Key Points
Expression and function of vitamin A metabolizing enzymes are increased in the intestine and mesenteric lymph nodes during GVHD.
Inhibiting donor T-cell RAR signaling reduces Th1 differentiation, gut homing, and GVHD while preserving graft-versus-lymphoma effects.
Abstract
Graft-versus-host disease (GVHD) is a critical complication after allogeneic bone marrow transplantation. During GVHD, donor T cells are activated by host antigen-presenting cells and differentiate into T-effector cells (Teffs) that migrate to GVHD target organs. However, local environmental factors influencing Teff differentiation and migration are largely unknown. Vitamin A metabolism within the intestine produces retinoic acid, which contributes to intestinal homeostasis and tolerance induction. Here, we show that the expression and function of vitamin A–metabolizing enzymes were increased in the intestine and mesenteric lymph nodes in mice with active GVHD. Moreover, transgenic donor T cells expressing a retinoic acid receptor (RAR) response element luciferase reporter responded to increased vitamin A metabolites in GVHD-affected organs. Increasing RAR signaling accelerated GVHD lethality, whereas donor T cells expressing a dominant-negative RARα (dnRARα) showed markedly diminished lethality. The dnRARα transgenic T cells showed reduced Th1 differentiation and α4β7 and CCR9 expression associated with poor intestinal migration, low GVHD pathology, and reduced intestinal permeability, primarily via CD4+ T cells. The inhibition of RAR signaling augmented donor-induced Treg generation and expansion in vivo, while preserving graft-versus-leukemia effects. Together, these results suggested that reagents blunting donor T-cell RAR signaling may possess therapeutic anti-GVHD properties.
Introduction
Graft-versus-host disease (GVHD) accounts for substantial morbidity and mortality after allogeneic bone marrow transplantation (BMT).1 Injury sustained from a conditioning regimen can create a proinflammatory environment that recruits donor T effectors (Teff), resulting in gut injury and subsequent GVHD morbidity.2-4 Neutralization of proinflammatory cytokines reduces, but does not eliminate, gut injury,5 indicating the importance of alternative pathways.
Retinoic acid (RA) regulates intestinal immune homeostasis, including tolerance. Intestinal cells produce RA,6-9 which can enhance Teff differentiation, support inducible Treg (iTreg) generation, and influence Teff and iTreg expansion, gut homing, and stability.10-12 Retinoic acid receptors (RARs) bind to 1 of the 3 isoforms of retinoid X receptors (RXRs).13 The resulting RAR-RXR heterodimers interact with retinoic acid-response elements (RAREs) within the promoter regions of RA-inducible genes and activate transcription factors after agonist binds to the heterodimer’s RAR moiety.14
Dependent upon the GVHD model, contrasting effects for RA have been seen. For example, the RA analog Am80 shows a chronic GVHD inhibitory effect and a vitamin A–deficient diet reduces gut acute GVHD incidence.15,16 Here we demonstrate that exogenous RA supplementation during early post-BMT correlates with increased acute GVHD severity. Conversely, transgenic expression of dominant-negative RARα (dnRARα) in donor T cells, which inhibits RA signaling, ameliorates GVHD by reducing Th1 differentiation, thereby inducing Tregs and reducing Teff gut homing, while preserving the graft-versus-lymphoma (GVL) effect. Additionally, we provide data as to the source of vitamin A metabolizing enzyme production and T cell sensing of RA during GVHD.
Materials and methods
Our data regarding experimental mice, BMT, histology, and immunohistochemistry of GVHD tissues, cell isolation, cell culture, flow cytometry, and carboxyfluorescein diacetate succinimidyl ester assays, assessment of GVL activity, bioluminescent imaging (BLI) studies, vitamin A metabolism quantification, and fluorescein isothiocyanate (FITC)-dextran assays are detailed in the supplemental Data, available on the Blood Web site. Institutional Animal Care and Use Committee study 1205A14681 was approved July 10, 2012.
Statistical analysis
The Kaplan-Meier product-limit method was used to calculate survival. Differences between groups were determined using log-rank statistical analysis. Group comparisons were made using Student t test or 1-way analysis of variance with a Tukey’s multiple comparison test. A P value of ≤ .05 was considered statistically significant.
Results
Vitamin A metabolism is upregulated during GVHD
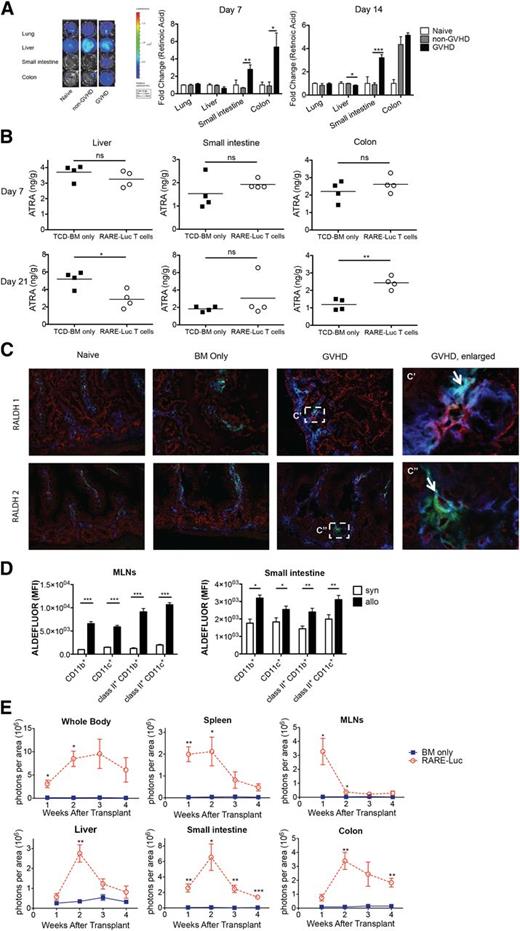
RA levels were quantified in GVHD-affected organs using a B16 tumor line modified to express RAREluc (B16-DR5 assay) to assess RA signaling as a surrogate assay for RA production.17 A difference of 30 pM can be detected by BLI (supplemental Figure 1A). For in vivo RA quantification, lethally irradiated B10.BR mice were administered B6 T-cell-depleted (TCD) bone marrow (BM) with/without splenocytes (15 × 106) to induce GVHD. Lung, liver, small intestine, and colon samples were analyzed on post-BMT days 7 and 14 (Figure 1A; supplemental Figure 1B). RARE-luc signaling induced by small-intestine tissue extracts obtained from GVHD mice was significantly higher on days 7 and 14 than in non-GVHD mice and naive controls. Colon extracts isolated on day 7 from GVHD mice compared with non-BMT or non-GVHD mice displayed higher RARE-luc signaling. GVHD mice had the same or higher RARE signaling compared with naive mice and non-GVHD mice on day 14. In contrast, RARE-luc signaling in day 14 liver extracts was significantly lower in GVHD mice than in non-GVHD mice (Figure 1A, P < .02).
