kiCafRICI7Af6Kfae6kH2ASyUxfjviBfKR9ABkxZYFfbGcg2JtPtetegLKJUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Pan-AKIs block TRAIL-induced canonical and noncanonical NF-κB activation in MM cells. (A) HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) and after 24 hours nuclear extracts were prepared. (i) Nuclear extracts from RPMI 8226 and RPMI 8226/R5 were immunoblotted against NF-κB p65, NF-κB1 p50, NF-κB2 p52, c-Rel, RelB, and laminin or histone H2B as nuclear loading control. Bands were subjected to densitometric scanning using the TINA 2 software, and the number below each lane represents the relative amount of the indicated proteins normalized to laminin or histone H2B expression. (ii) HMCL nuclear extracts were tested for DNA binding activity of the NF-κB p65, p50, p52, c-Rel, and RelB subunits using the TransAM NF-κB enzyme-linked immunosorbent assay kit. Results were normalized to the untreated control. Values represent mean ± SD of 3 separate experiments performed in triplicate. (B) HMCLs were cultured with pan-AKIs and/or TRAIL as previously described. Endogenous p-IKKα/β, p-NF-κB2 p100, p-IκB-α, IKKβ, and IKKα from whole cell lysates were revealed by western blot analysis. Anti-actin immunoblotting was performed as loading control. Bands were subjected to densitometric scanning and p-IKKα/β, p-NF-κB2 p100, and p-IκB-α protein expression levels were normalized to actin; the number below each lane represents the relative amount of the indicated proteins. (Ci-ii) HMCLs were electroporated with IκB-α or nontargeting siRNA (CONT), and 24 hours after siRNA transfection the cells were treated with MK-0457. After 24 hours of treatment, cytoplasmic and nuclear extracts were prepared. (i) Cytoplasmic cell lysates were immunoblotted against IκB-α and tubulin as a marker of cytoplasmic separation as well as loading control. (ii) Nuclear extracts were tested for DNA binding activity of the NF-κB p65 subunit using the TransAM NF-κB enzyme-linked immunosorbent assay kit. Results were normalized to the untreated control. Values represent mean ± SD of 3 separate experiments performed in triplicate. (iii) Transfection of IκB-α, but not the unrelated nonspecific control siRNA, led to a decrease in IκB-α protein expression in HMCLs without affecting the levels of the unrelated protein actin. At 24 hours after siRNA transfection, HMCLs were treated with pan-AKIs for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) for 24 hours, and the percentages of annexin-V+ apoptotic cells were measured. Values are mean ± SD of 3 independent experiments (*P < .01 vs pan-AKIs/TRAIL nonspecific siRNA [CONT]; Dunnet test). (D) Transfection of Aurora A or B, but not the unrelated nonspecific control siRNA, led to a decrease in Aurora kinase protein expression in RPMI 8226 and RPMI 8226/R5 without affecting the levels of the unrelated protein actin. At 48 hours after siRNA transfection, cells were lysed for immunoblot analysis to monitor the expression of Aurora A and B, p-IKKβ, p-IKKα, p-IκB-α, p-NF-κB2 p100, IKKβ, IKKα, and actin as loading control. Bands were subjected to densitometric scanning and p-IKKα/β, p-NF-κB2 p100, and p-IκB-α protein expression levels were normalized to actin. The number below each lane represents the relative amount of the indicated proteins.