In this issue of Blood, Mathewson et al characterized targeting a novel intracellular pathway with an agent known to induce potent antitumor effects but now also appears to potently suppress inflammatory responses by dendritic cells (DCs) and prevent graft-vs-host disease (GVHD). Identifying agents that can suppress GVHD yet not compromise graft-vs-tumor (GVT) effects remains a paramount goal in allogeneic hematopoietic stem cell transplantation (HSCT).1
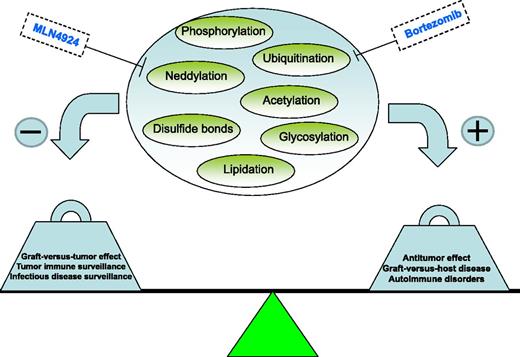
So-called “small molecules” have started to dominate the clinical practice of cancer treatment with the promise of targeting the underlying molecular changes that are responsible for specific malignant phenotypes and avoiding the side effects of systemic chemotherapies. Increasing evidence has emerged to indicate that these drugs also can exert profound immunomodulatory effects on the immune system which makes them attractive in targeting GVHD.2 The study by Mathewson et al1 continues in that vein. MLN4924 was first described regarding its potent antitumor effects affecting a wide array of tumor types.3 The current experiments, however, show that the process of neddylation (in which a ubiquitin-like protein, NEDD8, is conjugated to target proteins much like ubiquitination and is the predominant pathway blocked by MLN4924) has wide-reaching effects on the immune system. Posttranslational modification (PTM) represents a key component of all cellular control and can involve biochemical alterations such as acetylation, phosphorylation, and ubiquitination.4 Many of these small-molecule therapeutics currently used as potential cancer treatments target PTM and often converge on nuclear factor–κB (NF-κB) as the master controller for cellular responses to a variety of cellular stimuli such as cytokine production and cell survival. It has become increasingly apparent that other pathways are also involved in PTM; all contributing to the “net” effects on NF-κB and cellular signaling in general. Neddylation is one such a pathway. Through the use of a specific inhibitor of neddylation, MLN4924, which inhibits the NEDD8 activating enzyme, Mathewson et al demonstrate that MLN4924 treatment on DCs resulted in potent inhibition of both the canonical and noncanonical NF-κB pathways via stabilization of inhibitor of kappaB kinase beta, and yet did not inhibit the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathways. The suppressive effect of MLN4924 on NF-κB was greater than what was observed with dexamethasone or bortezomib. Toll-receptor signaling was markedly suppressed and inhibition of mixed lymphocyte responses in both human and mouse systems resulted. Importantly, the systemic administration of the inhibitor also prevented GVHD in mice. This study was particularly notable for the mechanistic molecular dissection, the use of both human and mouse systems, and the elegant in vivo GVHD model in which it was delineated that the predominant protective effects were due to the direct effect of MLN4924 on DCs.
There has been intense interest in targeting NF-κB and other molecular signaling pathways in GVHD. Proteasome inhibition, histone deacetylase (HDAC) inhibition, as well as other similar agents have all shown impressive preclinical efficacy and some have made their way into the clinic with encouraging results.2 Some agents appear to predominantly affect T cells whereas others act on DCs, but the effects on both cell types likely contribute to the overall “net” result.2 MLN4924 markedly impacted DCs but the data demonstrated also direct, albeit lesser, suppression on T cells as well. Importantly, all of the aforementioned molecular targeting agents were first evaluated for their direct antitumor effects. As allogeneic HSCT is predominantly used for hematologic malignancies, it makes sense that many promising antitumor agents would subsequently be assessed in HSCT in order to eradicate residual disease; any immunomodulatory effects they induce that could suppress GVHD would be an added bonus. The predominant issue in GVHD/GVT has remained unchanged for decades, that is, the separation of the 2 by suppressing GVHD without compromising GVT. The attractiveness of using small-molecule agents, as opposed to global immunosuppression by steroids and chemotherapeutics, is the potential for direct antitumor effects (see figure). The trick is balancing the immunosuppression such that GVT effects can still occur outside of the direct antitumor effects of the agent. This may be difficult to discern other than possibly determining whether continued antitumor effects occur well after cessation of drug administration. Nonetheless, given the potent antitumor effects attributed to MLN4924,3,5 it is encouraging that the suppression of GVHD may still result in antitumor effects. Another pitfall with the usage of immunosuppressive approaches in HSCT may be seen with regards to opportunistic infections as one walks a tightrope between too much and too little suppression. Timing is likely also key. It will be of particular interest, given the effect of targeting neddylation on Toll-like receptor (TLR) responses, to ascertain the effects on infectious responses, especially in the setting of existing immunosuppression such as after HSCT where such responses may be more critical. Additionally, the sparing effect on lipopolysaccharide (LPS)–induced activator protein-1 (AP-1)–mediated MAPK/ERK activation that Matthewson et al observed may be particularly important to reduce the side effects of these drugs in comparison with proteasome inhibitors, for example. Bortezomib can result in the upregulation of AP-1, which will enhance the activation of the LPS-induced MAPK/ERK pathway and associated (eg, tumor necrosis factor-α) cytokine release thus possibly accounting for toxicities with prolonged administration.6
The study by Mathewson et al also indicates another potential use for molecular targeting agents like MLN4924, which have not escaped notice of the biomedical community: suppression of other general inflammatory disease states. In this case, targeting neddylation may be attractive given the profound effect on TLR signaling in DCs and possibly other innate pathways. However, given the numerous, and at times opposing, effects of various HDAC inhibitors on immune responses,7 more work is needed to delineate the drug-specific response from the general pathway effect. As targeting agents affecting different pathways (such as bortezomib and suberoylanilide hydroxamic acid) have also shown increased effects when given in combination,2 the addition of agents targeting neddylation may also add synergistic effects. Important parameters such as the reversibility of the inhibitor, pharmacokinetics, and off-target effects still need to be explored. However, these studies now add yet another exciting pathway in our arsenal of targeting inflammatory disorders and GVHD.
Conflict-of-interest disclosure: M.A. is on the speaker’s bureau for Millennium Pharmaceuticals. The remaining author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal