Key Points
The integrin-activating adaptor Kindlin-3 promotes firm lymphocyte adhesion to inflamed blood vessels.
However, Kindlin-3 is not required for leukocyte extravasation through these inflamed blood vessels.
Abstract
Kindlin-3 is an integrin-binding focal adhesion adaptor absent in patients with leukocyte and platelet adhesion deficiency syndrome and is critical for firm integrin-dependent leukocyte adhesion. The role of this adaptor in leukocyte diapedesis has never been investigated. In the present study, the functions of Kindlin-3 in this process were investigated in effector T lymphocytes trafficking to various lymphoid and nonlymphoid tissues. In vitro, Kindlin-3–deficient T cells displayed severely impaired lymphocyte function antigen-1–dependent lymphocyte adhesion but partially conserved very late antigen-4 adhesiveness. In vivo, the number of adoptively transferred Kindlin-3–deficient T effectors was dramatically elevated in the circulating pool compared with normal effectors, and the Kindlin-3 mutant effectors failed to enter inflamed skin lesions. The frequency of Kindlin-3–deficient T effectors arrested on vessel walls within inflamed skin-draining lymph nodes was also reduced. Strikingly, however, Kindlin-3–deficient effector T cells accumulated inside these vessels at significantly higher numbers than their wild-type lymphocyte counterparts and successfully extravasated into inflamed lymph nodes. Nevertheless, on entering these organs, the interstitial motility of these lymphocytes was impaired. This is the first in vivo demonstration that Kindlin-3–stabilized integrin adhesions, although essential for lymphocyte arrest on blood vessels and interstitial motility, are not obligatory for leukocyte diapedesis.
Introduction
Leukocyte integrins function in leukocyte arrest on distinct vascular beds and enable diapedesis across these structures.1 Integrin-blocking studies both in vivo and in vitro strongly suggest that functionally intact integrins are necessary for arrested leukocytes to crawl, and cross endothelial barriers under physiological shear flow.2-7 Shear-resistant integrin-dependent leukocyte adhesions to vascular endothelium require correct associations of leukocyte integrins with 2 focal adhesion adaptors, talin1 and Kindlin-3.8 Mutations introducing a stop codon in Kindlin-3 underlie a rare integrin-dependent leukocyte and platelet adhesion deficiency syndrome, called LAD-III,9-11 which is associated with severe bleeding defects, defective neutrophil-endothelial interactions in vivo,12 and impaired integrin-dependent adhesion of leukocytes to inflamed endothelia and antigen-presenting cells in vitro.13,14
Recent data on neutrophils have suggested that although full activation of lymphocyte function antigen-1 (LFA-1)–mediated arrest requires both talin1 and Kindlin-3, LFA-1 can still mediate weakly adhesive rolling interactions, even in the absence of Kindlin-3.15 Furthermore, in human effector T cells lacking Kindlin-3, very late antigen-4 (VLA-4) supports both normal rolling adhesions and partial firm adhesions on vascular cell adhesion molecule-1 (VCAM-1).13 Nevertheless, in the same lymphocytes, Kindlin-3 was found critical for chemokine-induced arrest on both VLA-4 and LFA-1 ligands present at low site densities.13 Therefore, we hypothesized that effector T lymphocytes may use their Kindlin-3–independent integrin activities to arrest and extravasate specific vascular beds. To test this hypothesis in vivo, we compared the migratory properties of cotransferred murine normal and Kindlin-3–deficient T cells inside inflamed skin and inflamed skin-draining lymph nodes, shown to recruit large numbers of both Th1 and Tc1 effector cells via their high endothelial venules (HEVs).16,17 Intravital microscopy of these adoptively transferred effectors revealed a reduced ability to arrest on inflamed HEVs, but with time, the numbers of Kindlin-3–deficient T cells entering lymph nodes surpassed those of wild-type (wt) effector T cells. Taken together, our results suggest that despite its role in bidirectional integrin signaling12 necessary for optimal firm integrin-mediated adhesiveness,12,18 Kindlin-3 is not obligatory for lymphocyte diapedesis.
Materials and methods
All in vitro methodology is described in supplemental Materials (available on the Blood Web site). All animal procedures were carried out on mice of C57BL/6 background and were approved by the Animal Research Committee at the Weizmann Institute of Science.
Preparation of Kindlin-3 null effector T cells
CD45.1-recipient mice were irradiated at 950 rad, and reconstituted with 2 to 5 × 106 bone marrow cells from either Kindlin-3fl/fl (wt), or from CD4CrexKindlin-3fl/fl, (Kindlin-3–deficient) animals, both from a CD45.2 background, generated as described in supplemental Materials. Spleens were removed after 8 weeks, purified over a Novamed column, and stimulated on plates coated with 1 µg/mL of anti-CD3 and 5 µg/mL of anti-CD28 for 48 hours. CD45.2 T cells were purified by negative depletion using a CD45.1 purification kit and were expanded for 5 additional days with 10 U/mL of IL-2.
Induction of lymph node and skin inflammation
Inflammation within the inguinal and popliteal lymph nodes was induced by subcutaneous injection of Complete Freund’s Adjuvant (CFA) (2.5 mg/mL, 50-100 μL) to both the footpad and flank, 48 hours before adoptive transfer experiments. Under these conditions, both types of lymph nodes were rendered inflamed, as described previously.16 Skin inflammation in the flank was induced 48 hours before adoptive transfer by multizone intradermal CFA injections (2.5 mg/mL, 10 μL per injection), as described previously.19
Analysis of T-cell accumulation in blood, spleen, lung, and liver
wt or K3-deficient effector T cells (2 × 107 cells) were each labeled with (5-(and -6-)-((4-chloromethyl)benzoyl)amino) tetramethylrhodamine (CMTMR; 10 μM, 30 minutes), carboxyfluorescein diacetate succinimidyl ester (CFSE; 10 μM, 5 minutes), or 2,3,6,7-tetrahyfro-9-bromomethyl-1H,5H-quinolozino-(9,1-gh)-coumarin (Celltracker Violet; 50 μM, 25 minutes), mixed at a 1:1 ratio and injected intracardially (left lower ventricle) to anesthetized wt-recipient mice. Mice were bled 30 to 40 minutes after injection, and differently labeled T cells were enumerated by fluorescence-activated cell sorter (FACS). To determine T-cell accumulation in liver, spleen, and lungs, single-cell suspensions were prepared by mechanical disruption, as described previously.20 Reversal of these cell tracker dyes did not alter the relative entry of the labeled T-cell populations. To block lymphocyte responsiveness to chemokine signals, each experimental group was pretreated ex vivo with either pertussis toxin (PTx; 100 ng/mL) or medium for 4 hours. To block specific integrins, each population (2 × 107 cells in 0.1 mL) was suspended with 10 μg of an integrin-blocking monoclonal antibody (mAb) or control anti-CD45 mAb for 20 minutes on ice, washed once, mixed, and coinjected.
Analysis of T-cell accumulation and extravasation through skin vessels
Differently labeled T cells were coinjected as described above. Mice were euthanized 4 hours later, and either inflamed skin tissues (CFA injection spots) or noninflamed tissues (>10 mm from these sites) were removed and immediately fixed in 4% paraformaldehyde and 2% sucrose. Whole-mount samples were stained with rat anti-murine platelet endothelial CAM-1 mAb in 0.1% Triton X-100/0.2% bovine serum albumin overnight followed by Alexa-647–labeled goat anti-rat Ab. Immunostained tissues were imaged in a Delta Vision fluorescence microscope, as described previously,19 and intraluminal vs perivascular dye-labeled T cells were enumerated in multiple fields.
Intravital microscopic imaging of effectors entering inflamed lymph nodes
To enumerate the number of injected T cells that accumulated either intravascularly or extravascularly in different inguinal and popliteal lymph nodes, we used 2-photon microscopy (Ultima multiphoton microscope attached to an upright platform (Olympus BX61WI) equipped with 20× (NA 0.95) water immersion objective) of live recipient mice imaged 10 to 300 minutes after intracardiac injection of effector T-cell and Qtracker655 quantum dots. Excitation wavelength of 850 nm was used for simultaneous imaging of Celltracer Violet, CMTMR, and Qtracker655. To create a typical time-lapse sequence, a 50-µm–thick section was scanned at 5-µm Z-steps every 36 seconds. Cell movement was analyzed with Volocity software (PerkinElmer, Waltham, MA).
Results
Kindlin-3–deficient effector T cells exhibit defective LFA-1 and VLA-4 adhesiveness under shear flow conditions
Because Kindlin-3 deficiency in mice restricts T-cell development and impairs the generation of naïve T cells,12 we dissected its functions in in vitro-generated effector Th1 and Tc1 cells, expanded from spleen-derived CD3 T cells.19 To generate Kindlin-3–deficient T cells, we used a T-cell–specific conditional ablation, ie, Cre expressed under a CD4 promoter crossed with Kindlin-3fl/fl mice to obtain Kindlin-3 deletion during the CD4/CD8 double-positive stage. Spleen T cells were polyclonally stimulated in vitro and were expanded in IL-2. The resulting Kindlin-3–deficient effector T cells (Figure 1A) expressed normal levels of both α4 β1 and β2 integrins, as well as of prototypic G-protein coupled receptors (Figure 1B, and data not shown).
Impaired integrin adhesiveness of Kindlin-3–deficient effector T cells in vitro. (A) Deficient Kindlin-3 expression on T effectors derived from CD4CrexKindlin-3fl/fl mice and their control Kindlin-3fl/fl littermates. Lysates were immunoblotted with anti–Kindlin-3 Ab or anti-tubulin Ab. (B) FACS staining of the major integrin subunits critical for lymphocyte-endothelial interactions and of the inflammatory G-protein coupled receptor CXCR3 on wt and Kindlin-3–deficient effector T cells. (C) Resistance to detachment of Kindlin-3–deficient or wt effector T cells by the indicated shear forces after settling for 1 minute under shear-free conditions on ICAM-1 or on VCAM-1 coated at 760 sites/μm2. Results are the mean ± range of 2 measurements. *P < .05. (D) Resistance of wt and Kindlin-3–deficient T cells accumulating on low-density P-selectin (left panel) or E-selectin (right panel) to detachment by progressively elevated shear stresses. T cells were allowed to accumulate for 40 seconds on low-density P- or E-selectins (coated at 95 and 25 sites/μm2, respectively) at a shear stress of 1 dyn/cm2, and the flow was increased by stepwise increments every 5 seconds. The number of cells bound at the end of each interval was determined in 2 fields and was expressed as a percentage of initially accumulated lymphocytes. A representative of 3 experiments is shown. (E) Chemotaxis of Kindlin-3–deficient and wt effector T cells determined in a transwell assay. Unless indicated, all chemokines were placed at 10 nM at the lower well. Pore size: 5 µm. Results are the mean ± standard error of the mean (SEM) of 4 to 6 independent measurements.
Impaired integrin adhesiveness of Kindlin-3–deficient effector T cells in vitro. (A) Deficient Kindlin-3 expression on T effectors derived from CD4CrexKindlin-3fl/fl mice and their control Kindlin-3fl/fl littermates. Lysates were immunoblotted with anti–Kindlin-3 Ab or anti-tubulin Ab. (B) FACS staining of the major integrin subunits critical for lymphocyte-endothelial interactions and of the inflammatory G-protein coupled receptor CXCR3 on wt and Kindlin-3–deficient effector T cells. (C) Resistance to detachment of Kindlin-3–deficient or wt effector T cells by the indicated shear forces after settling for 1 minute under shear-free conditions on ICAM-1 or on VCAM-1 coated at 760 sites/μm2. Results are the mean ± range of 2 measurements. *P < .05. (D) Resistance of wt and Kindlin-3–deficient T cells accumulating on low-density P-selectin (left panel) or E-selectin (right panel) to detachment by progressively elevated shear stresses. T cells were allowed to accumulate for 40 seconds on low-density P- or E-selectins (coated at 95 and 25 sites/μm2, respectively) at a shear stress of 1 dyn/cm2, and the flow was increased by stepwise increments every 5 seconds. The number of cells bound at the end of each interval was determined in 2 fields and was expressed as a percentage of initially accumulated lymphocytes. A representative of 3 experiments is shown. (E) Chemotaxis of Kindlin-3–deficient and wt effector T cells determined in a transwell assay. Unless indicated, all chemokines were placed at 10 nM at the lower well. Pore size: 5 µm. Results are the mean ± standard error of the mean (SEM) of 4 to 6 independent measurements.
Next, we assessed to what extent the loss of Kindlin-3 impairs the adhesive properties of these integrins. Whereas the intrinsic adhesiveness of LFA-1 in Kindlin-3–deficient murine effector T cells was practically eliminated (Figure 1C), VLA-4 adhesiveness to VCAM-1 was impaired only at high shear stresses (Figure 1C). Nevertheless, the ability of Kindlin-3–deficient effector T cells to roll on low-density P- or E-selectins, implicated in effector T-cell migration to inflamed skin and skin-draining lymph nodes,17,21 remained intact (Figure 1D and not shown). Interestingly, the chemotactic activity of Kindlin-3–deficient effector T cells toward optimal levels of the 2 main CXCR3 chemokines, CXCL9 and CXCL10, implicated in T-cell trafficking to inflamed tissues including lymph nodes22 was slightly impaired despite normal CXCR3 expression (Figure 1B,E). Likewise, despite normal CCR5 and CCR7 expression, the chemotactic activity of Kindlin-3–deficient effector cells toward the cognate ligands, CCL5 and CCL21, respectively, was also partially impaired (Figure 1E). Taken together, these results show that although Kindlin-3 is not required for primary selectin-mediated lymphocyte adhesions and chemotaxis, it plays a critical role in shear-resistant adhesiveness of LFA-1 and VLA-4 integrins.
Kindlin-3–deficient effector T cells fail to enter inflamed skin but recruit to skin-draining lymph nodes
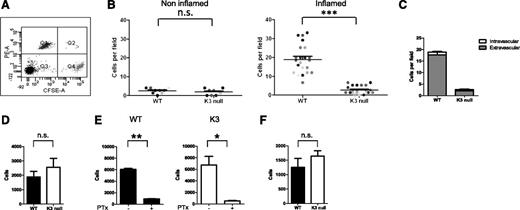
Next, we compared the ability of wt effectors and their Kindlin-3–deficient counterparts to enter inflamed skin.19 CFSE-labeled wt cells mixed with CMTMR-labeled Kindlin-3–deficient T cells were coinjected intracardially at a 1:1 ratio (Figure 2A) into mice that had been intradermally challenged with CFA into their flank 48 hours earlier. Although wt effector T cells readily accumulated in CFA-inflamed skin lesions shortly after transfer, Kindlin-3–deficient T cells accumulated poorly at these sites (Figure 2B-C). CFA injection into the skin also resulted, within 48 hours, in massive inflammation in the lymph nodes that drain the injected lesions.23 These inflamed lymph nodes readily attract effector T cells via their HEVs16,17,23 ; both intracellular adhesion molecule-1 (ICAM-1), the inflammatory CXCR3 chemokine, CXCL9, and the endothelial inducible selectin, P-selectin, have been implicated in this recruitment.16,17,22 Strikingly, in sharp contrast to CFA-inflamed skin, Kindlin-3–deficient effector T cells normally entered CFA-inflamed inguinal lymph nodes (Figure 2D) and did so in a PTx-sensitive manner (Figure 2E), consistent with a key role for chemokine signals in this accumulation. Kindlin-3–deficient effector T cells also normally accumulated in CFA-inflamed popliteal lymph nodes (Figure 2F). These unexpected results suggest that Kindlin-3, although critical for T-cell entry to inflamed skin, is dispensable for T-cell entry to inflamed lymph nodes.
Kindlin-3–deficient effectors fail to accumulate in inflamed skin but efficiently accumulate in various lymph nodes draining the inflamed skin tissues. (A) FACS of a 1:1 mixture of CFSE-labeled wt T effectors (Q4) mixed with CMTMR-labeled Kindlin-3–deficient T effectors (Q1) prior to intracardiac injection into recipient wt mice. (B) The number of wt and Kindlin-3–deficient T cells (injected at a 1:1 ratio) accumulated in noninflamed or inflamed skin flank 4 hours after intracardiac injection, determined by fluorescence microscopy of fixed skin sections. Skin inflammation was induced by an intradermal injection of CFA. Each dot represents a separate field (0.18 mm2 in size) within inflamed or noninflamed flank areas. Results are from multiple fields of 2 mice. ***P < .0001. (C) The partition of CFSE wt or CMTMR Kindlin-3–deficient T cells inside and outside of the skin vessels determined by platelet endothelial CAM-1 immunostaining of skin vessels. Mean values ± SEM of data from the indicated fields shown in B (n = 23). (D) Relative accumulation of wt or Kindlin-3–deficient T cells in inguinal lymph nodes 4 hours after intracardiac injection determined by FACS of harvested suspensions of lymph nodes collected from 2 mice; n.s., not significant. One experiment is representative of 3 experiments. (E) Relative accumulation of sham or PTx pretreated wt or Kindlin-3–deficient T cells determined as in panel D. *P < .01; **P < .002. (F) Relative accumulation of wt or Kindlin-3–deficient T cells in popliteal lymph nodes 4 hours after intracardiac injection. n = 3; n.s., not significant. The values in panels D to F are the mean ± SEM.
Kindlin-3–deficient effectors fail to accumulate in inflamed skin but efficiently accumulate in various lymph nodes draining the inflamed skin tissues. (A) FACS of a 1:1 mixture of CFSE-labeled wt T effectors (Q4) mixed with CMTMR-labeled Kindlin-3–deficient T effectors (Q1) prior to intracardiac injection into recipient wt mice. (B) The number of wt and Kindlin-3–deficient T cells (injected at a 1:1 ratio) accumulated in noninflamed or inflamed skin flank 4 hours after intracardiac injection, determined by fluorescence microscopy of fixed skin sections. Skin inflammation was induced by an intradermal injection of CFA. Each dot represents a separate field (0.18 mm2 in size) within inflamed or noninflamed flank areas. Results are from multiple fields of 2 mice. ***P < .0001. (C) The partition of CFSE wt or CMTMR Kindlin-3–deficient T cells inside and outside of the skin vessels determined by platelet endothelial CAM-1 immunostaining of skin vessels. Mean values ± SEM of data from the indicated fields shown in B (n = 23). (D) Relative accumulation of wt or Kindlin-3–deficient T cells in inguinal lymph nodes 4 hours after intracardiac injection determined by FACS of harvested suspensions of lymph nodes collected from 2 mice; n.s., not significant. One experiment is representative of 3 experiments. (E) Relative accumulation of sham or PTx pretreated wt or Kindlin-3–deficient T cells determined as in panel D. *P < .01; **P < .002. (F) Relative accumulation of wt or Kindlin-3–deficient T cells in popliteal lymph nodes 4 hours after intracardiac injection. n = 3; n.s., not significant. The values in panels D to F are the mean ± SEM.
