Abstract
The transcription factor signal transducers and activators of transcription 5 (STAT5) has an important and unique role in Breakpoint Cluster Region - Abelson 1 (BCR-ABL1)–driven neoplasias. STAT5 is an essential component in the signaling network that maintains the survival and growth of chronic myeloid leukemia (CML) cells. In contrast, the function of the prototypical upstream kinase of STAT5, the Janus kinase JAK2, in CML is still under debate. Although there is widespread agreement that JAK2 is part of the signaling network downstream of BCR-ABL1, it is unclear whether and under what circumstances JAK2 inhibitors may be beneficial for CML patients. Recent studies in murine models have cast doubt on the importance of JAK2 in CML maintenance. Nevertheless, JAK2 has been proposed to have a central role in the cytokine signaling machinery that allows the survival of CML stem cells in the presence of BCR-ABL1 tyrosine kinase inhibitors. In this review, we summarize the current debate and provide an overview of the arguments on both sides of the fence. We present recent evidence showing that CML stem cells do not depend on BCR-ABL1 kinase activity but require the continuous support of the hematopoietic niche and its distinct cytokine environment and suggest that it has the potential to resolve the dispute.
Introduction
The Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway represents one of the best-characterized signaling pathways in cell biology. JAK-STAT signaling was only discovered ∼20 years ago, and subsequent study has provided many valuable insights into the process by which extracellular information is transmitted through the cell membrane to the nucleus.1 We now know that the JAK-STAT pathway is involved in signaling downstream of >50 growth factors and cytokines, thereby participating in vital cellular functions such as proliferation, differentiation, apoptosis, survival, and migration.2,3 The mammalian family of Janus kinases is composed of 4 members, JAK1, JAK2, JAK3, and the tyrosine kinase 2 (Tyk2), all of which share a structure characterized by 7 JAK homology domains.4 JAK2 was initially shown to play a crucial part in immune cell development and hematopoiesis.5 Shortly afterward, it was found to be activated in the initiation and maintenance of cancer, but the exact mechanisms by which it contributes to pathogenesis remain obscure.6 The discovery that a single point mutation within the nonreceptor tyrosine kinase JAK2, leading to the substitution of a valine residue by phenylalanine at amino acid 617 (JAK2V617F), is responsible for driving a subset of myeloproliferative neoplasia (MPN) dramatically increased interest in JAK2.7-10 Point mutations and insertions/deletions within exon 12 of JAK2 have subsequently been identified in nearly all patients with JAK2V617F-negative polycythemia vera, as well as in some cases of acute myeloid leukemia, systemic mastocytosis, chronic myelomonocytic leukemia, and myelodysplastic syndrome.11 JAK2 has also been implicated in the formation of tyrosine kinase fusion genes in a variety of hematologic malignancies, mainly acute leukemias.12 The fusion proteins show a common mechanism of constitutive activation, in which JAK2’s 3′ kinase domain is translocated to a partner gene that confers oligomerization properties, namely BCR, PCM1, ETV6, PAX5, RPN1, or SSBP2.13 Increasing evidence of the involvement of JAK2 in various forms of leukemia has suggested that JAK2 might be an essential component of Breakpoint Cluster Region - Abelson 1 (BCR-ABL1)–driven leukemogenesis.14
The BCR-ABL1 oncogene results from the t(9;22)(q34;q11) reciprocal translocation generating the Philadelphia chromosome.15,16 BCR-ABL1+ chronic myeloid leukemia (CML) is a stem cell–derived disease that progresses in 3 distinct phases: chronic phase (CP), which may last for several years; accelerated phase (AP); and finally blast crisis (BC), which is refractory to therapy.17 CML patients have an excellent treatment option based on the small molecule inhibitor imatinib mesylate and related substances. However, these substances largely prevent expression of symptoms rather than addressing the cause of the disease. Curing CML would require the eradication of the cancer stem cell expressing BCR-ABL1. Remarkably, 6 patients initially treated with interferon (IFN)-α but subsequently switched to imatinib mesylate showed a surprisingly high rate of complete long-term remission,18 suggesting that there are unknown mechanisms of disease eradication. Support for this idea stems from studies that described patients who remained free of symptoms on tyrosine kinase inhibitor (TKI) discontinuation.19 These patients may be cured in the sense of eradication of all CML cells, but this is impossible to prove. Although eradication of all CML cells remains the ideal, operational cure could be achieved even with detectable residual disease, if the relapse risk is close to zero. Despite this glimmer of hope, a considerable number of patients are resistant to the inhibitors imatinib, nilotinib, and dasatinib, which have been approved for first-line therapy of BCR-ABL1. The appearance of the T315I gatekeeper mutation puts an end to these treatment options because it is resistant to all first- and second-generation TKIs. The recent approval of the third-generation inhibitor ponatinib by the US Food and Drug Administration offers the possibility to treat such cases, although ponatinib is also likely to suffer from limitations: its effectiveness may be constrained by the development of multi-TKI resistance or BCR-ABL1–independent resistance.20 The search for additional therapeutic targets will thus remain an important task.
The recent introduction of JAK2-specific inhibitors has coincided with the appearance of a number of excellent reviews summarizing the state-of-the-art knowledge of how JAK2V617F-mutated (and nonmutated) patients may benefit.21-24 The reports have raised the issue of whether and how the new substances may influence BCR-ABL1–targeted therapy in CML. The precise role of the JAK2-STAT5 proteins in the pathogenesis, maintenance, and progression of CML has been a matter of debate for more than a decade. In this review, we summarize the current understanding, focusing on the role of JAK2 as this protein is the object of intense discussions as a possible therapeutic option for CML patients.
STAT5: a key player with an undefined therapeutic potential
The longstanding lack of appropriate transgenic Stat5 knockout mouse models has limited experimental efforts because deletion of the Stat5 gene is associated with high perinatal lethality from anemia and lung abnormalities.25 The few surviving Stat5-deficient mice show normal levels of hematopoietic stem cells (HSCs) but exhibit severe lymphoid and moderate myeloid repopulation defects.26 Overexpression of a constitutively active STAT5 protein in total bone marrow and long-term HSCs suffices to induce CML that closely resembles a BCR-ABL1–induced disease.27,28 The expression of dominant negative STAT529-32 and RNA interference–mediated knockdown of STAT533-35 in cell lines and primary patient samples strengthened the evidence that STAT5 has an essential role in CML.
Only with the introduction of more advanced molecular methods did it become possible to recombine the Stat5 locus in adult mice using a unique conditional-null allele.25 Deletion of Stat5 is well tolerated in adult mice and has almost no effect on hematopoiesis. The availability of inducible Stat5-deleted mice enabled us to investigate the role of STAT5 in BCR-ABL1–mediated CML leukemogenesis.
A study conducted in the Van Etten laboratory describes the transplantation of bone marrow transduced with a retrovirus encoding for BCR-ABL1 into lethally irradiated recipient mice.36 This well-characterized procedure induces a CML-like leukemia that originates from stem/progenitor cells with multilineage repopulating activity and can progress to BC. Complete deletion of the Stat5 gene locus using the conditional-null allele prevented the development of myeloid or lymphoid leukemia in primary recipients,37 despite the persistence of BCR-ABL1–expressing HSCs. The self-renewal capacity of the BCR-ABL1–expressing Stat5-deficient HSCs was tested by serial transplantation. The BCR-ABL1+Stat5-null bone marrow conferred radioprotection and allowed myeloid engraftment, although all secondary recipients succumbed to fatal acute lymphoblastic leukemia. This indicated that BCR-ABL1–expressing Stat5-deficient HSCs possess the ability to self-renew and that loss of Stat5 does not prevent the outgrowth of transformed lymphoid cells.37
An independent study by Hoelbl et al38 used a slightly different technical approach. The disease was established via BCR-ABL1 transduction/transplantation before inducing deletion of Stat5 and led to a massive reduction of BCR-ABL1–expressing cells, to the point where neoplastic cells could no longer be detected and signs of disease vanished. The disease eventually reappeared, caused by the outgrowth of STAT5-expressing “escaper” clones. Secondary recipients only engrafted with Stat5 wild-type cells and failed to engraft with the Stat5-deleted population. The result may be interpreted in 2 ways. Either STAT5 is required for the engraftment and repopulation of BCR-ABL1+ leukemia in secondary recipients, in contrast to previous results, or Stat5 wild-type leukemic stem cells harbor a survival advantage and rapidly outcompete Stat5-null leukemic stem cells. Hoelbl et al38 also found no outgrowth of Stat5-deficient BCR-ABL1+ lymphoid cells: deletion of Stat5 in lymphoid BCR-ABL1+ cells was incompatible with cell viability.
There is little doubt of the requirement for STAT5 in the establishment of a CML-like leukemia. However, it is not clear whether deletion of Stat5 leads to eradication of the BCR-ABL1+ HSCs or whether the stem cells persist and allow progression to lymphoid BC. The discrepancy in the results from the 2 groups may stem from differing experimental setups (Table 1) such as susceptibilities to leukemogenesis in the mouse strains used: whereas the Sexl laboratory worked with C57BL/6J mice, the Van Etten laboratory used Balb/c animals, which are more prone to lymphoid malignancies. The debate on the potential of STAT5 inhibition to block CML stem cells and lymphoid expansion will only be settled by the development of STAT5 inhibitors and their use in human patients.
Similarities and differences in the experimental setup used to determine the in vivo effect of Stat5 deficiency in BCR-ABL1–induced leukemogenesis
| Experimental setup . | Walz et al . | Hoelbl et al . |
|---|---|---|
| Mouse strain | Balb/c | C57/B6 |
| Time point of STATS deletion | Disease induction | Established disease |
| Viral titer* | Higher | Lower |
| Number of cells injected (intravenously) | 5 × 105 | 1 × 106 |
| Induction of Mx1-Cre transgene | plpC 250 μg (4×) | pIpC 400μg (l×)/IFN-β (1000 U/mL) |
| Age of donor and recipient mice | 6 wk | 6 wk |
| 5-FU treatment dose | 150 mg/kg | 150 mg/kg |
| Retroviral vector | pMSCV-p210-IRES-eGFP | pMSCV-p210-IRES-eGFP |
| Experimental setup . | Walz et al . | Hoelbl et al . |
|---|---|---|
| Mouse strain | Balb/c | C57/B6 |
| Time point of STATS deletion | Disease induction | Established disease |
| Viral titer* | Higher | Lower |
| Number of cells injected (intravenously) | 5 × 105 | 1 × 106 |
| Induction of Mx1-Cre transgene | plpC 250 μg (4×) | pIpC 400μg (l×)/IFN-β (1000 U/mL) |
| Age of donor and recipient mice | 6 wk | 6 wk |
| 5-FU treatment dose | 150 mg/kg | 150 mg/kg |
| Retroviral vector | pMSCV-p210-IRES-eGFP | pMSCV-p210-IRES-eGFP |
IFN-β, interferon β; pIpC, polyinosinic polycytidylic acid; 5-FU, 5-fluorouracil.
V Sexl and R Van Etten, personal communication, 2010.
STAT5 in TKI resistance
Further work has identified STAT5 not only as an integral player in CML pathogenesis but also as an important modulator in the response of BCR-ABL1–expressing cells to therapy with kinase inhibitors. Whereas low levels of STAT5 protein are associated with increased sensitivity of BCR-ABL1+ cells to imatinib in vitro, enhanced STAT5 expression leads to a reduction of imatinib-induced cytotoxicity.34 These results have been confirmed in vivo: mice injected with Abelson virus (v-ABL)–transformed cells acquired resistance to imatinib treatment if STAT5 was ectopically expressed. STAT5 mRNA expression and protein levels are consistently increased in more advanced phases of CML, as well as in samples from TKI-resistant patients. The emergence of imatinib resistance is strictly dependent on the transcriptional activity of STAT5 and may be mediated by increased expression of the antiapoptotic STAT5 downstream target genes BclXL and Bcl-2, possibly building up a barrier against apoptosis and cytotoxicity.34 A recent publication described a highly significant correlation between the level of STAT5A mRNA and the occurrence of BCR-ABL1 mutations in a cohort of 50 CML patients, possibly mediated by the enforced production of reactive oxygen intermediates.39 Further support for a link between STAT5 activity and TKI response is provided by a recent phosphoprotein-profiling study that found a significant correlation between the level of phosphorylated STAT5 and the response to TKI treatment.40 It will be of interest to study whether and how STAT3 induces TKI resistance as STAT3 can compensate for STAT5 under certain circumstances, and there is preliminary evidence to implicate STAT3 in drug resistance in CML.41,42
JAK2: a promising candidate in CML therapy?
Initial evidence for the involvement of JAK signaling downstream of the Abelson oncogene dates to 1995, when Danial et al43 reported a physical interaction of v-ABL with JAK1 and JAK3. Using a temperature-sensitive mutant of v-ABL, they showed a tight correlation of JAK activity with the presence of oncogenic Abelson tyrosine kinase. One year later, BCR-ABL1 was shown to phosphorylate JAK2 constitutively in cell lines expressing p210BCR-ABL1. These observations sparked considerable interest, and it was not long before JAK kinases were being discussed as potential therapeutic targets in hematological malignancies including CML.44,45 Subsequently, activation of JAK2 was verified in several human and murine cell lines expressing distinct forms of BCR-ABL1, as well as in leukemic cells derived from CML patients.46 Imatinib treatment of CML cell lines was able to reduce JAK2 tyrosine phosphorylation, substantiating the link between BCR-ABL1 and JAK2 activity.46 Over the past years, the “signallosome” surrounding BCR-ABL1 has been discovered, and coimmunoprecipitation experiments in murine and human BCR-ABL1+ cell lines have shown JAK2 to be 1 of the components.
BCR-ABL1/JAK2 network
JAK2 interacts physically with the C terminus of BCR-ABL1, whereas the SH2 domain of BCR-ABL1 is required for the efficient phosphorylation of JAK2 on tyrosine residue Y1007, a prerequisite for JAK2 activation.46 It has been proposed that the BCR-ABL1/JAK2 complex is essential for full-blown v-myc myelocytomatosis viral oncogene homolog (c-MYC) induction downstream of BCR-ABL1 by 3 independent mechanisms. First, JAK2 increases c-MYC mRNA levels by phosphorylating v-akt murine thymoma viral oncogene homolog, thereby causing deactivation of glycogen synthase kinase-3β, a negative regulator of c-MYC expression.47 This process may involve β-catenin as glycogen synthase kinase-3β–mediated phosphorylation of β-catenin causes its degradation, which leads to the down-regulation of target genes such as cyclin D1, c-JUN, and c-MYC.48-50 Second, JAK2 activation maintains a high level of c-MYC protein by inhibiting ubiquitin/26S proteasome-dependent degradation.51 Finally, JAK2 deactivates the phosphatase protein phosphatase 2A (PP2A) (see below), preventing c-MYC’s dephosphorylation and degradation.52,53 The phosphorylation of BCR-ABL1 and JAK2 is reciprocal. Besides v-Src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (SRC) kinase family members like v-yes-1 Yamaguchi sarcoma viral related oncogene homolog (LYN),54-58 JAK2 is able to phosphorylate BCR-ABL1 on tyrosine residue 177.52 This particular residue is critical for BCR-ABL1–induced disease maintenance as it allows binding of the SH2/SH3 domain-containing growth factor receptor-bound protein 2 (GRB2) protein and the rat sarcoma (RAS)-activating nucleotide exchange factor son-of-sevenless (SOS), critical components of the pathway by which tyrosine kinases induce RAS activation.59,60 Already 20 years ago, GRB2 and SOS were shown to link BCR-ABL1 activity to mitogen-activated protein kinase signaling.61,62 GRB2 directly binds BCR-ABL1 via its SH2 domain, resulting in a BCR-ABL1-GRB2-SOS complex that activates RAS. The GRB2 SH2 domain also allows binding to other phosphorylated proteins such as the receptor tyrosine kinase Src-homology collagen protein, which induces phosphatidylinositol 3-kinase signaling, providing a link of BCR-ABL1 to this essential survival pathway and allowing GRB2-independent RAS activation.62-66 Another protein found in a complex with BCR-ABL1/JAK2 is the proto-oncogene Abelson helper integration site 1 (Ahi-1). The enforced expression of Ahi-1 in hematopoietic cells suffices to induce a leukemic phenotype in vivo and collaborates with BCR-ABL1 to drive an aggressive form of leukemia.67 Ahi-1 not only enhances BCR-ABL1–dependent transformation but also reduces the TKI response of CML stem/progenitor cells, which can be overcome by combined treatment with JAK2 inhibitors.67,68
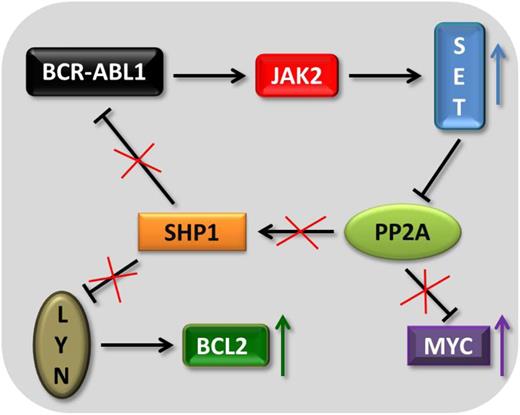
Suppression of the phosphatase PP2A has a central role in the pathogenesis of CML. PP2A activity is substantially impaired in CML-CP and barely detectable in CML-BC.69 BCR-ABL1–mediated inhibition of PP2A is crucial for the leukemic cells because PP2A, if active, would counteract and block BCR-ABL1 signaling via the downstream tyrosine phosphatase SHP1. SHP1 is capable of dephosphorylating and thus deactivating BCR-ABL1.69 The kinase BCR-ABL1 and the phosphatase PP2A share common targets; they both regulate v-akt murine thymoma viral oncogene homolog, mitogen-activated protein kinase, LYN, c-MYC, RB, STAT5, and JAK2. In BCR-ABL1+ cells, PP2A inhibition is achieved by BCR-ABL1–dependent up-regulation of the inhibitor proteins cancerous inhibitor of protein phosphatase 2A70 and SET nuclear oncogene (SET), a nuclear/cytoplasmic phospho-protein overexpressed in solid and hematological malignancies.71,72 The upregulation and activation of these proteins support CML cells to circumvent apoptosis.69,73 Accordingly, SET knockdown and PP2A-activating drugs restore PP2A activity and decrease BCR-ABL1 expression and activity, leading to apoptosis in CML cells.69 Jak2 TKI treatment and knockdown of JAK2 reduced SET protein levels, leading to the concept that JAK2 directly regulates SET.74 These studies defined PP2A deactivation as a key signaling event downstream of the BCR-ABL1-JAK2 axis. In addition, the SRC kinase LYN was identified as a JAK2 target regulated by the SET-PP2A-SHP1 pathway (Figure 1).
The BCR-ABL1-JAK2-PP2A network. The scheme depicts how the BCR-ABL1-JAK2–mediated up-regulation of the phosphatase SET helps to maintain BCR-ABL1 activity, BCL2 expression, and MYC stability.
The BCR-ABL1-JAK2-PP2A network. The scheme depicts how the BCR-ABL1-JAK2–mediated up-regulation of the phosphatase SET helps to maintain BCR-ABL1 activity, BCL2 expression, and MYC stability.
Impact of JAK2 TKIs on BCR-ABL1+ cells
The key role of JAK2 downstream of BCR-ABL1 was underlined by monitoring proliferation, apoptosis, and tumorogenicity of CML cells after treatment with JAK2 TKIs. The JAK2 inhibitor AG490 induced cell death in a dose-dependent manner in 32Dp210 and K562 cells, as well as in imatinib-resistant BCR-ABL1+ Ba/F3 cells.47,51 This finding supported the idea that JAK2 inhibitors might represent a novel way to treat imatinib-resistant CML patients.52 Colony formation of 32Dp210 cells that were imatinib sensitive or resistant to imatinib was drastically reduced on AG490 treatment. Similar results were obtained with the JAK2 inhibitor HBC90.74 Importantly, leukemic cells derived from CML patients in CP, AP, and BC underwent apoptosis on treatment with 1 of these inhibitors, irrespective of whether they were sensitive to imatinib.74 Clinical studies have only recently become possible with the availability of more specific JAK2 inhibitors (Table 2).75-86 The IC50 values of TG101209 (a precursor of TG101348 currently in clinical studies for use in the treatment of JAK2V617F+ MPN) required to induce apoptosis in imatinib-sensitive and -resistant murine and human cell lines are within the low micromolar range, approaching values that may be reached in patients.74 Moreover, CD34+ cells derived from CP and BC imatinib-resistant CML patients proved sensitive to TG101209 treatment.52 Treatment of leukemic mice with JAK2 inhibitors induced a significant therapeutic response.52 It should be noted that all cell viability studies were undertaken with JAK2 TKIs: to date, no studies have used small interfering/short hairpin RNA–mediated knockdown of JAK2.
JAK kinase specificity profile and clinical trials of distinct TKIs
| Drug name . | IC50 (nM) . | Clinical trial . | Reference . | |||
|---|---|---|---|---|---|---|
| JAK1 . | JAK2 . | JAK3 . | TYK2 . | |||
| INCB18424 (Ruxolitinib) | 3.3 | 2.8 | 428 | 19 | FDA approved for MF; phase II for CML-BC; recruiting for phase I/II for CML under nilotinib treatment | 75 |
| TQ101348 (SAR302503) | 115 | 3 | 1002 | 405 | Phase I/II in MPN | 76 |
| CYT387 | 11 | 18 | 155 | NA | Phase I/II in MPN | 77 |
| SB1518 (Pacritinib) | 1280 | 23 | 520 | 50 | Phase II in MPN | 78 |
| CEP7O1 (Lestaurtinib) | NA | 0.9 | 3 | NA | Phase II in MPN | 79 |
| LY2784544 | NA | 3 | 48 | NA | Phase I ongoing in MPN | 80 |
| NS-018 | 33 | 1 | 39 | 22 | Phase I/II ongoing in MPN | 81 |
| AZD1480 | 1.3 | 0.3 | 3.9 | NA | Phase I/II ongoing in MPN | 82 |
| BMS-911543 | 356 | 1 | 73 | 66 | Phase I/II ongoing in MPN | 83 |
| LY3009104 (Baricitinib) | 5.9 | 5.7 | 560 | 53 | Phase II ongoing in rheumatoid arthritis | 84 |
| TG101209 | NA | 6 | 169 | NA | In vitro use only | 85 |
| JAK inhibitor 1 | 15 | 1 | 5 | 1 | In vitro use only | 86 |
| Drug name . | IC50 (nM) . | Clinical trial . | Reference . | |||
|---|---|---|---|---|---|---|
| JAK1 . | JAK2 . | JAK3 . | TYK2 . | |||
| INCB18424 (Ruxolitinib) | 3.3 | 2.8 | 428 | 19 | FDA approved for MF; phase II for CML-BC; recruiting for phase I/II for CML under nilotinib treatment | 75 |
| TQ101348 (SAR302503) | 115 | 3 | 1002 | 405 | Phase I/II in MPN | 76 |
| CYT387 | 11 | 18 | 155 | NA | Phase I/II in MPN | 77 |
| SB1518 (Pacritinib) | 1280 | 23 | 520 | 50 | Phase II in MPN | 78 |
| CEP7O1 (Lestaurtinib) | NA | 0.9 | 3 | NA | Phase II in MPN | 79 |
| LY2784544 | NA | 3 | 48 | NA | Phase I ongoing in MPN | 80 |
| NS-018 | 33 | 1 | 39 | 22 | Phase I/II ongoing in MPN | 81 |
| AZD1480 | 1.3 | 0.3 | 3.9 | NA | Phase I/II ongoing in MPN | 82 |
| BMS-911543 | 356 | 1 | 73 | 66 | Phase I/II ongoing in MPN | 83 |
| LY3009104 (Baricitinib) | 5.9 | 5.7 | 560 | 53 | Phase II ongoing in rheumatoid arthritis | 84 |
| TG101209 | NA | 6 | 169 | NA | In vitro use only | 85 |
| JAK inhibitor 1 | 15 | 1 | 5 | 1 | In vitro use only | 86 |
NA, not available.
Cons: JAK2 TKIs kill Jak2-deficient cells
Interpretation of experiments with inhibitors is complicated by the fact that all of them hit >1 target,87,88 so dose-dependent effects on the off-targets need to be taken into consideration. To test the role of JAK2 in BCR-ABL1–induced leukemogenesis, we generated complete knockout and conditional Jak2 mice.89,90 Although JAK2 was essential for the initial transformation of lymphoid cells by v-ABL and p185BCR-ABL1, the initial transformation of myeloid cells by p210BCR-ABL1 was unaffected by the lack of JAK291 To investigate the role of JAK2 in the maintenance and survival of BCR-ABL1+ cells, we generated Jak2fl/fl mx1-Cre–positive cell lines. The CRE-mediated deletion of Jak2 in either lymphoid or myeloid BCR-ABL1+ cell lines had no impact on cell proliferation, cell cycle progression, or induction of apoptosis.91 In line with these in vitro findings, we observed no differences in disease latency on deletion of Jak2 in vivo.91 The experiment should be interpreted with caution: no long-term studies were performed, so we cannot exclude the possibility that JAK2 TKI inhibition provokes CML stem cell exhaustion; we cannot be certain that our transgenic mice represent a true model of the human disease or that retroviral infections faithfully mimic disease development; and the generation of gene-targeted mice might interfere with microRNAs that contribute to disease development. Despite these caveats, the results cast doubt on the significance of the proposed BCR-ABL1/JAK2 network for CML cell survival and proliferation.
All studies supporting the conclusion that JAK2 has a central role in CML cell survival relied on the use of JAK2 TKIs, whose off-target effects would provide an obvious explanation for the effects. We tested the effects of a panel of 5 distinct JAK2 TKIs (AG490,92 JAK inhibitor I, TG101209,85 TG101348,76 and INCB-01842475 ) on wild-type and Jak2-deficient BCR-ABL1+ cell lines. Three of the inhibitors (AG490, TG101209, and TG101348) induced cell death in BCR-ABL1+ cells, irrespective of whether JAK2 was expressed.91 As JAK inhibitor I in the concentration applied is a potent pan-JAK inhibitor86 and had no impact on CML cell survival, it is safe to conclude that JAKs are not involved in apoptosis induction by JAK inhibitors but that other off–targets induce cell death in BCR-ABL1–transformed cells, accounting for the discrepant observations. Remarkably, all 3 inhibitors that induced CML cell death were also able to inhibit BCR-ABL1 kinase activity. The choice of JAK2 TKI thus determines the outcome of the experiment. We consistently found that only inhibitors that target and inhibit BCR-ABL1 kinase were able to induce apoptosis in the low micromolar range (≤2 µM). JAK2 TKIs that do not target BCR-ABL1 failed to do so (JAK inhibitor I and INCB-018424).91
JAK2-independent activation of STAT5
Several investigations of the role of JAK2 in STAT5 activation in CML have reached similar conclusions: phosphorylation of tyrosine residue 694 and the resulting activation of STAT5 are independent of JAK2 in BCR-ABL1+ cells. An early study found STAT1 and STAT5 constitutively phosphorylated in BCR-ABL1–transformed cell lines but failed to detect a parallel increase in the steady-state tyrosine phosphorylation of JAK kinases, which is indicative of their activation.93 A similar mismatch between the levels of constitutive JAK2 and STAT5 tyrosine phosphorylation in BCR-ABL1–transformed Ba/F3 cells was observed by Ilaria and Van Etten; the extent of STAT5 activation was comparable to that in interleukin (IL)-3–stimulated maternal Ba/F3 cells, whereas JAK activation by BCR-ABL1 was considerably lower.94 Most importantly, the expression of dominant negative JAK2 mutants failed to interfere with the constitutive activation of STAT5 in BCR-ABL1+ cells but significantly decreased IL-3–dependent STAT5 activation.46,94 Evidence that SRC kinases are involved in the pathogenesis of BCR-ABL1–driven leukemia came from the finding of activated SRC kinases in a complex with BCR-ABL1 in myeloid cells.54,95 Although hemopoietic cell kinase (HCK) was shown to phosphorylate STAT5B on tyrosine 699 in murine 32D cells transfected with BCR-ABL1, the HCK STAT5B link could not be confirmed in human CML cell lines.91 As the SRC inhibitors PP1 and CGP76030 do not impact pSTAT5 levels in 32D cells expressing BCR-ABL1-T315I, the inhibition of pSTAT5 in wild-type BCR-ABL1–expressing cells is most likely caused by off-target effects.96 However, a role for SRC kinases in STAT5 activation cannot be entirely excluded; in murine Ph+ lymphoid leukemia, the SRC family kinases LYN, HCK, and Gardner-Rasheed feline sarcoma viral (V-Fgr) oncogene homolog are activated by p185BCR-ABL1.97 Disease latency was significantly enhanced in p185BCR-ABL1-triggered disease on deletion of ≥2 of these SRC kinases in genetically modified mice. In contrast, the induction of CML was not affected.
Our data support the concept that signaling in BCR-ABL1+ cells is “rewired” and that STAT5 activation becomes uncoupled from JAK2. The deletion of both Jak2 alleles did not affect the pSTAT5 level in BCR-ABL1–transformed cells. Moreover, treatment of BCR-ABL1–expressing Ba/F3 cells with the JAK2 TKIs INCB-018428, JAK inhibitor I, TG101209, and TG101348 (at a dosage that does not interfere with BCR-ABL1) did not alter the level of tyrosine-phosphorylated STAT5, despite abolishing IL-3– and JAK2-dependent STAT5 phosphorylation in the parental cells.91 siRNA-mediated knockdown of all 4 JAK kinases individually or in combination in Ku812 and K562 cells failed to change pSTAT5 levels. Finally, in vitro ABL kinase assays revealed a Km for STAT5 of ∼100 µM, within the range for the well-defined BCR-ABL1 target V-Crk sarcoma virus CT10 oncogene homolog (CRKL) under identical experimental conditions.91 The BCR-ABL1 target and adaptor protein CRKL physically interacts with BCR-ABL1 via its SH3 domain98 and is required for BCR-ABL1–induced STAT5 phosphorylation.99 It is attractive to speculate that BCR-ABL1 interacts with STAT5 via CRKL, although convincing experimental evidence is still lacking.
Bridging the gap: an essential role of canonical, cytokine-activated JAK2 signaling in CML?
Our data show that JAK2 is not essential for CML induced by retrovirally expressed BCR-ABL1. Nevertheless, it is possible that the canonical JAK2-STAT5 pathway is important for more primitive CML stem/progenitor cells that may rely on cytokine-activated JAK-STAT signaling in addition to BCR-ABL1 signaling. It has been postulated that “sanctuaries” such as the bone marrow provide a protective environment, thereby accounting for CML stem cell resistance to TKIs. There is a general consensus that TKIs inhibit BCR-ABL1 activity in primitive lineage CD34+CD38− cells.100,101 In contrast to progenitor cells, CML stem cells are able to survive in vitro for prolonged periods of time despite complete oncogene inactivation.101 To cure a patient, it is necessary to eliminate cells that are either not or only partially dependent on BCR-ABL1 signaling. It is conceivable that the bone marrow microenvironment contains a distinct milieu of cytokines and growth factors that allow BCR-ABL1–independent survival and thus TKI drug resistance. In the presence of cytokines, short-term BCR-ABL1 kinase inhibition with 100 nM dasatinib fails to reduce CD34+-dependent colony formation. Remarkably, the inhibition of JAK activity using JAK inhibitor I re-established the sensitivity of CML progenitors to BCR-ABL1 inhibition despite the presence of cytokines.102 Similarly, K562 cells become resistant to BCR-ABL1 TKIs when cultured in bone marrow stroma–derived conditioned medium (CM), highlighting the importance of the microenvironment.41 Stroma-induced drug resistance correlates with increased pSTAT3 levels.41 Reducing the expression of JAK2 and TYK2 by siRNA or inhibiting JAK kinase activity by INCB-018424 blocked CM-mediated STAT3 activation and sensitized CML cells to nilotinib-mediated cell death.42 Accordingly, the combined treatment of Lin−34+ patient-derived cells with INCB-018242 and nilotinib potentiated cell death and apoptosis when cocultured with bone marrow stromal cells.42
A recent study highlights the influence of the microenvironment for the survival of CML cells.103 High concentrations of the JAK2-activating factors IL-6, granulocyte–colony-stimulating factor, and granulocyte-macrophage–colony-stimulating factor (GM-CSF) are present in CM and enhance survival of CML CD34+ cells under imatinib treatment. Only in the presence of CM does the combination of imatinib and the JAK2 inhibitor TG101209 or CYT387 increase apoptosis.103 This observation unequivocally links the CM-protective effect to JAK2 signaling and provides a rationale for combining imatinib and JAK2 TKIs in patient treatment. In a murine CML model, JAK2 inhibition with high doses of TG101209 (200 mg/kg per day) only moderately prolonged survival, suggesting that monotherapy merely delays disease progression. In contrast, combined treatment with nilotinib and high doses of TG101209 was more effective against BCR-ABL1+ cells than nilotinib alone, although the beneficial effect was nullified by toxic effects to nonleukemic cells.103 As combination treatments with low-dose TG101209 (75 mg/kg per day) did not demonstrate any advantage compared with treatment with nilotinib alone, the window for combining JAK2 and ABL inhibitor therapy is probably very narrow.103
The microenvironment is not the only source of cytokines, and autocrine production of IL-3 and granulocyte–colony-stimulating factor by CD34+ CML cells has been reported. Both cytokines activate STAT5 in a JAK2-dependent manner, which provides an additional rationale for JAK2 TKI treatment in clinical settings.104 Secretion of GM-CSF has also been shown in CD34+ cells derived from imatinib-resistant patients: GM-CSF induces BCR-ABL1–independent activation of JAK2-STAT5 and thus counteracts TKI-based apoptosis. Cotreatment of the cells with a JAK2 inhibitor restores imatinib response.105
In summary, the 2 opposing opinions may not be as contradictory as they initially appear. There is convincing genetic evidence that JAK2 is not absolutely required for the maintenance of BCR-ABL1+ leukemia; BCR-ABL1 itself appears capable to circumvent the requirement for JAK2 by directly activating the critical downstream transcription factor STAT5 (Figure 2). Nevertheless, it is possible that JAK2 inhibitors may be valuable in the treatment of BCR-ABL1–driven diseases, particularly those involving leukemic stem cells where BCR-ABL1 appears to have a subordinate role and JAK2 could be critical for cell survival (Figure 3). We are currently unable to predict the effects of JAK2 inhibition on stem and progenitor cells, although our limited information suggests a critical role for JAK2-dependent signaling in both cellular compartments.106 Identifying the JAK2 vulnerabilities in stem/progenitor cells and defining the potential therapeutic window remains a major challenge. Further studies are required to understand the combined effects of BCR-ABL1 and JAK2 TKIs in mice and humans. More detailed answers will only result from transplantation of bone marrow samples from treated individuals into NOD-scid IL2rγnull recipients. Two clinical trials are currently planned to recruit CML patients to test the efficacy of the JAK1/2 inhibitior ruxolitinib (INCB-018424) in combination with approved BCR-ABL1 TKIs (http://www.clinicaltrials.gov/ct2/results?term=ruxolitinib&pg=1). It is hoped that the results of the trials will show conclusively whether inhibition of JAK1/2 can benefit CML patients.
CML progenitor cell treatment with BCR-ABL1 TKIs leads to an abrogation of STAT5 signaling essential for survival and proliferation of the cell.
CML progenitor cell treatment with BCR-ABL1 TKIs leads to an abrogation of STAT5 signaling essential for survival and proliferation of the cell.
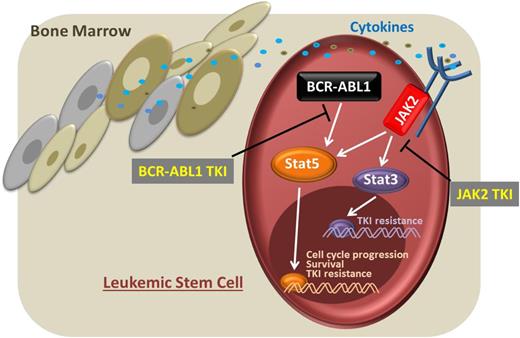
Leukemic stem cell. The presence of a cytokine-enriched microenvironment leads to a BCR-ABL1–independent activation of STAT3 and STAT5 via JAK2. Targeting both pathways via BCR-ABL1 and JAK2 TKIs would interfere with these essential survival signals.
Leukemic stem cell. The presence of a cytokine-enriched microenvironment leads to a BCR-ABL1–independent activation of STAT3 and STAT5 via JAK2. Targeting both pathways via BCR-ABL1 and JAK2 TKIs would interfere with these essential survival signals.
Acknowledgments
The authors thank Graham Tebb for scientific discussions and critical reading of the manuscript.
Work in the laboratory of V.S. was supported by the Austrian Science Fund (FWF).
Authorship
Contribution: W.W., C.W., and V.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for W.W. is Cambridge Institute for Medical Research and Department of Haematology, University of Cambridge, Cambridge, United Kingdom.
Correspondence: Veronika Sexl, Veterinaerplatz 1, A-1210 Vienna, Austria; e-mail: veronika.sexl@vetmeduni.ac.at.