Vitamin A metabolism is upregulated during acute GVHD. (A) Lethally irradiated B10.BR recipients were injected with 107 T-cell–depleted (TCD) BM cells and 1.5 × 107 splenocytes from fully MHC-mismatched B6 mice. On days 7 and 14 after BMT, tissues from lung, liver, small intestine, and colon were harvested and analyzed using a B16-DR5 assay (n = 4/group). The fold change was calculated by dividing the value of the non-GVHD group or GVHD group by the value of naive mouse group. (B) Lethally irradiated B10.BR recipients injected with 107 wt B6 TCD-BM cells and 3 × 106 purified RARE-luc B6 T cells. On days 7 and 21 after BMT, tissues from liver, small intestine, and colon were harvested and analyzed using a LC-(MS)-MS assay (n = 4/group). (C) Lethally irradiated B10.BR recipients were injected with 107 BM cells and 1.5 × 107 B6 splenocytes from wt B6 mice. Recipient mice (n = 4/group) were sacrificed on day 14 along with 4 naive B10.BR control mice, and sections from frozen tissue blocks were analyzed for expression of CD11c+ (green), CD45+ (blue), and either retinaldehyde dehydrogenase 1 (RALDH1) (red) or RALDH2 (red). The boxed area in (C) is indicated at higher magnification in (C′, C″). Arrows points out triple labeling (CD11c/CD45/RALDH1 or CD11c/CD45/RALDH2). Data shown are representative of 4 mice/group. Fluorescence was detected using an Olympus FluoView 1000 BX2 Upright confocal laser scanning microscope. (Original magnification ×400.) (D) Lethally irradiated B6-Ly5.2/Cr or B10.BR recipients were injected with 107 BM cells and 1.5 × 107 splenocytes from fully MHC-mismatched B6 mice. Lamina propria lymphocytes from the small intestine and lymphocytes from the MLNs were isolated on day 14 and donor (CD45.1− or H2kb+) CD11c+ and CD11b+ cells were evaluated for ALDH enzymatic activity by measuring the Aldefluor mean fluorescence intensity (MFI) (n = 4/group). (E) Lethally irradiated B6 or B10.BR recipients were transplanted with 107 BM cells and 3 × 106 purified RARE-luc B6 T cells. RARE-luc T cells were quantified by emitted photons over the total body area and within individual organs at serial time points after BMT (n = 3-4/group). *P > .05; **P > .01; and ***P > .001.
Vitamin A metabolism is upregulated during acute GVHD. (A) Lethally irradiated B10.BR recipients were injected with 107 T-cell–depleted (TCD) BM cells and 1.5 × 107 splenocytes from fully MHC-mismatched B6 mice. On days 7 and 14 after BMT, tissues from lung, liver, small intestine, and colon were harvested and analyzed using a B16-DR5 assay (n = 4/group). The fold change was calculated by dividing the value of the non-GVHD group or GVHD group by the value of naive mouse group. (B) Lethally irradiated B10.BR recipients injected with 107 wt B6 TCD-BM cells and 3 × 106 purified RARE-luc B6 T cells. On days 7 and 21 after BMT, tissues from liver, small intestine, and colon were harvested and analyzed using a LC-(MS)-MS assay (n = 4/group). (C) Lethally irradiated B10.BR recipients were injected with 107 BM cells and 1.5 × 107 B6 splenocytes from wt B6 mice. Recipient mice (n = 4/group) were sacrificed on day 14 along with 4 naive B10.BR control mice, and sections from frozen tissue blocks were analyzed for expression of CD11c+ (green), CD45+ (blue), and either retinaldehyde dehydrogenase 1 (RALDH1) (red) or RALDH2 (red). The boxed area in (C) is indicated at higher magnification in (C′, C″). Arrows points out triple labeling (CD11c/CD45/RALDH1 or CD11c/CD45/RALDH2). Data shown are representative of 4 mice/group. Fluorescence was detected using an Olympus FluoView 1000 BX2 Upright confocal laser scanning microscope. (Original magnification ×400.) (D) Lethally irradiated B6-Ly5.2/Cr or B10.BR recipients were injected with 107 BM cells and 1.5 × 107 splenocytes from fully MHC-mismatched B6 mice. Lamina propria lymphocytes from the small intestine and lymphocytes from the MLNs were isolated on day 14 and donor (CD45.1− or H2kb+) CD11c+ and CD11b+ cells were evaluated for ALDH enzymatic activity by measuring the Aldefluor mean fluorescence intensity (MFI) (n = 4/group). (E) Lethally irradiated B6 or B10.BR recipients were transplanted with 107 BM cells and 3 × 106 purified RARE-luc B6 T cells. RARE-luc T cells were quantified by emitted photons over the total body area and within individual organs at serial time points after BMT (n = 3-4/group). *P > .05; **P > .01; and ***P > .001.
Vitamin A metabolites (all-trans-retinoic acid [ATRA]; 13-cis-RA, not shown), capable of signaling via RAR-RXR heterodimer interactions with RAREs, were quantified by liquid chromatography/mass spectrometry LC-(MS)-MS in GVHD-affected organs 1 and 3 weeks post-BMT. In the liver, ATRA levels were at normal levels on day 7 and significantly reduced by day 21 (Figure 1B, n = 4/group), consistent with B16-DR5 assay results on days 7 and 14. Although small-intestine ATRA levels were not augmented at either time-point, a minority (25%) of GVHD mice showed >3.5-fold higher ATRA levels on day 14 post-BMT and significantly higher day 7 post-BMT 13-cis-RA levels than non-GVHD mice (data not shown); the latter was consistent with the B16-DR5 assay results. Colonic ATRA levels also were significantly higher in GVHD mice than in non-GVHD mice by 3 weeks post-BMT. These findings concur with recent study results.18
Vitamin A is metabolized by alcohol dehydrogenases into retinal and irreversibly converted to RA by selectively expressed retinaldehyde dehydrogenases (RALDHs) in dendritic cells (DCs) and stromal cells in both Peyer’s patches and mesenteric lymph nodes (MLNs).7-9,19,20 To determine whether expression and functional activities of vitamin A–metabolizing enzymes were influenced by GVHD, we first analyzed RALDH1 and RALDH2 expression in the small intestine by immunohistochemical analysis of samples on day 14 post-BMT. Figure 1C and supplemental Figure 1C show both GVHD-induced RALDH1 and RALDH2 expression in both nonhematopoietic (epithelial/stromal) and hematopoietic cells. Instead, there was no difference in RALDH1 or RALDH2 expression in the liver in all groups (supplemental Figure 1D). Furthermore, both host and donor cells showed both RALDH1 and RALDH2 activity (supplemental Figure 1E). Aldehyde dehydrogenase (ALDH) enzymatic activity was determined by ALDEFLUOR staining.21 At day 14 post-BMT, up to 99% of hematopoietic cells in the MLNs and 91% cells of hematopoietic cells in the small intestine were donor-derived (data not shown). The mean fluorescence intensity of donor DCs and macrophages from MLNs and lamina propria lymphocytes (LPLs) was significantly greater in GVHD mice than in non-GVHD mice on day 14 post-BMT (Figure 1D; supplemental Figure 2), indicative of functional vitamin A–metabolizing capacity.