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/15/10.1182_blood-2013-02-482356/4/m_2641f3.jpeg?Expires=1766009072&Signature=xuxj29nRIeB0A5kvcYzH1R3DtG4cjLKugLdjQy1CaVOE-5iIm7TfsRUUlDOgTvhZKL7tgN1O-ZCWytEdIocoo0vGjhIKXXi5fL~GKf-71WcT~wed4eKxL9rnae5zEKwYtq2E11IeqGOnwlJoJnAAjWgntG4s4bLFpHBfL3UVDBMxSiDpGQFYcoC8SIYKMA19wm-OOsqPub94YvfzcZgLa5n8dxN32ovKuti7YN67iIS2gi4fR93vgyEXMprooFFP8~G2hJCrN80oNxCrIQMZb1~ZZKiwoseL7KhADTnzLCGmj36kN2nsxHhLnBf2j2Gow-f2hPnGBTV6oGPv~~JWMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Pan-AKIs sensitize MM cells to TRAIL by inhibiting the expression of TRAIL-induced NF-κB target genes. (A) HMCLs were treated with MK-0457 for 3 hours and then incubated with TRAIL (2.4 ng/mL in RPMI 8226 and OPM-2, 9.6 ng/mL in RPMI 8226/R5 and U266, 300 ng/mL in JJN3) for 24 hours, after which endogenous A1/Bfl-1 and Mcl-1 were revealed by immunoblotting analysis. Blots were subsequently reprobed for actin expression to ensure equivalent loading and transfer of protein. Histograms represent the mean value ± SD of the ratio of A1/Bfl-1 or Mcl-1/actin normalized to the untreated control from blots of 3 independent experiments (*P < .001; Tukey-Kramer test). (B) Transfection of A1/Bfl-1 or Mcl-1, but not the unrelated nonspecific control siRNA (CONT), led to a decrease in A1/Bfl-1 or Mcl-1 protein expression in HMCLs without affecting the levels of the unrelated protein actin (insets). At 30 minutes after siRNA transfection, the cells were treated with TRAIL at the indicated doses (ng/mL) for the indicated time. Apoptosis was measured by annexin-V staining. Values are mean ± SD of 3 independent experiments (*P < .005; **P < .001 vs non-specific control siRNA [CONT]; Dunnet test). (C) RPMI 8226/R5 cells were infected with an empty lentiviral vector or lentivirus expressing A1/Bfl-1 or Mcl-1. All lentviral expression vectors coexpressed red fluorescent protein to monitor the infection by flow cytometry. Plot represents comparable red fluorescent protein expression of RPMI 8226/R5s infected with the parental retroviral vector or those encoding for Mcl-1 or A1/Bfl1. Whole-cell lysates of uninfected or virus-infected RPMI 8226/R5 cells were prepared and analyzed by western blot to confirm the overexpression of A1/Bfl-1 or Mcl-1 (inset). Pools of RPMI 8226/R5 were expanded after infection and treated with PHA-680632 (0.8 μM) and then incubated with TRAIL at the indicated doses for 24 hours. Cell death was measured by annexin-V staining. Values are mean ± SD of 3 independent experiments (*P < .005, vs PHA/TRAIL empty vector; Dunnet test). (D) HMCLs were treated as described in A and endogenous cIAP1, cIAP2, XIAP, and actin proteins were revealed by immunoblotting analysis. Bands were subjected to densitometric scanning, and histograms represent the mean value ± SD of the ratio of cIPA1, cIAP2, or XIAP/actin normalized to the untreated control from blots of 3 independent experiments (*P < .01; **P < .001; Tukey-Kramer test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/15/10.1182_blood-2013-02-482356/4/m_2641f6.jpeg?Expires=1766009072&Signature=OMkWP4PaOQoBWDp0FDqhi8WKgdX3-0OM82RDCgdkMd6K-UfIq8dLc-01VrLoVsY-nUNRhQOpXgGms4H-6EwhkBFaN3tWr59RhhP9Okw~JP60eARjvfS5oiwbEbLkOZ3F75nyC2dvRRMYslSxADWZczc6lnhcu90zbkGG9EnKQ80Fis6CtLZMm93eFmxrHrUV0P966Mw02wmcOfmcCESW-lC7bgF~5d2yWK~HgE6rAmwqQoc-Qfnf0IJZVj4yhwL5GviKX2pZaDfrQcSP7-VlNuRNCkiHhGn1302zrdiW~as~ZplhX8lDVLCw0Rte5I6wWx9DFfokJgiqhEP7ujR2tA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)