Kindlin-3–deficient, but not PTx-pretreated, effector T cells are enriched in blood after adoptive transfer
Next, we compared the number of Kindlin-3–deficient and wt T cells that remained circulating in the blood shortly after adoptive transfer. Surprisingly, as early as 30 to 45 minutes after adoptive transfer, although injected at a 1:1 ratio (Figure 2A), the number of circulating Kindlin-3–deficient effector T cells was fourfold higher than that of coinjected wt effector T cells (Figure 3A). A threefold-higher number of Kindlin-3–deficient T cells also persisted in the circulation 4 hours after injection (data not shown), both in inflamed and naïve recipient mice (supplemental Figure 1). Consistently, fourfold-higher numbers of Kindlin-3–deficient effector T cells were recovered in the spleen of recipient mice (Figure 3B), in agreement with a largely integrin-independent lymphocyte entrapment in this organ.24 In contrast, PTx pretreatment of wt T effectors, known to interfere with chemokine-stimulated integrin-dependent leukocyte extravasation,19 did not result in elevated recovery of these lymphocytes in the blood of either CFA-treated mice or naïve mice (Figure 3C; supplemental Figure 2). Thus, we reasoned that the reduced numbers of wt effector T cells relative to the kindlin-3 mutant in the circulation could not be attributed to their preferential extravasation from the circulation. Rather, the higher fraction of injected Kindlin-3–deficient effector T cells in the circulation was the result of their reduced ability, relative to their wt counterparts, to engage with vascular LFA-1 and α4-integrin ligand, because mAb blocking of these integrins released both Kindlin-3–deficient and wt T cells to the circulating pool (Figure 3D-E). Many blood capillaries in the lungs, liver, skin, kidney, and heart express integrin ligands in a constitutive manner25-30 (supplemental Figure 3). The pulmonary vasculature is a particularly extensive network known to sequester numerous circulating leukocytes31 and entrap adoptively transferred leukocytes.28,32,33 Notably, 40 minutes after injection, 15% to 20% fewer adoptively transferred Kindlin-3–deficient T effectors were recovered from recipient lungs relative to adoptively transferred wt T effectors (Figure 3F; supplemental Figure 4). Fewer adoptively transferred Kindlin-3–deficient T effectors were also recovered from resting lymph nodes (see below). In contrast, more Kindlin-3–deficient T cells were recovered from the liver 40 minutes after transfer (Figure 3F). Therefore, our findings collectively suggest that Kindlin-3–deficient effector T cells preferentially remain in the circulation probably because of their poor global retention by the numerous vascular LFA-1 and α4-integrin ligands constitutively expressed by distinct blood vessels.
Kindlin-3–deficient effector T cells are retained in the circulation at higher numbers than wt T cells. (A) Relative abundance of coinjected CFSE-labeled wt and CMTMR-labeled Kindlin-3–deficient T cells in blood 40 minutes after intracardiac transfer. The indicated numbers of total T cells were determined by FACS of blood samples, as explained in “Materials and methods.” Mean values ± range of 2 mice. A representative of 4 experiments. ***P < .0002. (B) Accumulation of dye-labeled wt or Kindlin-3–deficient T cells in spleens recovered 40 minutes after intracardiac injection determined by FACS analysis. Mean values ± range of 2 mice. A representative of 4 experiments. Average values ± range. ***P < .0004. (C) The numbers of sham and PTx-pretreated T cells (labeled with either CFSE or CMTMR) recovered in blood 40 minutes after intracardiac injection determined as in panel A. n = 2. n.s., not significant. (D) Effect of pretreatment of wt effector T cells with either LFA-1 or α4-blocking mAb or with a nonblocking mAb (anti-CD45) on the concentration of T cells circulating in blood. Dye-labeled wt T cells were incubated with each of the mAbs, washed, and coinjected at a 1:1 ratio. The blood concentration of each mAb-treated population was determined as in panel A, and the ratios between each indicated groups are depicted. Results are given as mean ± SEM of 5 to 9 experiments. (E) Effects of pretreatment of dye-labeled Kindlin-3–deficient T cells with either LFA-1 or α4-blocking mAbs on lymphocyte numbers in blood. Results are mean ± SEM of 3 experiments. (F) wt (CFSE-labeled) or Kindlin-3–deficient (CMTMR-labeled) T cells were coinjected at a 1:1 ratio, and their relative accumulation in the lung (left) and liver (right) was enumerated by FACS analysis 40 minutes later n = 3. **P < .002; ***P < .0002.
Kindlin-3–deficient effector T cells are retained in the circulation at higher numbers than wt T cells. (A) Relative abundance of coinjected CFSE-labeled wt and CMTMR-labeled Kindlin-3–deficient T cells in blood 40 minutes after intracardiac transfer. The indicated numbers of total T cells were determined by FACS of blood samples, as explained in “Materials and methods.” Mean values ± range of 2 mice. A representative of 4 experiments. ***P < .0002. (B) Accumulation of dye-labeled wt or Kindlin-3–deficient T cells in spleens recovered 40 minutes after intracardiac injection determined by FACS analysis. Mean values ± range of 2 mice. A representative of 4 experiments. Average values ± range. ***P < .0004. (C) The numbers of sham and PTx-pretreated T cells (labeled with either CFSE or CMTMR) recovered in blood 40 minutes after intracardiac injection determined as in panel A. n = 2. n.s., not significant. (D) Effect of pretreatment of wt effector T cells with either LFA-1 or α4-blocking mAb or with a nonblocking mAb (anti-CD45) on the concentration of T cells circulating in blood. Dye-labeled wt T cells were incubated with each of the mAbs, washed, and coinjected at a 1:1 ratio. The blood concentration of each mAb-treated population was determined as in panel A, and the ratios between each indicated groups are depicted. Results are given as mean ± SEM of 5 to 9 experiments. (E) Effects of pretreatment of dye-labeled Kindlin-3–deficient T cells with either LFA-1 or α4-blocking mAbs on lymphocyte numbers in blood. Results are mean ± SEM of 3 experiments. (F) wt (CFSE-labeled) or Kindlin-3–deficient (CMTMR-labeled) T cells were coinjected at a 1:1 ratio, and their relative accumulation in the lung (left) and liver (right) was enumerated by FACS analysis 40 minutes later n = 3. **P < .002; ***P < .0002.
Kindlin-3 is critical for efficient arrest of effectors on inflamed lymph node vessels
Next, we used intravital microscopy to determine the frequency of wt or Kindlin-3–deficient effector T cells accumulating on inflamed lymph node vessels soon after adoptive transfer. Whereas negligible wt or Kindlin-3–deficient effector T cells arrested on resting lymph nodes 40 minutes after intracardiac injection (Figure 4A), wt effector T cells arrested on inflamed skin-draining lymph node vessels, at a much higher frequency than Kindlin-3–deficient effector T cells (Figure 4A; supplemental Figure 5). As expected, blocking either LFA-1 or α4 integrins significantly reduced the frequency of wt T cells arrested on inflamed lymph node vessels (Figure 4B and not shown) (Figure 4B). Interestingly, PTx pretreatment did not interfere with the arrest frequency of wt effector T cells on identical lymph node vessels (Figure 4C). Notably, the few Kindlin-3–deficient T cells that arrested on inflamed lymph node vessels used their integrins for this firm adhesion, as demonstrated by the sensitivity of their arrests to inhibition of either LFA-1 or α4 integrins (Figure 4D). Thus, Kindlin-3–deficient effectors express poorly adhesive but partially functional LFA-1 and α4 integrins.
Arrest efficiency of Kindlin-3–deficient effectors on inflamed lymph nodes vessels is lower compared with wt effectors (A) Accumulation efficiency of coinjected CFSE wt and CMTMR-labeled Kindlin-3 mutant effector T cells inside resting and inflamed inguinal lymph node vessels as determined by multiphoton intravital microscopy at 40 minutes after intracardiac injection. CFA was injected subcutaneously 48 hours before adoptive transfer of T cells. Blood vessels were visualized by Qtracker655 quantum dots. Each dot represents a single field of view (0.23 mm2). For each experimental group, the number of accumulated T cells per field was normalized to the number of T cells in the corresponding group found to circulate in the blood (ie, the flux of freely flowing T cells). Each colored dot represents a single field of view. Results are accumulated from 3 independent experiments. ***P < .0001. (B) Effect of pretreatment of wt effector T cells with either LFA-1–blocking mAb (left), α4-blocking mAb (middle), with a nonblocking anti-CD45 mAb (all panels), or a nonbinding mAb (control, right panel) on accumulation of wt effector T cells inside inflamed lymph nodes 40 minutes after intracardiac injection. Differently dye-labeled T cells were pretreated with either mAb 20 minutes before injection, washed, mixed, and coinjected at a 1:1 ratio. Each colored dot represents a single field of view. The numbers of cells were normalized to the T-cell flux as in panel A. Results are accumulated from 3 independent experiments. ***P < .0001. (C) Effect of PTx on the accumulation of wt effector T cells inside inflamed lymph nodes 40 minutes after intracardiac injection. Sham-treated CFSE-labeled T cells were coinjected with PTx-pretreated CMTMR-labeled T cells, as in Figure 3C. Each dot represents a single field of view. The numbers of cells were normalized to the T-cell flux as in panels A-B. One experiment is representative of 3 experiments. (D) Effect of LFA-1 blocking on early accumulation (40 minutes after intracardiac injection) of Kindlin-3 null T cells in inflamed lymph node vessels. Differently labeled Kindlin-3–deficient effector T cells were pretreated with either LFA-1–blocking mAb (left), α4-blocking mAb (right), or with a nonblocking anti-CD45 mAb, as in panel B. Each dot represents a single field of view. The numbers of cells were normalized to the T-cell flux as in panel A. **P < .0032; ***P < .0001; n.s., not significant.
Arrest efficiency of Kindlin-3–deficient effectors on inflamed lymph nodes vessels is lower compared with wt effectors (A) Accumulation efficiency of coinjected CFSE wt and CMTMR-labeled Kindlin-3 mutant effector T cells inside resting and inflamed inguinal lymph node vessels as determined by multiphoton intravital microscopy at 40 minutes after intracardiac injection. CFA was injected subcutaneously 48 hours before adoptive transfer of T cells. Blood vessels were visualized by Qtracker655 quantum dots. Each dot represents a single field of view (0.23 mm2). For each experimental group, the number of accumulated T cells per field was normalized to the number of T cells in the corresponding group found to circulate in the blood (ie, the flux of freely flowing T cells). Each colored dot represents a single field of view. Results are accumulated from 3 independent experiments. ***P < .0001. (B) Effect of pretreatment of wt effector T cells with either LFA-1–blocking mAb (left), α4-blocking mAb (middle), with a nonblocking anti-CD45 mAb (all panels), or a nonbinding mAb (control, right panel) on accumulation of wt effector T cells inside inflamed lymph nodes 40 minutes after intracardiac injection. Differently dye-labeled T cells were pretreated with either mAb 20 minutes before injection, washed, mixed, and coinjected at a 1:1 ratio. Each colored dot represents a single field of view. The numbers of cells were normalized to the T-cell flux as in panel A. Results are accumulated from 3 independent experiments. ***P < .0001. (C) Effect of PTx on the accumulation of wt effector T cells inside inflamed lymph nodes 40 minutes after intracardiac injection. Sham-treated CFSE-labeled T cells were coinjected with PTx-pretreated CMTMR-labeled T cells, as in Figure 3C. Each dot represents a single field of view. The numbers of cells were normalized to the T-cell flux as in panels A-B. One experiment is representative of 3 experiments. (D) Effect of LFA-1 blocking on early accumulation (40 minutes after intracardiac injection) of Kindlin-3 null T cells in inflamed lymph node vessels. Differently labeled Kindlin-3–deficient effector T cells were pretreated with either LFA-1–blocking mAb (left), α4-blocking mAb (right), or with a nonblocking anti-CD45 mAb, as in panel B. Each dot represents a single field of view. The numbers of cells were normalized to the T-cell flux as in panel A. **P < .0032; ***P < .0001; n.s., not significant.
Kindlin-3 is not required for diapedesis of effector T cells across inflamed lymph node vessels
The poor arrest of Kindlin-3–deficient effector T cells on inflamed inguinal lymph node vessels 40 minutes after tranfser (Figure 4) could not explain the high PTx-sensitive accumulation of these cells 4 hours after transfer (Figure 2D-F). Therefore, we analyzed, by 2 photon microscopy, the fraction of accumulating T cells that successfully extravasated through the lymph node vessels. Surprisingly, whereas 30 minutes after adoptive transfer of wt and Kindlin-3–deficient effector T cells, neither lymphocyte type extravasated vessels of inflamed inguinal lymph nodes (Figure 5A), 90 minutes later, a substantial number of Kindlin-3–deficient lymphocytes were found in the parenchyma of these inflamed inguinal lymph nodes and to a greater extent than wt effector T cells (Figure 5A). The entry of Kindlin-3–deficient effector T cells into these lymph nodes could not be attributed to extravasation of afferent skin-draining lymphatics, because Kindlin-3–deficient T cells failed to enter the inflamed skin (Figure 2B-C). Therefore, these findings are a first demonstration that Kindlin-3–deficient T cells can efficiently complete diapedesis across inflamed lymph node vessels. Furthermore, this diapedesis was completely abrogated by PTx pretreatment (Figure 5B; supplemental Videos 1 and 2). However, in contrast to PTx-pretreated wt effector T cells (Figure 5B), PTx-pretreated Kindlin-3–deficient T cells failed to even temporarily stick to the inflamed lymph node vessels (Figure 5B). Thus, despite their poor integrin-dependent adhesions, Kindlin-3–deficient T cells could integrate chemokine signals and develop sufficient adhesive contacts for successful diapedesis across inflamed lymph node vessels.
Accumulation and diapedesis of Kindlin-3–deficient effector T cells in inflamed lymph nodes are elevated compared with their wt counterparts. (A) The number of wt and Kindlin-3–deficient effector T cells arrested (intravascular) or emigrating (extravascular) from inflamed inguinal lymph node vessels was determined 30, 60, and 120 minutes after coinjection of these T cells (CFSE or Violet and CMTMR prelabeled, as in previous figures) by multiphoton intravital microscopy. Blood vessels were visualized by Qtracker655 quantum dots. Results are the mean ± SEM of 3 fields of view (0.23 mm2); n = 3. (B) Left: The fraction of sham-treated or PTx-pretreated wt T effectors that accumulated and successfully extravasated inflamed inguinal lymph node vessels 210 minutes after injection. The mean ± SEM of 3 fields of view. n = 3. Right: The fraction of accumulated sham or PTx pretreated Kindlin-3–deficient T effectors that successfully extravasated inflamed lymph node vessels. The mean ± SEM of 3 fields; n = 3. (C) Selected frames from supplemental Video 5 depicting individual wt (green arrows) and Kindlin-3–deficient T cells (pink arrows) undergoing diapedesis through HEVs of inflamed popliteal lymph nodes (blue). The time elapsed from the initial recording is indicated in each frame. T = 0 was set 80 minutes after coinjection of the labeled T cells. (D) The numbers of wt and Kindlin-3–deficient effector T cells arrested (intravascular) or emigrating (extravascular) out of inflamed popliteal lymph node vessels were determined 60 minutes and 240 minutes after coinjection at a 1:1 ratio. One experiment is representative of 3 independent experiments. (E) The numbers and fraction of sham-treated or PTx-pretreated wt (left) and Kindlin-3–deficient effector T cells (right) emigrating out of inflamed popliteal lymph node vessels determined 240 minutes after coinjection at a 1:1 ratio. Data shown are the mean ± SEM of 3 fields of view; n = 3.
Accumulation and diapedesis of Kindlin-3–deficient effector T cells in inflamed lymph nodes are elevated compared with their wt counterparts. (A) The number of wt and Kindlin-3–deficient effector T cells arrested (intravascular) or emigrating (extravascular) from inflamed inguinal lymph node vessels was determined 30, 60, and 120 minutes after coinjection of these T cells (CFSE or Violet and CMTMR prelabeled, as in previous figures) by multiphoton intravital microscopy. Blood vessels were visualized by Qtracker655 quantum dots. Results are the mean ± SEM of 3 fields of view (0.23 mm2); n = 3. (B) Left: The fraction of sham-treated or PTx-pretreated wt T effectors that accumulated and successfully extravasated inflamed inguinal lymph node vessels 210 minutes after injection. The mean ± SEM of 3 fields of view. n = 3. Right: The fraction of accumulated sham or PTx pretreated Kindlin-3–deficient T effectors that successfully extravasated inflamed lymph node vessels. The mean ± SEM of 3 fields; n = 3. (C) Selected frames from supplemental Video 5 depicting individual wt (green arrows) and Kindlin-3–deficient T cells (pink arrows) undergoing diapedesis through HEVs of inflamed popliteal lymph nodes (blue). The time elapsed from the initial recording is indicated in each frame. T = 0 was set 80 minutes after coinjection of the labeled T cells. (D) The numbers of wt and Kindlin-3–deficient effector T cells arrested (intravascular) or emigrating (extravascular) out of inflamed popliteal lymph node vessels were determined 60 minutes and 240 minutes after coinjection at a 1:1 ratio. One experiment is representative of 3 independent experiments. (E) The numbers and fraction of sham-treated or PTx-pretreated wt (left) and Kindlin-3–deficient effector T cells (right) emigrating out of inflamed popliteal lymph node vessels determined 240 minutes after coinjection at a 1:1 ratio. Data shown are the mean ± SEM of 3 fields of view; n = 3.
To validate our observations, we compared the ability of wt and Kindlin-3–deficient effectors coinjected at a 1:1 ratio to enter and extravasate popliteal lymph nodes. As observed in inflamed inguinal lymph nodes, much higher numbers of Kindlin-3–deficient T cells entered the parenchyma of these inflamed lymph nodes (Figure 5C-D; supplemental Videos 3 and 4). Similar to inguinal lymph nodes, the extravasation rates of both wt and Kindlin-3–deficient T cells across inflamed popliteal vessels were high (Figure 5C; supplemental Video 5) and were totally abrogated by PTx pretreatment (Figure 5E; supplemental Video 6). These results collectively indicate that even in the absence of Kindlin-3 and optimal integrin adhesiveness, effector lymphocytes can successfully integrate chemotactic signals critical for diapedesis through blood vessels under physiological shear flow.
Chemokine-stimulated effector T cells deficient in Kindlin-3 exhibit impaired motility inside inflamed lymph nodes
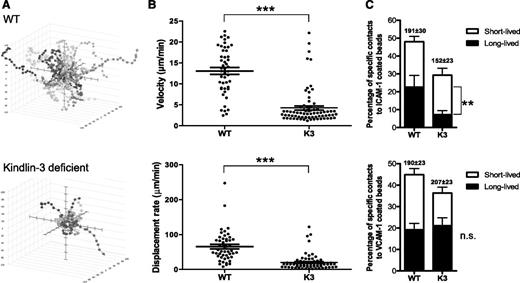
Next, we monitored the interstitial motility properties of Kindlin-3–deficient effector T cells inside popliteal lymph nodes after diapedesis. Despite the normal ability of the vast majority of Kindlin-3–deficient cells to cross the blood vessels, the interstitial motility of these effector T cells that entered the popliteal lymph nodes was significantly slower than that of their wt T-cell counterparts (Figure 6A-B; supplemental Figure 6; supplemental Videos 7 and 8). Thus, the ability to cross endothelial barriers and the ability to subsequently migrate inside the lymph nodes are distinct in their Kindlin-3 dependence.
Interstitial motility of Kindlin-3–deficient T cells inside lymph nodes is severely impaired. (A) Interstitial motility of extravasating wt and Kindlin-3–deficient T cells inside inflamed popliteal lymph nodes measured 4 hours after adoptive transfer. Cell trajectories were determined at 36-second intervals, as detailed in “Materials and methods.” Three-dimensional paths tracking T-cell movement are depicted as if emanating from a common starting point. (B) Scatterplots illustrating the mean velocities and displacement rates of the individual T-cell tracks as depicted in panel A (n = 75 for k3-null; n = 48 for wt). Both parameters were significantly (P < .0001) lower for Kindlin-3–deficient T cells (one experiment representative of 3 experiments is shown). (C) LFA-1 adhesiveness to ICAM-1– or VCAM-1–coated beads (each containing 4200 CAM sites/μm2) on wt and Kindlin-3–deficient effector T cells migrating over CXCL9, determined under shear-free conditions. The frequencies of T-cell collisions with productive short-lived (≤180 seconds) and long-lived (≥190 seconds) contacts are depicted for each group and are expressed as a percentage of all lymphocyte bead collision events. No stable contacts to ICAM-1 or VCAM-1 beads could be observed in the absence of cations. Values are for >90 cells in 5 fields of view. One experiment representative of 3 experiments is shown. The mean contact duration is depicted in parentheses. The distribution of all T-cell bead contact durations is depicted in supplemental Figure 6. Further details are provided in “Materials and methods.”
Interstitial motility of Kindlin-3–deficient T cells inside lymph nodes is severely impaired. (A) Interstitial motility of extravasating wt and Kindlin-3–deficient T cells inside inflamed popliteal lymph nodes measured 4 hours after adoptive transfer. Cell trajectories were determined at 36-second intervals, as detailed in “Materials and methods.” Three-dimensional paths tracking T-cell movement are depicted as if emanating from a common starting point. (B) Scatterplots illustrating the mean velocities and displacement rates of the individual T-cell tracks as depicted in panel A (n = 75 for k3-null; n = 48 for wt). Both parameters were significantly (P < .0001) lower for Kindlin-3–deficient T cells (one experiment representative of 3 experiments is shown). (C) LFA-1 adhesiveness to ICAM-1– or VCAM-1–coated beads (each containing 4200 CAM sites/μm2) on wt and Kindlin-3–deficient effector T cells migrating over CXCL9, determined under shear-free conditions. The frequencies of T-cell collisions with productive short-lived (≤180 seconds) and long-lived (≥190 seconds) contacts are depicted for each group and are expressed as a percentage of all lymphocyte bead collision events. No stable contacts to ICAM-1 or VCAM-1 beads could be observed in the absence of cations. Values are for >90 cells in 5 fields of view. One experiment representative of 3 experiments is shown. The mean contact duration is depicted in parentheses. The distribution of all T-cell bead contact durations is depicted in supplemental Figure 6. Further details are provided in “Materials and methods.”
Lymphocyte motility in the T-cell zone of inflamed lymph nodes involves an encounter of chemokines within a dense array of resident immune and stromal cells, including dendritic cells and fibroblastic reticular cells, which express both ICAM-1 and VCAM-1.34-38 Having confirmed high expression levels of both ligands by stromal cells lining inflamed HEVs (data not shown), we next hypothesized that the reduced interstitial motility of Kindlin-3–deficient effector T cells inside inflamed lymph nodes could result from their deficient integrin-dependent recognition of these 2 stromal ligands under the low hydrodynamic flow inside lymph nodes. Therefore, we next determined the ability of Kindlin-3–deficient T cells encountering a surface-bound chemokine to recognize bead-immobilized ICAM-1 or VCAM-1 under shear-free conditions. Indeed, a significant fraction of wt effector T cells stimulated by a surface-bound chemokine established stable contacts with both bead-bound ICAM-1 or VCAM-1 (Figure 6C). Surprisingly, whereas the fraction of Kindlin-3–deficient effector T cells that productively recognized surface-bound ICAM-1 after encounter of surface-bound chemokine was reduced (Figure 6C; supplemental Figure 7A), both the fraction of T effectors that stably bound VCAM-1 beads and the duration of these contacts were not altered by Kindlin-3 deficiency (Figure 6C; supplemental Figure 7B). Collectively, these results suggest that under shear-free conditions, chemokine-stimulated Kindlin-3–deficient effector T cells inefficiently recognize surface-bound ICAM-1 but interact normally with surface-bound VCAM-1. Thus, the reduced interstitial motility of Kindlin-3–deficient effector lymphocytes could result from their inefficient recognition of ICAM-1, rather than VCAM-1, in the extravascular space of the inflamed lymph node.
Discussion
Leukocyte diapedesis across inflamed postcapillary venules requires initial arrest, a step shown to be strictly integrin dependent.1,39 As arrested leukocytes need to scan the endothelial cells for chemotactic diapedesis cues,40 these cells must maintain high resistance to detachment by venular shear forces.41 This balance involves short-lived integrin-dependent focal adhesions.6 Indeed, genetic interference with this balance via cytoplasmic mutations that slow down integrin-ligand bond dissociation, strongly perturbs leukocyte diapedesis.42 On the other hand, the effects of integrin bond destabilization on leukocyte migration on and through endothelial barriers have been very difficult to dissect because such destabilization often results in leukocyte detachment from the original recruitment site. Two major focal adhesion adaptors implicated in all types of integrin adhesions, including leukocyte-endothelial interactions, are talin1 and Kindlin-3.8 Whereas talin1-deficient leukocytes appear indistinguishable from integrin-deficient leukocytes,43 our previous in vitro studies have suggested that Kindlin-3–deficient lymphocytes maintain some integrin adhesiveness.13 Therefore, we anticipated that these lymphocytes, even if deficient in optimal firm adhesions, might still be able to use their residual integrin adhesiveness to scan blood vessels for chemotactic signals and successfully enter tissues.
In the present study, we addressed this intriguing possibility by comparing the migratory properties of Kindlin-3–deficient effector T cells at different sites of inflammation. Although highly enriched in the circulation, Kindlin-3–deficient effector T cells failed to accumulate in and extravasate through inflamed dermal microvessels. Remarkably, however, these same T cells could readily accumulate in postcapillary venules of inflamed inguinal and popliteal lymph nodes and could successfully transmigrate through these vessels. Importantly, as evident from their high sensitivity to PTx pretreatment, which perturbs leukocyte responsiveness to all inflammatory chemokines,19 Kindlin-3–deficient effector T cells could also readily respond to chemokine signals presented to them within these lymph node vessels during their crawling and diapedesis. Therefore, our study is the first to demonstrate that effector lymphocytes can, in fact, generate significant shear-resistant integrin adhesions, even in the absence of Kindlin-3, probably because Kindlin-3–deficient effector T cells express sufficiently high levels of integrins that may engage their lymph node endothelial ligands via abundant intermediate affinity bonds, in analogy to Kindlin-3–deficient neutrophils.15 Furthermore, Kindlin-3–deficient effector cells influx better into lymph nodes because of their reduced entrapment by peripheral blood vessels. Once arrested, Kindlin-3–deficient effector T cells can also normally integrate chemokine signals presented both by the luminal and subluminal aspects of the lymph node vessels, which sequentially facilitate their spreading and promote their protrusions through the endothelial barrier and their final transendothelial migration.6,41 Therefore, our results are the first to demonstrate that leukocyte diapedesis can occur in the absence of a key cytoskeletal adaptor responsible for the acquisition of high-affinity integrin bonds.15
Effector T cells have been shown to disseminate in multiple organs including uninfected ones, consistent with their robust capacity to mediate immunosurveillance and their ability to patrol both inflamed and noninflamed tissues.44-47 Our study confirms reports indicating that Th1 and Tc1 effector cells released from primary sites of antigenic activation and proliferation can migrate not only to sites of infection and inflammation but also can reenter the inflamed lymph nodes that drain these sites.19,48,49 Our data are also consistent with the notion that these 2 key trafficking routes face competition by the numerous integrin ligands expressed constitutively on many vascular sites.48 Interestingly, whereas this entrapment was reversed by blocking of LFA-1 or α4 integrins, PTx pretreatment of these T cells did not interfere with this entrapment and did not increase the circulating pool as did integrin blocking, suggesting that global lymphocyte entrapment is a chemokine-independent, integrin-dependent process. Importantly, we show that Kindlin-3–deficient T cells that express weakly adhesive integrins may reach their target sites more effectively that wt effector T cells that express highly adhesive integrins, probably because these lymphocytes are less readily entrapped by the global pools of constitutively expressed vascular integrin ligands. Remarkably, the partially adhesive integrins expressed by Kindlin-3–deficient effector T cells, although insufficient for trafficking to inflamed skin, were sufficient to promote both the accumulation and extravasation of these effector T cells through the postcapillary venules of skin-draining inflamed lymph nodes. A possible explanation for this dichotomy is that lymph node vessels express abundant ICAM-1 and 2 as well as VCAM-1,50,51 elevate P-selectin,17 and may use the cuboidal topography of their HEVs to stabilize the weak adhesions of Kindlin-3–deficient T cells. Notably, VLA-4 of Kindlin-3–deficient effector T cells retained full ability to bind VCAM-1 under shear-free conditions and could, thus, support the interactions of these effector T cells with both endothelial and subendothelial VCAM-1. This and other partial integrin activities retained by Kindlin-3–deficient effector T cells may be sufficient for their efficient diapedesis across blood vessels within inflamed lymph nodes.
Our study highlights another intriguing outcome regarding the trafficking routes taken by effector T cells released into the circulation from their primary site of activation and proliferation: because of their expression of adhesive integrins, these T cells likely get retained inside noninflamed vascular beds45 before being gradually released to the circulation and enter inflamed organs. Although these integrins promote firm adhesion in the absence of integrin-activating chemokines,19 they also temporally trap effector lymphocytes in many vascular beds and restrict their arrival at specific sites of inflammation. The local production and efficient presentation of proper inflammatory chemokines at target vascular beds, although not essential for initial arrest of effector T cells,19,28 are critical for postarrest crawling and protrusion events that underlie their diapedesis through the inflamed endothelial barriers. Furthermore, although optimal integrin activation is benefited from the simultaneous presence of the 2 key cytoplasmic integrin coactivators, talin1 and Kindlin-3, after their arrest, Kindlin-3–deficient effector T cells can still efficiently incorporate endothelial chemokine signals for crawling and diapedesis. Therefore, our study is the first in vivo example to show that suboptimal integrin activation by its cytoplasmic partners, although insufficient for optimal leukocyte arrest, is, in fact, sufficient for leukocyte diapedesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr V. Kalchenko for assistance with 2-photon microscopy and Dr S. Schwarzbaum for editorial assistance.
R.A. is an incumbent of the Linda Jacobs Chair in Immune and Stem Cell Research, and his research is supported by the German-Israeli Foundation; the Israel Science Foundation; the US Israel Bi-national Science Foundation; the Flight Attendant Medical Research Institute Foundation; and the Minerva Foundation, Germany.
Authorship
Contribution: S.J.C. designed the models, performed most of the in vivo experiments, analyzed data, and assisted in manuscript preparation; I.G. performed and analyzed intravital microscopy experiments; S.W.F. performed all in vitro studies and assisted in data analysis and manuscript preparation; E.P. assisted in FACS staining and intravital microscopy; M.M. generated the CD4 conditional Kindlin-3 KO mice; G.S. supervised parts of the multiphoton imaging experiments; R.F. provided the Kindlin-3–deficient mice; and R.A. supervised experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronen Alon, Department of Immunology, The Weizmann Institute of Science, Rehovot, Israel, 76100; email: ronen.alon@weizmann.ac.il.
References
Author notes
I.G. and S.W.F. contributed equally to this study.