To determine GVHD’s influence on donor T-cell RA signaling, we employed a novel B6 transgenic strain in which RARE was positioned upstream of a luc reporter.22 Lethally irradiated allogeneic B10.BR mice were administered B6 BM with or without 3 × 106 B6 RARE-luc transgenic T cells. Sequential BLI was performed weekly. Total-body BLI signals increased in GVHD mice between weeks 1 and 3 (Figure 1E). By week 4, the signals modestly decreased, suggesting that alloreactive T cells either destroyed or inhibited the host-cell RA-producing capacity. In support of this hypothesis, donor T-cell RAR signaling in MLNs peaked at week 1 and rapidly declined afterward (Figure 1E). Signaling of colonic donor RARE-luc T cells markedly increased between weeks 1 and 2, consistent with increased donor T-cell infiltration and/or increased donor T-cell RARE-luc signaling on a per-cell basis. To determine whether RARE-luc signaling was upregulated because of alloreactivity, we performed a study using syngeneic B6 or allogeneic B10.BR mice as recipients. A similar pattern of high RARE-luc donor T-cell signaling was observed in allogeneic B10.BR mice, but not in syngeneic B6 mice (supplemental Figure 3A). Furthermore, we evaluated photons emitted per T cell in the small intestine and colon (supplemental Figure 3B). T-cell numbers in the small intestine and colon in allogeneic mice decreased between weeks 2 and 3. Photons emitted per T cell in the small intestine did not decrease and were higher in the allogeneic than in the syngeneic BMT group, except for the colon at week 3. These results suggested that photons in the small intestine decreased as a result of lower T-cell number and the vitamin A metabolite sensing in allogeneic T cells was more active than in syngeneic T cells, indicating that allogeneic BMT influences vitamin A metabolism.
High RA levels lead to GVHD acceleration
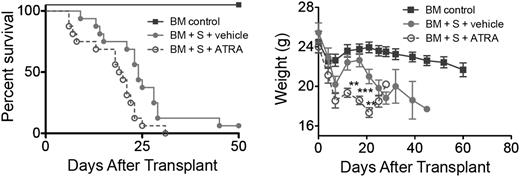
To determine whether heightened vitamin A signaling could augment acute GVHD, B10.BR recipients were administered BM with/without 5 × 106 splenocytes from B6 mice that were administered ATRA (200 μg/mouse intraperitoneally) daily from day 0 to day 28. Recipients administered ATRA showed a significant increase (P = .017) in GVHD lethality and accelerated weight loss compared with recipients administered a vehicle (Figure 2). A similar reduction in survival rates (P = .013) and weight loss was observed with administration of 15 × 106 splenocytes (supplemental Figure 4). These findings indicated that heightened vitamin A levels enhanced GVHD lethality.
Increased RA levels and RAR signaling exacerbates GVHD. Survival and weight curves of lethally irradiated B10.BR recipients injected with 107 BM cells and 5 × 106 splenocytes from B6 mice are shown (n = 16/group). Subgroups were treated with vehicle (filled circles) or ATRA (open circles); P = .017. Data are combined from 2 experiments with similar results. **P > .01; ***P > .001.
Increased RA levels and RAR signaling exacerbates GVHD. Survival and weight curves of lethally irradiated B10.BR recipients injected with 107 BM cells and 5 × 106 splenocytes from B6 mice are shown (n = 16/group). Subgroups were treated with vehicle (filled circles) or ATRA (open circles); P = .017. Data are combined from 2 experiments with similar results. **P > .01; ***P > .001.
Inhibition of RAR signaling in donor T cells prevents GVHD lethality and target-organ injury
Considering GVHD acceleration in ATRA-treated mice, we hypothesized that blocking RAR signaling in donor T cells would reduce GVHD incidence. Because RARα expression by activated CD4+ and CD8+ T cells has been confirmed by reverse transcriptase–polymerase chain reaction,23,24 we investigated whether RARs have unique functions in a murine GVHD model. To circumvent the severe phenotype of RAR-null mutant mice, donor mice expressing dnRAR were used to restrict RAR signaling deficiency to T cells.25
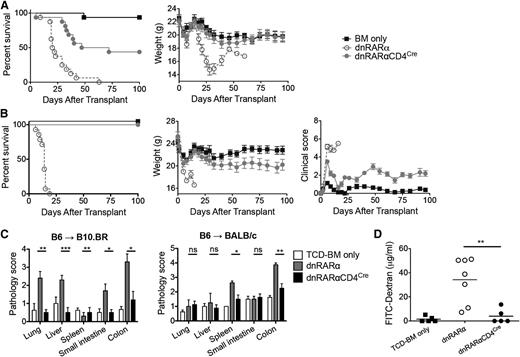
Lethally irradiated B10.BR mice were administered B6 BM with or without 10 × 106 splenocytes from dnRARα-CD4Cre, dnRARα (no CD4Cre, RAR signaling intact), or wild-type (wt) mice. Whereas all dnRARα and wt controls died of GVHD, 44% mice administered dnRARα-CD4Cre survived >100 post-BMT (P < .001), with mean weight curves similar to BM-only controls (Figure 3A; supplemental Figure 5A). To determine whether the impact of vitamin A signaling in GVHD was strain-dependent, BALB/c recipients were administered dnRARα-CD4Cre, dnRARα, or wt splenocytes. Although recipients administered dnRARα or wt splenocytes experienced rapid lethality and mean weight loss, no dnRARα-CD4Cre recipient died by d100 (P < .001), and mean weight curves were comparable to BM-only controls for the first month post-BMT (Figure 3B; supplemental Figure 5B). Surviving recipients of dnRARα-CD4Cre splenocytes showed significantly lower clinical GVHD scores than BM controls, which was confirmed by histopathological analysis of lung, spleen, liver, small intestine, and colon tissues from B10.BR recipients of B6 cells and the spleen and colon tissues from BALB/c mouse recipients of B6 cells on day 21 (Figure 3C). Using the FITC-dextran assay to measure intestinal epithelial cell barrier function, recipients of dnRARα-CD4Cre splenocytes had reduced intestinal barrier function on day 14 post-BMT compared with recipients of dnRARα splenocytes (Figure 3D; n = 5 to 7/group; P = .007).
Inhibiting RAR signaling in donor T cells prevents GVHD lethality. (A) Survival and weight curves of lethally irradiated B10.BR recipients of 107 BM cells and 107 splenocytes from B6 mice. Recipients of splenocytes from dnRARα-CD4Cre mice (filled circles) survived significantly longer than recipients of dnRARα splenocytes (open circles; P < .001; n = 16/group). (B) Survival, weight, and clinical GVHD scores curves of lethally irradiated BALB/c recipients of 107 BM cells and 5 × 106 splenocytes from B6 mice. Recipients of splenocytes from dnRARα-CD4Cre mice (filled circles) survived significantly longer than recipients of dnRARα splenocytes (open circles) (P < .001; n = 13-14/group). (C) Tissues (lung, liver, spleen, small intestine, and colon) from B10.BR recipients or BALB/c recipients were harvested on day 21 posttransplant, stained with hematoxylin and eosin, and scored for GVHD (means ± standard error). (D) FITC-dextran was orally administered to BALB/c recipients on day 21. Serum FITC-dextran levels were measured 4 hours later. Mice received wt BM cells unless otherwise indicated (P = .007). Data are combined from 2 experiments with similar results or 1 experiment each with 4 to 5 (C) or 5 to 7 (D) mice per group. (C-D) *P > .05; **P > .01; and ***P > .001.
Inhibiting RAR signaling in donor T cells prevents GVHD lethality. (A) Survival and weight curves of lethally irradiated B10.BR recipients of 107 BM cells and 107 splenocytes from B6 mice. Recipients of splenocytes from dnRARα-CD4Cre mice (filled circles) survived significantly longer than recipients of dnRARα splenocytes (open circles; P < .001; n = 16/group). (B) Survival, weight, and clinical GVHD scores curves of lethally irradiated BALB/c recipients of 107 BM cells and 5 × 106 splenocytes from B6 mice. Recipients of splenocytes from dnRARα-CD4Cre mice (filled circles) survived significantly longer than recipients of dnRARα splenocytes (open circles) (P < .001; n = 13-14/group). (C) Tissues (lung, liver, spleen, small intestine, and colon) from B10.BR recipients or BALB/c recipients were harvested on day 21 posttransplant, stained with hematoxylin and eosin, and scored for GVHD (means ± standard error). (D) FITC-dextran was orally administered to BALB/c recipients on day 21. Serum FITC-dextran levels were measured 4 hours later. Mice received wt BM cells unless otherwise indicated (P = .007). Data are combined from 2 experiments with similar results or 1 experiment each with 4 to 5 (C) or 5 to 7 (D) mice per group. (C-D) *P > .05; **P > .01; and ***P > .001.
Because RAR signaling affects CD4+ and CD8+ T-cell function,24,26 we aimed to determine whether either CD4+ and/or CD8+ T cells ameliorated the severity of GVHD. To test this hypothesis, B6 into BALB/c and B6 into bm1 GVHD models were used as CD4- and CD8-dependent models, respectively.27 Weight curves and mortality rates in BALB/c mice administered dnRARα-CD4Cre CD4+ T cells improved compared with dnRARα controls (supplemental Figure 5C). In contrast, mean weight curves in dnRARα-CD4Cre CD8+ T cells showed no beneficial effects compared with dnRARα controls (supplemental Figure 5D). Neither group demonstrated mortality (not shown). These results indicated that RAR signaling in donor CD4+ T cells impacted GVHD-related mortality and morbidity.
Gut-homing receptor expression and Th1 phenotype are modulated by inhibition of donor T-cell RAR signaling
To elucidate the contributions of RAR signaling inhibition on donor T-cell proliferation in the development of GVHD, day 4 post-BMT T-cell expansion and apoptosis were evaluated in BALB/c recipients using carboxyfluorescein diacetate succinimidyl ester labeling. dnRARα-CD4Cre compared with dnRARα, donor CD4+, and CD8+ T cells did not exhibit reduced early post-BMT proliferation (supplemental Figure 6A-C). Both divided and nondivided CD4+ splenocytes in the dnRARα-CD4Cre group were significantly less apoptotic than in the dnRARα group (supplemental Figure 6B), although absolute CD4+ and CD8+ T-cell numbers on days 4 or 6 were similar (supplemental Figure 6D-E), which we speculate may be related to differences in T-cell egress from the spleen between these groups.
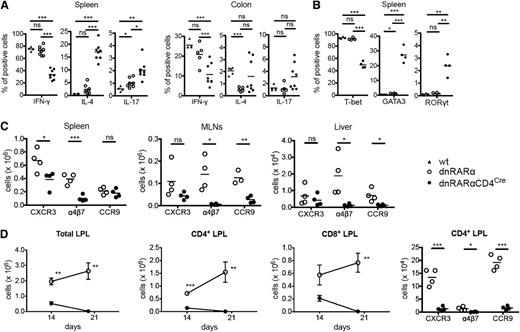
To determine whether RAR signaling affected Th generation, splenocytes on day 6 and colonic LPLs on post-BMT day 14 from BALB/c recipients of dnRARα-CD4Cre or dnRARα T cells were analyzed. The frequency of interferon-γ (IFN-γ)-secreting splenic and colonic LPL CD4+ T cells was lower in dnRARα-CD4Cre donors than in wt or dnRARα donors, whereas that of splenic interleukin (IL)-4-secreting CD4+ T cells was higher (Figure 4A). Although several reports have suggested that RAR signaling decreased Th17 generation,10-12 the frequency of IL-17–secreting CD4+ T cells in spleen was higher with a more modest increase in colonic LPLs (Figure 4A). Correlating with cytokine data, a lower frequency of splenic CD4+T-bet+ T cells was found in dnRARα-CD4Cre donors than in wt or dnRARα donors, whereas the frequencies of CD4+GATA3+ and CD4+RORγt+ T cells were higher (Figure 4B). Also, we assessed the frequencies of natural killer T (NKT) cells in the donor inoculum on the day of transplantation and in the liver, a location for NKT cells, at day 6 post-BMT. The frequencies of NKT cells were similar in the 3 groups (supplemental Figure 7).
Blocking RAR signaling in donor T cells impairs integrin and chemokine expression and skews T-cell polarity toward a Th2 phenotype. (A-D) Lethally irradiated BALB/c recipients were transplanted with B6 107 BM cells and 1.5 × 106 wt– (filled triangles), dnRARα− (open circles), or dnRARα-CD4Cre– (filled circles) purified T cells. Splenocytes, MLNs, liver cells, and colon lamina propria lymphocytes (LPLs) were isolated on indicated days and analyzed by fluorescence-activated cell sorter. Cells were gated on H-2Kb-positive events. (A) The frequency of CD4+ cells expressing IFN-γ, IL-4, and IL-17 from spleen and colon LPLs is shown. (B) The frequency of CD4+ cells expressing T-bet, GATA3, and RORγt from spleen is shown. (C) The absolute number of CD4+ cells expressing CXCR3, α4β7, and CCR9 in spleen, MLN, and liver cells is depicted. (D) The absolute number of total, CD4+, and CD8+ cells and the absolute number of CD4+ cells expressing CXCR3, α4β7, and CCR9 in colon LPLs are shown. (A) Data were combined from 2 experiments with similar results (n = 4 to 8/group). (B,D) Data were obtained from 1 experiment each with 4 mice per group. (C) Data were from 1 representative of 3 independent experiments. *P < .05; **P < .01; and ***P < .001.
Blocking RAR signaling in donor T cells impairs integrin and chemokine expression and skews T-cell polarity toward a Th2 phenotype. (A-D) Lethally irradiated BALB/c recipients were transplanted with B6 107 BM cells and 1.5 × 106 wt– (filled triangles), dnRARα− (open circles), or dnRARα-CD4Cre– (filled circles) purified T cells. Splenocytes, MLNs, liver cells, and colon lamina propria lymphocytes (LPLs) were isolated on indicated days and analyzed by fluorescence-activated cell sorter. Cells were gated on H-2Kb-positive events. (A) The frequency of CD4+ cells expressing IFN-γ, IL-4, and IL-17 from spleen and colon LPLs is shown. (B) The frequency of CD4+ cells expressing T-bet, GATA3, and RORγt from spleen is shown. (C) The absolute number of CD4+ cells expressing CXCR3, α4β7, and CCR9 in spleen, MLN, and liver cells is depicted. (D) The absolute number of total, CD4+, and CD8+ cells and the absolute number of CD4+ cells expressing CXCR3, α4β7, and CCR9 in colon LPLs are shown. (A) Data were combined from 2 experiments with similar results (n = 4 to 8/group). (B,D) Data were obtained from 1 experiment each with 4 mice per group. (C) Data were from 1 representative of 3 independent experiments. *P < .05; **P < .01; and ***P < .001.
RA has been shown to induce α4β7 and CCR9 upregulation in CD4+ and CD8+ T cells by anti-CD3 and anti-CD28 stimulation in vitro.7 Thus, RAR signaling in donor T cells might diminish chemokine receptor and integrin expression required for optimal GVHD initiation. To test this hypothesis, we examined the in vitro expression of chemokines and integrin in dnRARα as well as dnRARα-CD4Cre cells in a mixed lymphocyte reaction. CXCR3 and α4β7 expression was decreased in the dnRARα-CD4+/CRE T-cell group (supplemental Figure 8) and there was no difference in CCR5, CCR6, CXCR6, or CCR9 expression between dnRARα and dnRARα-CD4Cre cells. Next, we quantified the number and frequency of chemokine- and integrin-expressing donor CD4+ and CD8+ T cells post-BMT. Consistent with in vitro data and the improved survival of BALB/c recipients of dnRARα-CD4Cre compared with dnRARα donor T cells, on day 6 post-BMT there were significantly lower numbers of splenic CXCR3 and α4β7+ CD4+ T cells, MLN and liver α4β7+, and CCR9+ CD4+ T cells (Figure 4C) as well as splenic and MLN CXCR3+ α4β7+ and CCR9+ CD8+ (supplemental Figure 9A) T cells. Because CXCR3, α4β7, and CCR9 are important gut-homing receptors,7,28,29 we also quantified colonic LPL CD4+ and CD8+ T cells on days 14 and 20 post-BMT. Collectively, CD4+ and CD8+ T-cell numbers were both significantly reduced in recipients of dnRARα-CD4Cre compared with dnRARα T cells (Figure 4D). Moreover, the numbers of CXCR3-, α4β7-, and CCR9-expressing LPLs were significantly lower in recipients of dnRARα-CD4Cre than in dnRARα T cells (Figure 4D). Because there was no significant difference in the percentage of Ki67- and/or annexin V–positive cells in CD4+ and CD8+ cells isolated from the liver and colon of recipients of dnRARα and dnRARα-CD4Cre splenocytes (supplemental Figure 9B-D), in situ migration but not expansion in GVHD target organs is likely responsible for reduced infiltration in those sites. Thus, dnRARα-CD4Cre donor cells, compared with dnRARα donor T cells, have reduced chemokine receptor and integrin expression early post-BMT, which likely assists in their migration to these GVHD sites to initiate the GVHD process.
RAR signaling inhibition in dnRARα-CD4Cre donor T cells supports Treg induction and expansion
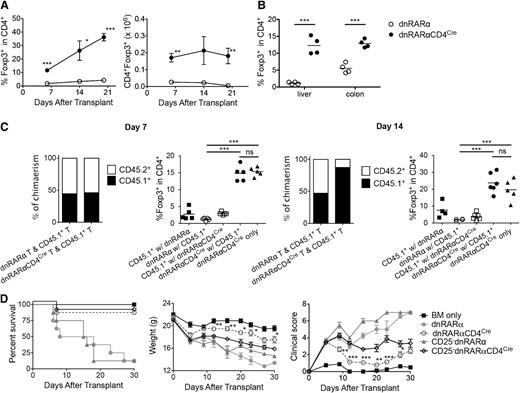
Although RA has been shown to promote transforming growth factor β–mediated Foxp3 conversion of naive T cells in iTregs,8,10,18,19,26,30 the mechanism by which RAR signaling in T cells affects Treg proliferation or expansion during acute GVHD remains unknown. Therefore, we analyzed iTreg generation using CD4+ T cells from dnRARα mice and dnRARα-CD4Cre mice in vitro. As expected, higher frequencies of CD4+Foxp3+ T cells were detected in the dnRARα group than in the dnRARα-CD4Cre group under commonly used culture conditions (supplemental Figure 10). Adding IL-21, produced during GVHD,31 reduced iTreg generation frequencies in both groups. Next, we quantified the frequency and number of CD4+Foxp3+ T cells in spleen tissues post-BMT. Surprisingly, compared with dnRARα T cells, BALB/c recipients administered dnRARα-CD4Cre had significantly higher frequencies and absolute numbers of splenic Foxp3+ cells on days 6, 14, and 20 (Figure 5A), and a significantly higher frequency of liver and colonic CD4+Foxp3+ T cells on day 14 post-BMT (Figure 5B).
Inhibition of RAR signaling in donor T cells increases Tregs in vivo. Lethally irradiated BALB/c recipients were transplanted with B6 107 BM cells and 1.5 × 106 dnRARα (open circles) or dnRARα-CD4Cre (filled circles) purified T cells. (A) The frequency of CD4+Foxp3+ cells and the absolute number of CD4+Foxp3+ cells in spleen are shown. (B) Liver cells and colon lamina propria lymphocytes were isolated on day 14 and analyzed using fluorescence-activated cell sorter. The frequency of CD4+Foxp3+ cells is shown. (C) Lethally irradiated BALB/c recipients were transplanted with CD45.2+ TCD B6 107 BM cells and 1 × 106 CD45.1+ B6 T cells along with 1 × 106 CD45.2+ T cells from either dnRARα (CD45.1 with dnRARα) or dnRARαCD4Cre (CD45.1 with dnRARα-CD4Cre) mice or were transplanted with CD45.2+TCD-B6 107 BM cells and 2 × 106 dnRARα-CD4Cre T cells alone. The percentages of chimerism of CD4+ cells and frequency of CD4+Foxp3+ cells gated on CD45.2+ (dnRARα or dnRARαCD4Cre) or CD45.1+ cells in spleens on day 14 after BMT are shown. (D) Lethally irradiated BALB/c recipients were injected with 107 BM cells and 5 × 106 CD25-depleted splenocytes or CD25-replete splenocytes from either dnRARα or dnRARα-CD4Cre donors and monitored for survival. (A-D) Data were obtained from 1 experiment each with 4 (A-B), 4 to 5 (C), or 8 (D) mice per group. *P < .05; **P < .01; and ***P < .001.
Inhibition of RAR signaling in donor T cells increases Tregs in vivo. Lethally irradiated BALB/c recipients were transplanted with B6 107 BM cells and 1.5 × 106 dnRARα (open circles) or dnRARα-CD4Cre (filled circles) purified T cells. (A) The frequency of CD4+Foxp3+ cells and the absolute number of CD4+Foxp3+ cells in spleen are shown. (B) Liver cells and colon lamina propria lymphocytes were isolated on day 14 and analyzed using fluorescence-activated cell sorter. The frequency of CD4+Foxp3+ cells is shown. (C) Lethally irradiated BALB/c recipients were transplanted with CD45.2+ TCD B6 107 BM cells and 1 × 106 CD45.1+ B6 T cells along with 1 × 106 CD45.2+ T cells from either dnRARα (CD45.1 with dnRARα) or dnRARαCD4Cre (CD45.1 with dnRARα-CD4Cre) mice or were transplanted with CD45.2+TCD-B6 107 BM cells and 2 × 106 dnRARα-CD4Cre T cells alone. The percentages of chimerism of CD4+ cells and frequency of CD4+Foxp3+ cells gated on CD45.2+ (dnRARα or dnRARαCD4Cre) or CD45.1+ cells in spleens on day 14 after BMT are shown. (D) Lethally irradiated BALB/c recipients were injected with 107 BM cells and 5 × 106 CD25-depleted splenocytes or CD25-replete splenocytes from either dnRARα or dnRARα-CD4Cre donors and monitored for survival. (A-D) Data were obtained from 1 experiment each with 4 (A-B), 4 to 5 (C), or 8 (D) mice per group. *P < .05; **P < .01; and ***P < .001.
To determine whether donor T cell–derived cytokines affect Tregs post-BMT, dnRARα or dnRARα-CD4Cre T cells were co-transferred with CD45.1 T cells (1:1) into BALB/c recipients and the frequency of splenic and MLN donor Tregs was analyzed. On days 7 and 14 post-BMT, a higher frequency of CD4+Foxp3+ T cells was evident in recipients of dnRARα-CD4Cre than in those of dnRARα T cells (with or without co-transferred T cells) (Figure 5C). Few CD4+Foxp3+ T cells were directly derived from CD45.1+ or dnRARα T cells, indicating that dnRARα-CD4Cre T cells had a higher propensity to support Tregs during GVHD, regardless of the presence of co-transferred or CD45.1+ T cells. Notably, dnRARα-CD4Cre compared with dnRARα T-cell chimerism was comparable to CD45.1+ T cells on day 7; however, compared with dnRARα T cells, dnRARα-CD4Cre T cells had reduced accumulation by day 14 post-BMT, consistent with a recent report regarding CD8+ T cells.24 To determine whether the GVHD-protective effect of dnRARα-CD4Cre compared with dnRARα donor T cells was dependent on the presence of donor Tregs, Tregs were depleted from the donor inoculum before transfer. Recipients of dnRARα-CD4Cre splenocytes had less severe GVHD than those receiving dnRARα-CD4CreTreg–depleted splenocytes. Mice in both groups survived significantly longer than recipients of dnRARα splenocytes or dnRARα Treg-depleted splenocytes (Figure 5D). The mean weights and clinical scores paralleled the survival curves; thus, Treg depletion reduced but did not eliminate the survival benefits of dnRARα-CD4Cre splenocytes. Taken together, we hypothesize that the lower GVHD caused by dnRARα-CD4Cre T cells results in reduced IL-21 production in vivo, leading to higher iTreg generation, which would contribute to reduced GVHD regardless of donor Treg content.31
GVL effects of dnRARα-CD4Cre donor T cells
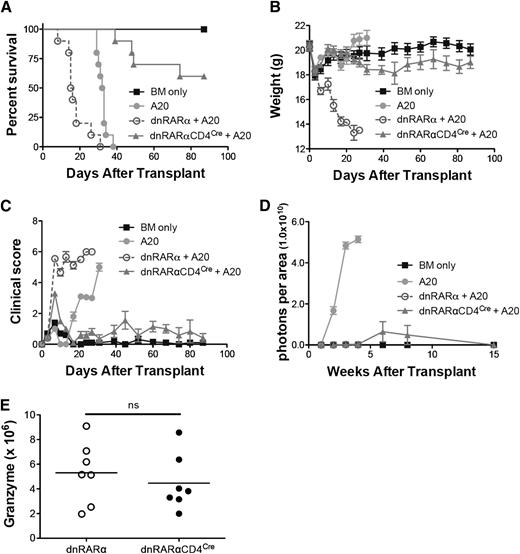
To determine whether dnRARα-CD4Cre donor T-cell expression adversely affected GVL activity, lethally-irradiated BALB/c recipients were administered B6 TCD-BM and A20luc cells on day 0. The groups were administered splenocytes from dnRARα or dnRARα-CD4Cre mice. All mice that were administered A20luc and TCD-BM alone died within 38 days because of tumor burden (Figure 6A-D); those administered dnRARα splenocytes all died of GVHD by day 31 before rapid weight loss (Figure 6B) and high clinical scores (Figure 6C), but showing no or minimal initial tumor growth (Figure 6D). Most mice administered A20luc and dnRARα-CD4Cre splenocytes showed minimal initial tumor growth followed by regression with minimal evidence of GVHD mean weight curves or clinical scores.
Blockade of RAR signaling in donor T cells does not abort the GVL effect. Lethally irradiated BALB/c recipients were transplanted with 107 T-cell–depleted BM cells with or without 3 × 105 A20-lymphoma cells on day 0. Subgroups were transplanted with 5 × 106 splenocytes from dnRARα (open circles) and dnRARα-CD4Cre (filled triangles) mice also on day 0. (A-C) Survival, weight, and clinical GVHD scores of lethally irradiated BALB/c recipients transplanted with BM cells only (filled squares), BM cells + A20 (filled circles), or with A20 + 5 × 106 splenocytes from dnRARα (open circles) or dnRARα-CD4Cre (filled triangles) donor mice. (D) Tumor growth was monitored by luciferase imaging at 1, 2, 3, 4, 6, 8, and 15 weeks after BMT. (E) The frequency of CD8+ cells expressing granzyme B was analyzed in spleen on day 14. Data were obtained from 1 experiment each with 5 to 10 (A-D) or 7 to 8 (E) mice per group. (A) BM cells + A20 vs dnRARα-CD4Cre + A20; P < .001. dnRARα+ A20 vs dnRARα-CD4Cre + A20; P < .001. (B-C) dnRARα+ A20 vs dnRARα-CD4Cre + A20 on day 6 to day 27; P < .001. (D) BM cells + A20 vs dnRARα-CD4Cre + A20 at 1, 2, 3, and 4 weeks; P < .001. dnRARα+ A20 vs dnRARα-CD4Cre + A20; P = not significant (ns).
Blockade of RAR signaling in donor T cells does not abort the GVL effect. Lethally irradiated BALB/c recipients were transplanted with 107 T-cell–depleted BM cells with or without 3 × 105 A20-lymphoma cells on day 0. Subgroups were transplanted with 5 × 106 splenocytes from dnRARα (open circles) and dnRARα-CD4Cre (filled triangles) mice also on day 0. (A-C) Survival, weight, and clinical GVHD scores of lethally irradiated BALB/c recipients transplanted with BM cells only (filled squares), BM cells + A20 (filled circles), or with A20 + 5 × 106 splenocytes from dnRARα (open circles) or dnRARα-CD4Cre (filled triangles) donor mice. (D) Tumor growth was monitored by luciferase imaging at 1, 2, 3, 4, 6, 8, and 15 weeks after BMT. (E) The frequency of CD8+ cells expressing granzyme B was analyzed in spleen on day 14. Data were obtained from 1 experiment each with 5 to 10 (A-D) or 7 to 8 (E) mice per group. (A) BM cells + A20 vs dnRARα-CD4Cre + A20; P < .001. dnRARα+ A20 vs dnRARα-CD4Cre + A20; P < .001. (B-C) dnRARα+ A20 vs dnRARα-CD4Cre + A20 on day 6 to day 27; P < .001. (D) BM cells + A20 vs dnRARα-CD4Cre + A20 at 1, 2, 3, and 4 weeks; P < .001. dnRARα+ A20 vs dnRARα-CD4Cre + A20; P = not significant (ns).
To elucidate the potential mechanisms of retained GVL effects in recipients administered A20luc and dnRARα-CD4Cre splenocytes, we tested the granzyme B and perforin cytotoxic pathways of donor T cells.32 Granzyme B expression in day 14 splenic CD8+ T cells was largely preserved in the dnRARα-CD4Cre group (Figure 6E); no difference occurred in CD8+ T-cell perforin expression (not shown). Furthermore, no significant differences in the number of tumor necrosing factor α–secreting cells, associated with GVL effects,33 were observed in day 6 splenic or hepatic dnRARα-CD4Cre compared with dnRARα-CD4+ and dnRARα--CD8+ T cells (supplemental Figure 11). To confirm and extend our findings, the P815 mastocytoma model was used to address whether GVL effects were maintained in another mouse strain and/or tumor combination. Similar to the A20 lymphoma model, there was no significant increase in BLI signaling in the dnRARα-CD4Cre group compared with the dnRARα group (supplemental Figure 12).
Discussion
Here, we demonstrate that GVHD-induced RA production via donor-derived DCs and macrophages in the intestines and MLNs, and that RAR signaling in donor T cells was upregulated, contributing to donor T-cell accumulation in GVHD target organs, thereby accelerating the disease. Conversely, inhibition of RAR signaling in donor T cells demonstrated reduced GVHD capacity associated with reduced intestinal-homing receptor expression, decreased Th1 Teff proinflammatory cytokine production, and greater Tregs content. Despite these beneficial effects, GVL effects were preserved.
RALDH, expressed in gut-associated DCs7 and intestinal epithelial cells,6 is a pivotal enzyme for RA synthesis. Here, we demonstrate that RALDH1 and RALDH2 expression was upregulated in GVHD mice in the small intestine on day 14 post-BMT. ALDEFLOUR staining confirmed the upregulation of ALDH enzymatic activities in day 14 post-BMT small intestinal tissue and MLN macrophages and DCs. RA concentrations and signaling in donor T cells were significantly increased, suggesting that blocking either RA production or donor T-cell RA signaling might be a useful approach to ameliorate GVHD. Regarding the latter, we report the novel observation that selective diminution of RAR signaling in donor T cells prevents GVHD lethality, whereas RA supplementation had the opposite effect.
Furthermore, we demonstrate that RA administration enhanced GVHD lethality in a major histocompatibility complex (MHC)-mismatched acute GVHD model, consistent with recent studies by Chen et al using a different strain combination.18 Additionally, their studies showed that RA affects expression of gut-homing molecules and inflammatory cytokine production. In other studies, RA was shown to modulate BM myeloid-derived suppressor cell proliferation, which was correlated to GVHD severity.34,35 Conversely, Nishimori et al demonstrated that Am80 ameliorated chronic GVHD because of downregulation of Th1 and Th17 cells.15 Although chronic and acute GVHD are pathologically distinct and often have opposing mechanisms, more research is required to determine the role of RA in GVHD.
Pathological analysis revealed the importance of RAR signaling in the development of intestinal GVHD; its protective effects in other organs may be due to reduced gut inflammation and permeability, which affects systemic GVHD-induced injury and lethality.1,2 Koenecke et al.16 reported that vitamin A deficiency reduces intestinal but exacerbates liver GVHD and does not offer protection from overall GVHD lethality. Notably, the vitamin A–deficient mice had a higher level of endogenous inflammation than nondeficient mice, which we overcame by employing a selective approach to prevent RAR signaling in donor T cells.
We determined that selective diminution of RAR signaling in donor T cells reduced early post-BMT expression of donor T-cell CXCR3, α4β7, and CCR9, which are critical factors in GVHD-induced gut injury.16,36-40 dnRARα-CD4Cre T cells had skewed Th2 and Th17 profiles with lower levels of Th1; paracrine production of cytokines from donor T cells had no impact on Th1/Th2 cell differentiation of dnRARα-CD4Cre T cells (data not shown), indicating that reduction in Th1 differentiation was T-cell intrinsic. Several studies have proposed that Th2 polarization regulates acute GVHD41 severity, although acute GVHD may not be ameliorated by a reduction in only Th1 and IFN-γ.42-44 Nonetheless, diminished Th1 differentiation of donor T cells from dnRARα-CD4Cre compared with that in dnRARα donor T cells may have contributed decreasing GVHD lethality. Because RA effects observed in dnRARα-CD4Cre may also influence donor T-cell signaling via RARα, RARβ, and RARγ resulting from effects of RXR sequestration by dnRARα, future studies will be also required to determine the role of each RAR and its associated RXRs on donor T cells and antigen-presenting cells in GVHD modulation.
RA was shown to promote transforming growth factor β–mediated Foxp3 conversion of naive T cells and stabilize Foxp3 expression in natural Tregs and iTregs.8,10,19,26,30 Somewhat surprisingly, dnRARα-CD4Cre donor T cells enhanced Treg generation from both donor BM and T cells in vivo, but not in vitro. Neither donor T-cell autocrine nor paracrine cytokine production inhibited Treg generation/expansion in recipients of dnRARα-CD4Cre T cells post-BMT. Because GVHD is markedly reduced when using dnRARα-CD4Cre as compared with dnRARα, we hypothesize that the former is associated with low IL-21 levels that favor the generation of iTregs, offsetting the effects of impaired RA signaling, which only modestly reduces iTreg generation vs the potent inhibitory effect of IL-21. These data further indicate that high inflammatory cytokines31,45,46 associated with GVHD per se are not conducive to in vivo Treg generation and that approaches to reduce GVHD will favor iTreg generation and/or expansion, offsetting any deleterious effect of diminished RAR signaling in donor T cells. The overall higher Treg frequencies in mice administered dnRARα-CD4Cre T cells may account for the greater reduction in pathologic scores of the gastrointestinal tract observed in the context of dnRARα-CD4Cre donor T cells, even though RA is known to promote α4β7 expression by inducible Tregs.47
Teffs from dnRARα-CD4Cre mice may be eliminated or fail to accumulate in GVHD target organs, as has been shown for CD8+ T-cell accumulation in the late, and not early, phase after antigenic stimulation.24 Also, it may be possible that NKT cells augment GVHD severity,48 although there is no significant difference in the percentage of NKT cells from dnRARα and dnRARα-CD4Cre in donor inoculum and splenocytes at day 6 post-BMT. Together, these findings indicate that reduced donor Th1 cytokine differentiation and T-cell expression by gut-homing receptors coupled with higher Treg numbers (and hence a favorable Treg:Teff ratio) may each contribute to the lower GVHD mortality observed in dnRARα-CD4Cre than dnRARα T-cell administration.
Importantly, GVL effects against A20 lymphoma and P815 mastocytoma were largely maintained, despite markedly decreased GVHD lethality in recipients of B6 dnRARα-CD4Cre splenocytes vs wt splenocytes, which was consistent with a recent report.18 However, this GVL effect does not appear to be susceptible to Treg-mediated suppression.49 The production of perforin-granzyme from CTL cells and transforming growth factor-α from dnRARα-CD4Cre donor T cells are preserved. In spite of our recent findings that diminished RAR signaling in CD8+ T cells dampened late CD8+ T-cell expansion and clonal accumulation in a tumor microenvironment, GVL activity appeared mostly intact. Explanations for these differences in antitumor responses of dnRARα-CD4Cre donor T cells may be explained by the required Teff mechanisms (eg, CD4+ T cells can recognize MHC class II+ A20 cells and high sensitivity to perforin-granzyme B–mediated cytolysis50 ) and the high frequency of alloresponsive (and hence A20 responsive) vs syngeneic antitumor-responsive T cells.
In conclusion, these studies demonstrate that enhanced RA synthesis and RAR signaling in donor T cells during GVHD augments disease severity. Because the inhibition of RAR signaling in donor T cells attenuated GVHD lethality while preserving GVL activity, selectively targeting the RAR signaling pathway may be useful for GVHD prevention or therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Klara Noble for excellent animal husbandry and technical assistance, all colleagues of Blazar’s Laboratory for technical assistance, Karina Pino-Lagos (The Geisel School of Medicine at Dartmouth) for providing dnRARα-CD4Cre splenocytes, and Enago (www.enago.jp) for the English language review.
This work was supported by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, National Heart, Lung, and Blood Institute, and National Cancer Institute (R01 AI34495, R01 HL56067, and P01 AI056299) (B.R.B.) and R01-CA062275 and the Mochida Memorial Foundation (K.A.). E.D. is an American Cancer Society Professor supported by a generous gift from the F. M. Kirby Foundation.
Authorship
Contribution: K.A. designed and performed research, analyzed the data, designed the figures, and wrote the paper; M.J.R. and L.M.F. performed research; A.S. and R.G.V. performed research, designed the figures, and edited the paper; J.T., A.P-M., and R.B. contributed data and edited the paper; P.A.T., C.A.K., G.S., D.H.M., W.J.M., J.S.S., T.T., and B.R.B. designed research and edited the paper; and R.A.C., E.D., Y.G., and R.J.N. designed research, provided reagents and advice, and edited the paper.
Conflict-of-interest disclosure: R.B. has an ownership interest (including patents) in Cgene; E.D. has ownership interest (including patents) in a pending patent; R.A.C. is employed (other than primary affiliation; eg, consulting) by Io Pharmaceuticals and is a consultant/advisory board member, holds title of Scientific Advisory Board Chair, and has ownership interest (including patents) in Io Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, University of Minnesota Cancer Center and Department of Pediatrics, Division of Bone Marrow Transplantation, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal