Key Points
B cells rapidly downregulate CD1d expression after EBV infection, thus abrogating iNKT cell recognition.
EBV-infected B cells induced to express CD1d elicit iNKT cell functions even in the absence of exogenous antigen.
Abstract
Individuals with X-linked lymphoproliferative disease lack invariant natural killer T (iNKT) cells and are exquisitely susceptible to Epstein-Barr virus (EBV) infection. To determine whether iNKT cells recognize or regulate EBV, resting B cells were infected with EBV in the presence or absence of iNKT cells. The depletion of iNKT cells increased both viral titers and the frequency of EBV-infected B cells. However, EBV-infected B cells rapidly lost expression of the iNKT cell receptor ligand CD1d, abrogating iNKT cell recognition. To determine whether induced CD1d expression could restore iNKT recognition in EBV-infected cells, lymphoblastoid cell lines (LCL) were treated with AM580, a synthetic retinoic acid receptor-α agonist that upregulates CD1d expression via the nuclear protein, lymphoid enhancer-binding factor 1 (LEF-1). AM580 significantly reduced LEF-1 association at the CD1d promoter region, induced CD1d expression on LCL, and restored iNKT recognition of LCL. CD1d-expressing LCL elicited interferon γ secretion and cytotoxicity by iNKT cells even in the absence of exogenous antigen, suggesting an endogenous iNKT antigen is expressed during EBV infection. These data indicate that iNKT cells may be important for early, innate control of B cell infection by EBV and that downregulation of CD1d may allow EBV to circumvent iNKT cell-mediated immune recognition.
Introduction
X-linked lymphoproliferative disease (XLP), caused by mutations of signaling lymphocyte activation molecule-associated protein (SAP), is a primary immunodeficiency syndrome marked by fulminant infectious mononucleosis, uncontrolled lymphoproliferation, and malignant B-cell lymphomas after infection with Epstein-Barr virus (EBV).1 SAP is critical for signaling in natural killer (NK) cells, CD4+ follicular helper T cells, and CD8+ cytotoxic T lymphocytes (CTL) and contributes to a broad number of lymphocyte functions including cytotoxicity, cytokine production, antibody class switching, and memory B-cell generation.2 Thus, the exquisite susceptibility of patients with XLP to EBV may be a consequence of SAP signaling defects in 1 or multiple immune cell subsets. Individuals with XLP, as well as SAP-deficient mice, have normal numbers of NK cells, B cells, and T cells but completely lack invariant NKT (iNKT) cells, suggesting this innate lymphocyte subset might have a role in the recognition and regulation of EBV-driven lymphoproliferation and transformation of B cells.3-5 This hypothesis is supported by several studies that have suggested a role for iNKT cells in controlling herpesvirus infections. First, 2 severe cases of disseminated chicken pox infection after immunization with an attenuated vaccine strain of varicella zoster herpesvirus were attributed to a selective loss of iNKT cells.6,7 Second, interleukin 2 (IL-2)–inducible T-cell kinase mutations that, similar to XLP, result in an absence of iNKT cells have been linked with EBV-associated lymphoproliferation.8 Third, individuals who lack iNKT cells as a result of deficient expression of X-linked inhibitor of apoptosis also develop an X-linked lymphoproliferative syndrome.9 Moreover, Kaposi sarcoma–associated herpesvirus and herpes simplex virus 1 both downregulate CD1d, implying that herpesviruses may have evolved mechanisms to suppress surface CD1d expression in an attempt to evade immunosurveillance by iNKT cells.10,11
NKT cells are nonconventional αβ T lymphocytes restricted by the conserved MHC class 1–related molecule CD1d, which presents exogenous and endogenous glycolipid antigens.12 Type 1, or iNKT, cells are strongly activated by the synthetic glycolipid, α-galactosylceramide (αGalCer), and express an invariant T-cell receptor α (TCRα) chain (Vα24-Jα18 in humans, Vα14-Jα18 in mice) coupled with a restricted repertoire of TCRβ chains (Vβ11 in humans and Vβ2, Vβ7, and Vβ8 in mice). iNKT cells activated by αGalCer are cytotoxic and rapidly secrete large quantities of cytokines, such as interferon γ (IFN-γ) and IL-4, that stimulate the expansion and maturation of dendritic cells (DC), NK cells, B cells, and conventional T cells.13 As a consequence, iNKT cell activation can initiate and enhance a wide range of immune responses that may include antiviral responses.14-17
The signaling pathways that regulate CD1d expression, and thereby iNKT cell activation, are incompletely understood. However, the nuclear retinoic acid receptor α (RARα) family, regulators of genes involved in lipid storage, metabolism, and transport, have been implicated in controlling CD1d expression, and RARα ligands, a class of vitamin A derivatives called retinoids, rapidly induce CD1d transcription in human DC and enhance iNKT cell activation.18 In addition, retinoids and αGalCer synergize to induce B-cell maturation and antibody production, suggesting that iNKT cells recognize lipid antigens on the B-cell surface and modulate their function in vivo.19 Recently, we have shown that CD1d levels are tightly regulated during B-cell differentiation, with expression being rapidly lost on activation in vitro.20 Notably, treatment with the RARα agonist AM580 was found to reestablish surface CD1d expression on activated B cells.20
Given that herpesviruses have varying immune evasion strategies to establish lifelong infections,16,17 we investigated the effect of EBV infection on CD1d expression and recognition by iNKT cells. By infecting B cells in vitro with EBV and incubating them with iNKT cell lines, we show that iNKT cells limit the viral transformation of resting human B cells to lymphoblastoid cell lines (LCL). However, transformation of B cells by EBV results in the complete loss of CD1d expression and the inability to activate iNKT cells even in the presence of exogenous αGalCer. These data suggest that iNKT cells have a role in the innate recognition and control of EBV on initial infection.
Methods
Tonsil samples and ethics statement
Deidentified tonsil samples were collected from patients undergoing elective surgery at British Columbia Children’s Hospital. Ethics approval was obtained from the University of British Columbia and the Children’s and Women’s Health Centre of British Columbia Review Board (H06-03256). All clinical investigations were conducted in accordance with the principles expressed in the Declaration of Helsinki. Informed consents were not required for this study because tonsil specimens were deidentified with no plan to subsequently link the specimen to an individual subject. Tonsil samples were mechanically homogenized, and cell suspensions were isolated by Ficoll-Paque (GE Healthcare) density centrifugation before cryopreservation.
EBV-transformation, LCL, and NKT cells
EBV infections and generation of LCL were carried out by resuspending tonsillar lymphocytes or peripheral blood mononuclear cells (PBMC) in supernatant from viral replication-permissive marmoset cell line B95-8 (VR-1492; ATCC)21 or a B95-8 derivative line that bears an enhanced green fluorescent protein (GFP) reporter inserted into the LMP2 gene (EBV-GFP) and transforms with equivalent efficiency to wild-type virus.22 CD1d-restricted human NKT cell lines BM2a.3, J3N.5, and M0 were maintained in culture, as described previously.23
Antibodies and flow cytometry
The following antibodies were used: anti-human CD1a (HI149), CD1b (M-T101), CD1d (CD1d42), CD3 (HIT39), CD4 (RPA-T4), CD8 (G42-8), CD19 (HIB19), HLA-DR, DP, DQ (TU39), and CD80 (BB1) (all from BD Biosciences) and anti-human HLA-A, HLA-B, and HLA-C (W6132) and CD86 (IT2.2) (eBioscience). Data were acquired using a FACSCalibur flow cytometer (BD Biosciences) and analyzed by FlowJo 8.7 (Treestar) software.
Depletion of iNKT cells
PBMC isolated from healthy donors with relatively high iNKT cell frequencies (>0.1% CD3+ T cells) were stained with phycoerythrin (PE)-conjugated αGalCer-loaded CD1d tetramer for 30 minutes on ice. PBMC were washed, and PE-labeled cells were depleted using EasySep Human PE Positive Selection kits (StemCell Technologies). Cell numbers were normalized between nondepleted and iNKT cell-depleted PBMC (5 × 106 cells/well) and cultured in 12-well plates (BD Biosciences), and EBV infection was performed as described earlier.
Transwell experiments
PBMC from healthy donors with high iNKT cell frequencies (>0.1% of CD3+ T cells) were depleted of iNKT cells, using PE-conjugated αGalCer-loaded CD1d tetramer and PE-positive selection kits (StemCell Technologies). PBMC depleted of iNKT cells were placed into 12-well plates, and iNKT cells were transferred back into transwell inserts (3 μm pore size, BD Biosciences), resuspended above the iNKT cell-depleted PBMC, and infected with EBV-GFP for 8 days.
IFN-γ neutralization and phospho-signal transducer and activator of transcription-1 detection
PBMC (5 × 106 cells/well) from healthy controls were infected for 8 days with EBV-GFP in 12-well plates in the presence or absence of iNKT cells. IFN-γ was neutralized during infection and coculture, using 20 μg/mL blocking IFN-γ antibody (clone NIB42; eBioscience) at 0, 2, 4, and 6 days. To ensure the efficacy of neutralizing IFNγ antibody, PBMC were stimulated with 500 U/mL recombinant human IFN-γ (eBioscience) alone or in the presence of 20 μg/mL blocking IFN-γ antibody (clone NIB42; eBioscience) for 15 minutes. For assessment of IFN-γ signaling, B cells were subsequently surface-labeled with CD19 antibody on ice, fixed using 2% paraformaldehyde, made permeable with 70% methanol, and stained intracellularly with PE-conjugated phospho-STAT1 monoclonal antibody (Tyr701, clone 58D6; Cell Signaling Technology) before flow cytometric analysis.
IFN-γ ELISA and chromium release assays
Tonsillar B cells were purified by magnetic bead negative selection (StemCell Technologies), and LCL were treated with 100 nM AM580 (Enzo Life Sciences) for 24 hours. Untreated and AM580-treated naive B cells or LCL were cocultured at a 1:1 ratio with iNKT cells (50 000 cells/well) in 96-well U-bottom plates for 48 hours. AM580 (100 nM), αGalCer (10 nM; Kirin Breweries), and blocking CD1d antibody (25 μg/mL, clone 42.1)24 were added to iNKT cells simultaneously. At 48 hours, IFN-γ production was measured using capture enzyme-linked immunosorbent assay (ELISA) anti–IFN-γ antibody (clone 2G1; Thermo Electron) and biotinylated anti–IFN-γ detection antibody (clone B133.5; Thermo Electron). Untreated LCL and LCL treated with AM580 (100 nM) for 72 hours were pulsed with 10 ng/mL αGalCer overnight. LCL were resuspended in 125 μCi 51Cr (PerkinElmer) for 1.5 hours, and 51Cr-labeled LCL (10 000 cells/well) were plated in triplicate to 96-well U-bottom plates. After 4 hours, supernatants were measured using a Wizard γ counter (PerkinElmer). iNKT cell-specific killing was calculated using the following equation: % specific killing = 100 × (experimental release – spontaneous release) / (maximum release – spontaneous release).
Quantitative real-time PCR and reverse-transcriptase PCR
Quantitative real-time polymerase chain reaction (PCR) was performed in triplicate on a 7500 Fast Real-Time PCR System (Applied Biosystems), using TaqMan Universal Master Mix (Applied Biosystems), according to the manufacturer’s instructions. Immediately before PCR, a 5′-FAM-labeled Taqman probe specific for the major viral matrix protein EBV-BNFR1 (5′-FAM-TCA ACG ACC TGG CGT CCC CG-BHQ1-3′) and oligonucleotide primers (EBVBN-F: 5′-CCC CTC GGT GGA CTC AAC T -3′; EBVBN-R: 5′-ATC ATC TCG GCG GTG GAA A-3′), diluted to the appropriate concentrations, were added to the Universal Master Mix. For each sample, a negative control (water) and a set of positive control standards calibrated against the first World Health Organization international standard for EBV (plasmid containing 62-base pair EBV PCR product titrated from a concentration of 6.65 × 104 c/μL to 6.65 × 101 c/μL) were performed. The threshold PCR cycle number, corresponding to the point of exponential amplification, was used to obtain quantitative values, and EBV DNA detected by PCR (copies/mL) was calculated by comparing threshold PCR cycle number values of the sample and positive control standards.
Reverse-transcriptase PCR
RNA from C1R cells,25 C1R-CD1d cells,24,25 untreated LCL, and LCL incubated for 72 hours with AM580 (100 nM) were purified using RNeasy Micro Kits (Qiagen), and cDNA was synthesized using SuperScript First-Strand (Invitrogen). The CD1d and cyclophilin A primer sequences have been previously described.26,27
Chromatin immunoprecipitation assays
LCL generated from unrelated tonsil samples were treated with or without AM580 for 4 days, fixed with 1% formaldehyde, lysed in chromatin immunoprecipitation P lysis buffer (10 mM Tris⋅HCL, 1 mM EDTA, 0.1% sodium dodecyl sulfate, 0.1% sodium deoxycholate, 1% Nonidet P40, and protease inhibitor cocktail from Roche) and sonicated (Misonix sonicator S-4000). Lysates precleared with protein A Dynabeads (Invitrogen) were incubated with anti-lymphoid enhancer-binding factor (LEF) antibody or isotype control antibody (Millipore) and protein A Dynabeads overnight at 4°C. DNA fragments bound to LEF-1 were quantified by using specific quantitative PCR primers for the CD1d promoter region (forward: CTG CTA AAG CAA GGA CTT TGA TCC; reverse: GCA GGA CAT AAG GTT GTG TCT GTG TT) and ABI 7500 Fast Real-Time PCR System (Applied Biosystems). The quantity of pull-down DNA was calculated by normalizing untreated LCL to corresponding AM580-treated LCL samples.
Statistical analysis
Data were tested for normal distribution using Stata (v12.1) Data Analysis and Statistical software (StataCorp LP). Statistical significance was calculated using paired Student t tests and Prism (GraphPad Software).
Results
iNKT cells limit EBV transformation of human B cells
To determine whether iNKT cells limit the transformation of human B cells, whole PBMC from healthy donors with a relatively high iNKT cell frequency (>0.1% CD3+ T cells) or PBMC depleted of iNKT cells by negative selection were infected with a recombinant EBV B95-8 strain (EBV-GFP), bearing a GFP reporter to facilitate detection of virus-infected cells.22 Total PBMC numbers (5 × 106 cells/well) were normalized before infection, and the frequency of CD19+ B cells was not significantly altered by iNKT cell depletion (Figure 1A-B and data not shown). After 8 days of culture, PBMC lacking iNKT cells displayed a larger expansion of EBV-infected B cells (2.4 ± 0.5-fold increase of GFP+ CD19+ cells; Figure 1C,E) and higher titers of EBV DNA relative to PBMC containing a full complement of iNKT cells (1.6 ± 0.5-fold increase; Figure 1F). To verify the fidelity of cell gating used for GFP positivity, PBMC with or without iNKT cell depletion were also infected with wild-type EBV B95-8 to account for changes in B-cell autofluorescence associated with blasting and transformation (EBV B95-8; Figure 1D). Given that the frequency of iNKT cells among total PBMC (∼0.4% of CD3+ T cells), it was striking to observe a significant increase in virally infected B cells when iNKT cells are removed. Collectively, these experiments suggest that iNKT cells may restrict the transformation of B cells by EBV.
iNKT cells limit the transformation of B cells by EBV. PBMC isolated from the blood of healthy controls were incubated in 12-well plates for 8 days with either EBV-GFP or wild-type EBV (EBV Β95-8) in the presence or absence of iNKT cells (depleted by negative selection). Representative dot plots (A) and cumulative data (B) indicating the percentage of iNKT cells (CD1d tetramer+/CD3+/CD19−/7-aminoactinomycin D−) in the peripheral T-cell population ex vivo or after iNKT cell depletion (−iNKT) (n = 7). (C-D) Fluorescence-activated cell sorter plots display CD19+7-aminoactinomycin D− PBMC. Gated numbers indicate the frequency of GFP+ and GFP− CD19+ B cells in the presence (+iNKT) or absence (−iNKT) of iNKT cells. B cells infected with wild-type EBV (EBV Β95-8) are shown as a GFP-negative control. Cumulative data indicating the relative proportion of GFP+CD19+ cells (E) and EBV DNA titer (F) (n = 7). **P < .01; ***P < .001. (G) PBMC depleted of iNKT cells were placed in 12-well plates. Isolated iNKT cells were transferred into transwell inserts and resuspended above iNKT cell-depleted PBMC. Fluorescence-activated cell sorter plots and gated numbers indicate the frequency of GFP+ and GFP– CD19+ B cells from PBMC (+iNKT), PBMC-depleted iNKT cells (−iNKT) and PBMC with iNKT cells separated by transwell inserts (+iNKT [transwell]) infected for 8 days with EBV-GFP. Dot plot shows the frequency of GFP+ B cells of 6 individual healthy controls (n = 6). ***P < .001.
iNKT cells limit the transformation of B cells by EBV. PBMC isolated from the blood of healthy controls were incubated in 12-well plates for 8 days with either EBV-GFP or wild-type EBV (EBV Β95-8) in the presence or absence of iNKT cells (depleted by negative selection). Representative dot plots (A) and cumulative data (B) indicating the percentage of iNKT cells (CD1d tetramer+/CD3+/CD19−/7-aminoactinomycin D−) in the peripheral T-cell population ex vivo or after iNKT cell depletion (−iNKT) (n = 7). (C-D) Fluorescence-activated cell sorter plots display CD19+7-aminoactinomycin D− PBMC. Gated numbers indicate the frequency of GFP+ and GFP− CD19+ B cells in the presence (+iNKT) or absence (−iNKT) of iNKT cells. B cells infected with wild-type EBV (EBV Β95-8) are shown as a GFP-negative control. Cumulative data indicating the relative proportion of GFP+CD19+ cells (E) and EBV DNA titer (F) (n = 7). **P < .01; ***P < .001. (G) PBMC depleted of iNKT cells were placed in 12-well plates. Isolated iNKT cells were transferred into transwell inserts and resuspended above iNKT cell-depleted PBMC. Fluorescence-activated cell sorter plots and gated numbers indicate the frequency of GFP+ and GFP– CD19+ B cells from PBMC (+iNKT), PBMC-depleted iNKT cells (−iNKT) and PBMC with iNKT cells separated by transwell inserts (+iNKT [transwell]) infected for 8 days with EBV-GFP. Dot plot shows the frequency of GFP+ B cells of 6 individual healthy controls (n = 6). ***P < .001.
To determine whether direct iNKT–PBMC cell contact was required for the control of EBV-infected B cells, iNKT cells were separated from iNKT cell-depleted PBMC, using transwell inserts, and infected with EBV-GFP for 8 days. The frequencies of GFP+ B cells were measured by flow cytometry and compared with PBMC that were infected with EBV-GFP in the presence (+iNKT) or complete absence (−iNKT) of iNKT cells. As expected, the frequencies of GFP+ B cells were significantly higher in the absence of iNKT cells (20.0% ± 4.4% vs 12.8% ± 2.8%; Figure 1G). However, the expansion of GFP+ B cells was similar in wells in which iNKT cells had been completely depleted, and in those where iNKT cells were present but were prevented from direct contact with infected PBMC by transwell inserts (20.0% ± 4.4% vs 23.7% ± 4.4%; Figure 1G). Next, we tested whether the addition of neutralizing IFN-γ antibody or recombinant IFN-γ to iNKT cell-sufficient and iNKT cell-depleted PBMC, respectively, could affect the expansion of EBV-infected B cells. Given that activity of antibody and cytokine were shown to positively and negatively regulate IFN-γ receptor signaling (phospho-STAT1 induction), respectively, these experiments suggested that IFN-γ alone does not limit the expansion of EBV-infected B cells (supplemental Figure 1, available on the Blood Web site). These results indicate that iNKT cells require direct contact with newly infected PBMC to regulate the expansion of EBV-infected B cells.
LCL express high levels of the costimulatory molecules CD80 and CD86 but fail to activate iNKT cells
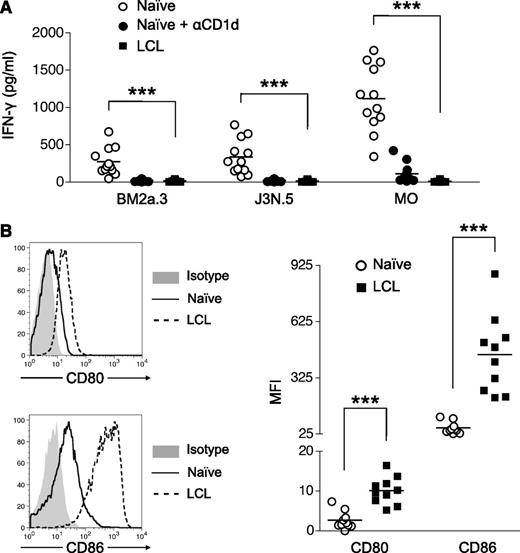
Given that iNKT cells limit expansion of EBV-infected B cells in a cell contact–dependent manner, we next assessed whether iNKT cells could directly recognize EBV-transformed B cells (LCL). Human tonsillar B cells or donor-matched LCL were cultured with 3 human iNKT cell lines, BM2a.3, J3N.5, and M0,23,28 in the presence or absence of exogenous αGalCer (Figure 2A). All iNKT cell lines incubated with resting B cells, along with αGalCer, secreted substantial amounts of IFN-γ, indicating that iNKT cells are capable of B-cell recognition on addition of exogenous antigen. However, all 3 iNKT cell lines failed to produce IFN-γ when stimulated with LCL plus αGalCer, despite the expression of higher levels of CD80 and CD86 costimulatory molecules (Figure 2B).
LCL express high levels of the costimulatory molecules CD80 and CD86 but fail to activate iNKT cells. (A) Untreated naive B cells (○) and naive B cells treated with blocking αCD1d (●) and LCL (▪) were loaded with αGalCer and cultured with human iNKT cell lines (BM2a.3, J3N.5, and M0) for 48 hours. IFN-γ secretion by iNKT cells was measured by ELISA performed in triplicate (n = 10). ***P < .001. (B) Representative CD80, CD86, and isotype control staining of naive B cells and EBV-transformed B cells (LCL). MFI values indicate the level of CD80 and CD86 expression above the isotype control on naive B cells (○) and LCL (▪) generated from unrelated tonsil samples (n = 10). ***P < .001.
LCL express high levels of the costimulatory molecules CD80 and CD86 but fail to activate iNKT cells. (A) Untreated naive B cells (○) and naive B cells treated with blocking αCD1d (●) and LCL (▪) were loaded with αGalCer and cultured with human iNKT cell lines (BM2a.3, J3N.5, and M0) for 48 hours. IFN-γ secretion by iNKT cells was measured by ELISA performed in triplicate (n = 10). ***P < .001. (B) Representative CD80, CD86, and isotype control staining of naive B cells and EBV-transformed B cells (LCL). MFI values indicate the level of CD80 and CD86 expression above the isotype control on naive B cells (○) and LCL (▪) generated from unrelated tonsil samples (n = 10). ***P < .001.
EBV transformation of human B cells results in the downregulation of CD1d
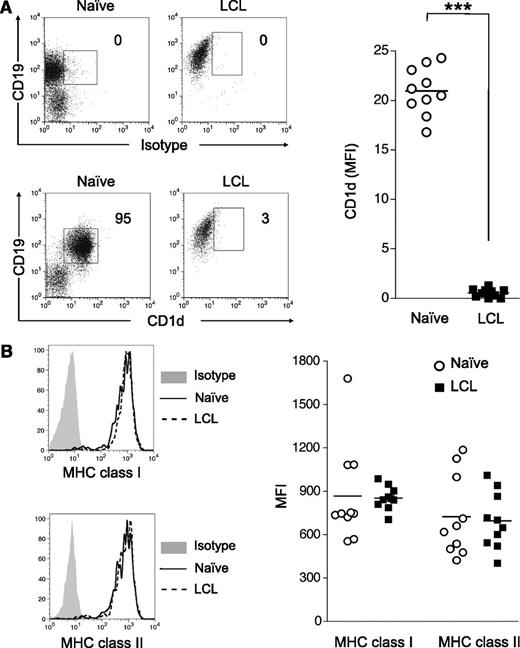
We previously demonstrated that CD1d expression is regulated by B-cell activation,20 and therefore we speculated that the inability of LCL to activate iNKT cells was a consequence of EBV transformation triggering B-cell activation pathways that shut down CD1d expression. Indeed, surface CD1d expression on LCL was negligible and dramatically lower than levels found on resting tonsillar or peripheral B cells (>50-fold decrease; mean fluorescence intensity [MFI], 29.3 ± 1.6 vs 0.55 ± 0.13; Figure 3A). Moreover, loss of CD1d expression on LCL does not appear to be result of ex vivo culture, as uninfected B cells incubated for 8 days in vitro maintain 40-fold higher expression than LCL (MFI, 21.0 ± 0.76 vs 0.55 ± 0.13). In contrast, the expression of classical antigen presentation molecules, MHC class 1 (HLA-A, HLA-B, and HLA-C) and MHC class 2 (HLA-DR, HLA--DQ, and HLA-DP), on LCL was comparable to levels seen on resting B cells directly ex vivo (Figure 3B). These observations indicate that EBV transformation is associated with a selective loss of CD1d expression and abrogation of iNKT cell recognition.
EBV transformation of B cells downregulates CD1d expression. Tonsillar mononuclear cells were incubated with or without EBV B95-8 supernatant for 8 days and analyzed for CD1d and MHC class 1 and class 2 expression. (A) Representative CD1d and isotype control staining of naive B cells and EBV-transformed B cells (LCL). MFI values indicate the level of CD1d expression above the isotype control (n = 10). ***P < .001. (B) MHC class 1 (HLA-A, HLA-B, and HLA-C) and MHC class 2 (HLA-DR, HLA-DP, and HLA-DQ) expression on naive B cells (dotted lines) and LCL (solid lines). Isotype control staining is shown as shaded regions. MFI values of MHC class 1 and MHC class 2 show net staining above the isotype controls for naive B cells (○) and LCL (■) (n = 10). ***P < .001.
EBV transformation of B cells downregulates CD1d expression. Tonsillar mononuclear cells were incubated with or without EBV B95-8 supernatant for 8 days and analyzed for CD1d and MHC class 1 and class 2 expression. (A) Representative CD1d and isotype control staining of naive B cells and EBV-transformed B cells (LCL). MFI values indicate the level of CD1d expression above the isotype control (n = 10). ***P < .001. (B) MHC class 1 (HLA-A, HLA-B, and HLA-C) and MHC class 2 (HLA-DR, HLA-DP, and HLA-DQ) expression on naive B cells (dotted lines) and LCL (solid lines). Isotype control staining is shown as shaded regions. MFI values of MHC class 1 and MHC class 2 show net staining above the isotype controls for naive B cells (○) and LCL (■) (n = 10). ***P < .001.
AM580 restores CD1d expression on LCL and inhibits association of LEF-1 to the CD1d promoter
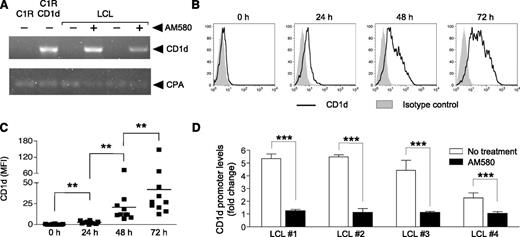
The finding that B cells extinguish CD1d expression after EBV transformation suggested that iNKT cells may be important for recognizing newly infected B cells during the brief temporal window before loss of CD1d expression in vivo. To address this hypothesis, we investigated whether iNKT cells could recognize LCL if surface CD1d expression were restored artificially using AM580, a RARα-agonist previously shown to upregulate CD1d transcription and surface expression on human DC and activated human B cells.18,20 Using parental C1R cells (HLA-A– and HLA-B–negative LCL-derived line) and CD1d-transfected (C1R-CD1d) cells24,25 as putative positive and negative controls, CD1d transcripts were only found in C1R-CD1d-transfected cells but not the parental C1R line or untreated LCL (Figure 4A). However, AM580 treatment induced readily detectable levels of CD1d message in different LCL lines at 72 hours posttreatment (Figure 4A and data not shown). Furthermore, a kinetic analysis of AM580 treatment revealed that surface CD1d expression was first observed after a 24-hour exposure and climbed steadily until reaching a maximum level at 72 hours posttreatment (Figure 4B-C). At this 72-hour point, CD1d expression was found to have increased 135-fold relative to untreated cells (MFI, 0.31 ± 0.13 vs 42.0 ± 12.8), which are levels similar to those present on resting tonsillar B cells. The ability of AM580 to induce CD1d expression on LCLs suggested the possibility that EBV transformation may interfere with RARα signaling pathways that govern CD1d expression. RARα is thought to modulate CD1d expression by altering the activity of LEF-1, a nuclear protein that associates with translocated β-catenin and binds to the distal region of the CD1d promoter.29-31 Furthermore, overexpression of LEF-1 in both K562 and Jurkat human leukemia T-cell lines silences the human CD1d promoter activity, suggesting that higher levels of LEF-1 expression in LCL may also downregulate CD1d expression.30 Given that EBV transformation of B cells resulted in a loss of CD1d that could be restored with AM580, we hypothesized that CD1d expression in LCL is silenced by the binding of LEF-1/β-catenin complex to the CD1d distal promoter on EBV infection and that AM580 treatment restores CD1d expression by inhibiting the accumulation of LEF-1 at the CD1d promoter. To test this hypothesis, chromatin immunoprecipitation analysis was performed on LCL in the presence or absence of AM580. LEF-1 was immunoprecipitated, and quantitative PCR was used to determine the levels of LEF-1 bound to the distal region of the CD1d promoter. Strikingly, there was a sharp decrease (∼4.5-fold) in the quantity of CD1d promoter segments pulled down in AM580-treated LCL compared with untreated LCL (Figure 4D). Together, these results suggest that increased LEF-1 occupancy on the CD1d promoter silences CD1d expression in EBV-transformed human B cells and that AM580 treatment promotes CD1d transcription by reducing the amount of LEF-1 bound to the CD1d promoter.
RARα agonist AM580 induces CD1d expression on LCL and inhibits LEF-1 binding to the CD1d promoter. (A) CD1d transcriptional levels measured by RT-PCR of LCL treated with (+) or without (−) AM580 for 72 hours. CD1d-transfected C1R (C1R-CD1d) and parental C1R cells shown as positive and negative controls for CD1d expression; RT-PCR loading control was cyclophilin A (CPA). (B) Representative surface CD1d (solid lines) and isotype control staining of LCL treated with AM580 for 0, 24, 48, and 72 hours. (C) MFI values (■) of CD1d indicate positive CD1d staining above the isotype control of LCL generated from unrelated individual tonsil samples (n = 10). **P < .01. (D) The specific binding of LEF-1 to the CD1d promoter in unrelated LCL (LCL 1-4) was measured by chromatin immunoprecipitation experiments performed in duplicate and is decreased compared with untreated LCL after incubation with AM580 (100 nM) for 96 hours. ***P < .001. Chromatin immunoprecipitation assays were repeated twice and completed independently at separate times, using LCL generated from 4 unrelated donors.
RARα agonist AM580 induces CD1d expression on LCL and inhibits LEF-1 binding to the CD1d promoter. (A) CD1d transcriptional levels measured by RT-PCR of LCL treated with (+) or without (−) AM580 for 72 hours. CD1d-transfected C1R (C1R-CD1d) and parental C1R cells shown as positive and negative controls for CD1d expression; RT-PCR loading control was cyclophilin A (CPA). (B) Representative surface CD1d (solid lines) and isotype control staining of LCL treated with AM580 for 0, 24, 48, and 72 hours. (C) MFI values (■) of CD1d indicate positive CD1d staining above the isotype control of LCL generated from unrelated individual tonsil samples (n = 10). **P < .01. (D) The specific binding of LEF-1 to the CD1d promoter in unrelated LCL (LCL 1-4) was measured by chromatin immunoprecipitation experiments performed in duplicate and is decreased compared with untreated LCL after incubation with AM580 (100 nM) for 96 hours. ***P < .001. Chromatin immunoprecipitation assays were repeated twice and completed independently at separate times, using LCL generated from 4 unrelated donors.
CD1d expression on LCL stimulates iNKT cells
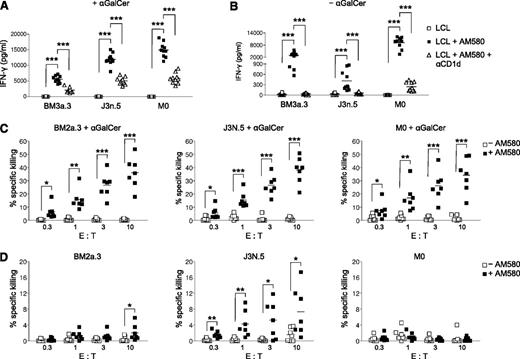
To determine whether LCL could activate iNKT cells if CD1d were present on the cell surface, LCL were pretreated with AM580 for 24 hours and cocultured with αGalCer and human iNKT cell lines for an additional 48 hours in the presence of AM580 to maximize CD1d expression (Figure 5A). In contrast to untreated LCL, AM580-treated, CD1d-expressing LCL loaded with αGalCer stimulated robust IFN-γ secretion by all 3 iNKT cell lines tested. The addition of CD1d blocking antibody suppressed IFN-γ production, demonstrating that the iNKT activation was CD1d-dependent. Remarkably, AM580-treated LCL induced strong IFN-γ responses by iNKT cells even in the absence of αGalCer (Figure 5B). In contrast, uninfected B cells treated with AM580 were not recognized by iNKT cell lines and did not produce IFN-γ (supplemental Figure 2). Next, we examined whether improved NKT cell responsiveness to AM580-treated LCL may be related to increased costimulatory molecule expression. However, we found that AM580 treatment of LCL did not alter CD80 or CD86 expression (supplemental Figure 3). Thus, iNKT cells are capable of recognizing EBV-infected, but not uninfected, B cells in the absence of exogenous antigen.
CD1d expression on LCL stimulates iNKT cell effector functions. Human iNKT cells (BM2a.3, J3N.5, and M0) were cultured for 48 hours with LCL loaded with (A) or without (B) αGalCer. LCL were pretreated for 24 hours with (■) or without (□) AM580 and blocking αCD1d antibody (△). IFN-γ secretion by iNKT cells was measured by ELISA performed in triplicate (n = 10). ***P < .001. LCL pulsed with (C) or without (D) αGalCer were pretreated for 72 hours with (■) or without (□) AM580 and cocultured with BM2a.3, J3N.5, and M0 for 6 hours at the indicated effector: target (E:T) ratios. 51Cr killing assays were performed in triplicate, using LCL generated from unrelated tonsil samples (n = 6). *P < .05; **P < .01; ***P < .001.
CD1d expression on LCL stimulates iNKT cell effector functions. Human iNKT cells (BM2a.3, J3N.5, and M0) were cultured for 48 hours with LCL loaded with (A) or without (B) αGalCer. LCL were pretreated for 24 hours with (■) or without (□) AM580 and blocking αCD1d antibody (△). IFN-γ secretion by iNKT cells was measured by ELISA performed in triplicate (n = 10). ***P < .001. LCL pulsed with (C) or without (D) αGalCer were pretreated for 72 hours with (■) or without (□) AM580 and cocultured with BM2a.3, J3N.5, and M0 for 6 hours at the indicated effector: target (E:T) ratios. 51Cr killing assays were performed in triplicate, using LCL generated from unrelated tonsil samples (n = 6). *P < .05; **P < .01; ***P < .001.
Next, we tested whether AM580-treated LCL could act as targets of iNKT cell cytotoxicity, using standard 51Cr-release assays. All 3 iNKT cell lines exhibited potent and specific killing of AM580-treated LCL that had been pulsed with αGalCer (Figure 5C). In contrast, untreated LCL failed to trigger iNKT cell cytotoxicity, despite the presence of αGalCer. Similar to secretion of IFN-γ, AM580-treated LCL incubated with effector iNKT cells in the absence of αGalCer-induced moderate cellular cytotoxicity by 1 of the 3 iNKT cell lines (J3N.5), with 2 of the iNKT cell lines (BM2a.3 and M0) showing low specific killing (Figure 5D). It is unclear why the different iNKT cell lines exhibited variable killing of AM580-treated targets, but the phenomenon may be attributed to their TCR specificities or their relative affinities for the CD1d–antigen complex. Together, these experiments indicate that CD1d-expressing LCL loaded with αGalCer are capable of eliciting iNKT cell effector functions and suggest iNKT cells may contribute to suppression of viral replication in vivo.
Discussion
Immune control of EBV has been ascribed primarily to responses by NK cells and virus-specific CTL.17 NK cells limit the early dissemination of virus through the rapid release of IFN-γ, preventing EBV transformation of oropharyngeal B cells,32 whereas the fundamental importance of EBV-specific CTL is underscored by their massive expansions during infectious mononucleosis 33 and by the benefit that infusions of virus-specific effector T cells have on EBV-associated malignancies.34 In XLP, both NK and CTL functions have been shown to be functionally impaired,35-37 and Hislop and colleagues have recently shown that the memory CTL response to EBV is defective in patients with XLP who survived primary infection.38 Conversely, Ho and associates have proposed that a subset of iNKT cells can suppress immune responses toward EBV.39 Specifically, the addition of αGalCer to PBMC cultures stimulated with EBV peptide was found to reduce the frequency of EBV-specific MHC class 1 tetramer+ CD8+ T cells.39 By testing different iNKT cell clones, they showed that a CD4− CD8αα+ iNKT clone was capable of suppressing antigen-specific T-cell proliferation through direct killing of CD1d-expressing-activated CD8 T cells or Ag-presenting cells.39
Our data indicate that iNKT cells may play an important role in controlling EBV infection by regulating viral replication during the earliest stages of B-cell infection. iNKT cells are possibly the first lymphocyte responders: they can elicit effector functions immediately on antigen exposure and mobilize rapidly into tissues.40,41 Although their in vivo role remains unclear, iNKT cells may directly recognize and regulate virus-infected cells before CD1d downregulation, as we have shown in vitro, or may provide critical early cross-talk, in the form of IFN-γ release, to NK cells,42 leading to DC maturation and activation.43 Either or both of these mechanisms would likely lead to earlier containment of infected B cells and may account for the difference between a manageable and an uncontrolled infection. As a consequence, humans lacking iNKT cells or optimal iNKT cell function may have complicated or fatal outcomes from EBV or other herpesvirus infections.8 Little is known about early, innate responses to primary EBV infection,44 partly because the long incubation period of the virus precludes early subclinical identification of patients and partly because there is no widely available small animal model of infection.45 The recent generation of humanized mouse models that possess a functional complement of iNKT cells46 will be useful in determining the role of iNKT cells in vivo and for evaluating whether specific targeting of iNKT cells may improve immune control of EBV.
B cells perform a diverse array of immunological functions including the antigen presentation of both peptides and lipids. Our previous study20 and the investigation presented here indicate that optimal CD1d expression on the B-cell surface is essential to generate iNKT cell responses, and the loss of CD1d levels on B-cell activation or B-cell transformation by EBV results in severe attenuation of iNKT cell effector functions. Recent work suggests that CD1d levels on human B cells, in particular immature CD1dhi B cells, are critical for iNKT cell homeostasis47 ; moreover, the removal of B cells from PBMC was shown to greatly impede the production of cytokines and expansion of iNKT cells when cultures were stimulated with αGalCer and IL-2.47 In addition, the reduced expression of CD1d by B cells from individuals with systemic lupus erythematosus is associated with decreased iNKT cell numbers and impaired iNKT cell functions.47 Together, the above findings, along with our own observations, emphasize the importance of crosstalk between CD1d-expressing B cells and iNKT cells for maintaining iNKT cell homeostasis and eliciting iNKT cell functions.
Retinoid-based therapies are currently used clinically to treat a wide spectrum of diseases, and ones having an activity resembling AM580 may prove valuable for targeting the immune system toward EBV-infected B cells. These include all-trans retinoic acid for promyelocytic leukemia48 and 9-cis retinoic acid for Kaposi's sarcoma.48,49 The exact mechanisms that make these drugs effective are not completely known and, in addition to their ascribed roles, may involve the action of iNKT cells. We have shown that AM580 reduces LEF-1 nuclear protein binding to the CD1d promoter and associates with upregulated expression of CD1d on LCL (Figure 4), suggesting that that LEF-1 is responsible for the suppression of CD1d levels on LCL. If so, currently licensed RARα agonists may serve as an additional tool to manipulate iNKT cell functions.
EBV may also regulate CD1d expression by increasing the cytoplasmic accumulation and nuclear translocation of β-catenin, which acts as a coactivator for LEF-1 and modulates its DNA-binding properties.29 β-Catenin, a member of the Wnt pathway, mediates major survival and proliferation programs associated with B-cell activation and cellular transformation.50 Virus-encoded latent membrane protein 2A has been shown to trigger Wnt signaling during EBV transformation,51 inducing the accumulation of cytoplasmic and nuclear β-catenin.52 Given our results and the interaction of the Wnt/β-catenin/LEF-1 pathway, we propose that CD1d is silenced on B-cell transformation by strong activation of the Wnt signaling pathway and translocation of LEF-1 to the nucleus. Thus, it may be possible to maintain or restore CD1d expression by inhibiting members of the Wnt signaling pathway, including phosphoinositide 3-kinase, the serine/threonine-specific kinase, Akt, and glycogen synthase kinase 3β.51,53 The management of EBV-associated malignancies and the development of EBV vaccines are 2 areas in which an approach of maintaining or restoring CD1d expression using RARα agonists or Wnt pathway inhibitors may prove fruitful. Alternatively, strategies aimed at augmenting CD1d expression may be combined with the boosting of iNKT responses through administration of αGalCer or other iNKT cell-agonists.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank our volunteer blood and tonsil donors and Yu-Hsuan Huang and Ashish Marwaha for their phlebotomy skills. The authors also thank Pamela Lutley for subject recruitment, Duc Nguyen (BC Children’s Hospital) for statistical assistance, Richard Longnecker (Northwestern University) for the GFP reporter EBV strain, Virginia Young (BC Children’s Hospital) for EBV DNA quantification, and the R.T. and P.v.d.E. laboratory members for helpful discussion.
The authors are members of the Canadian Institutes of Health Research (CIHR) SLED Team for Childhood Autoimmunity. R.T. is a Michael Smith Foundation Senior Scholar.
Authorship
Contribution: B.K.C., K.T., D.J.Z., J.C.N., and C.M.B. performed experiments; B.K.C., L.L.A., J.J.P., P.v.d.E., and R.T. designed the research and analyzed results; F.K.K. provided tonsil samples; M.R.H. designed the EBV PCR; B.K.C. produced the figures; and B.K.C., K.T., J.J.P., and R.T. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.K.C. is Department of Medical Genetics, University of British Columbia, Vancouver, Canada.
Correspondence: Rusung Tan, Child & Family Research Institute, British Columbia Children's Hospital, 950 West 28th Ave, Vancouver, British Columbia, Canada, V5Z 4H4; e-mail: roo@mail.ubc.ca.
References
Author notes
J.J.P. and R.T. contributed equally to this study.

![Figure 1. iNKT cells limit the transformation of B cells by EBV. PBMC isolated from the blood of healthy controls were incubated in 12-well plates for 8 days with either EBV-GFP or wild-type EBV (EBV Β95-8) in the presence or absence of iNKT cells (depleted by negative selection). Representative dot plots (A) and cumulative data (B) indicating the percentage of iNKT cells (CD1d tetramer+/CD3+/CD19−/7-aminoactinomycin D−) in the peripheral T-cell population ex vivo or after iNKT cell depletion (−iNKT) (n = 7). (C-D) Fluorescence-activated cell sorter plots display CD19+7-aminoactinomycin D− PBMC. Gated numbers indicate the frequency of GFP+ and GFP− CD19+ B cells in the presence (+iNKT) or absence (−iNKT) of iNKT cells. B cells infected with wild-type EBV (EBV Β95-8) are shown as a GFP-negative control. Cumulative data indicating the relative proportion of GFP+CD19+ cells (E) and EBV DNA titer (F) (n = 7). **P < .01; ***P < .001. (G) PBMC depleted of iNKT cells were placed in 12-well plates. Isolated iNKT cells were transferred into transwell inserts and resuspended above iNKT cell-depleted PBMC. Fluorescence-activated cell sorter plots and gated numbers indicate the frequency of GFP+ and GFP– CD19+ B cells from PBMC (+iNKT), PBMC-depleted iNKT cells (−iNKT) and PBMC with iNKT cells separated by transwell inserts (+iNKT [transwell]) infected for 8 days with EBV-GFP. Dot plot shows the frequency of GFP+ B cells of 6 individual healthy controls (n = 6). ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/15/10.1182_blood-2013-01-480665/4/m_2600f1.jpeg?Expires=1769144628&Signature=GGMu1I~9o~n8taQrRB82tBNNn2PljLGbDHA1QMLEMjStDyb5vSbtzBEYFbS-ZSNT8w0V-hi9fK43zzxwLPrGKuDzAAJebGxsfvSZV9Lp33knUvY3uIZXdyPyDRqwCjHBnFjSD1Vwv~tYgrUgzTkEz2jTQ6yfEPEhCAe9Lkbq-SG12drfjSs0ck7vbI7o5kl8xHa7qTjJ4~gYMYMctj7qVRltQWuaptYYLJMGDlCxrygFzklroC9a3RoZpecQRb198N5Ue87vlute8qlQePpQctHP0VlukmkXn10inWjvVoXSCH0~B2jwX4Zv05zy50gA3eXGpxMYpq58zUNZnVeakw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal