Key Points
Newly diagnosed and chronic ITP are most likely separate disease entities.
Abstract
Immune thrombocytopenia (ITP) is an autoimmune disease where platelets are destroyed prematurely. In the majority of children the disease resolves, but in some it becomes chronic. To investigate whether these 2 phases of the disease are molecularly similar or separate entities we performed DNA microarray analysis (GEO accession number: GSE46922) of T-cells from newly diagnosed children and children with chronic ITP. We found complete separation of the gene expression profiles between the 2 phases of the disease. Furthermore, the gene expression levels of several cytokines differed between the 2 phases of the disease. This was also reflected in plasma with increased levels of interleukin (IL)-16 and TNF-related weak inducer of apoptosis and lower levels of IL-4 in newly diagnosed compared with chronic ITP. Thus, our data indicate that chronic ITP in childhood is a separate disease entity, dissimilar in many aspects to the newly diagnosed phase.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease where the autoantibody opsonized platelets are destroyed prematurely in the reticuloendothelial system and directly by cytotoxic T-cells and by complement activation.1-3 ITP exists in a primary idiopathic and a secondary variant caused by various disorders. Primary ITP was previously split into acute and chronic ITP depending on the outcome. However, the condition is today divided into 3 phases depending on the duration of thrombocytopenia after the initial diagnosis, that is, newly diagnosed up to 3 months, persistent ITP between 3 and 12 months, and chronic ITP beyond 12 months.4
Clinical findings indicate that the development of chronic ITP in children is associated with older age, less mucosal bleedings, and an insidious onset. Furthermore, chronic ITP is less likely to have had a viral illness before onset and has higher platelet count at presentation than in children with a spontaneously resolving ITP.5 At presentation there are no clinical useful biomarkers that can separate a spontaneously resolving disease from the chronic variant. Nevertheless, Semple et al found increased serum levels of interleukin 2 (IL-2), IL-10, and interferon-γ in chronic ITP compared with acute ITP.6 Del Vecchio et al also found increased serum levels of IL-10 in chronic vs acute ITP.7 Recently, Zhang et al found increased mRNA expression of vanin-1 in whole blood from chronic ITP compared with acute ITP.8
Nevertheless, it is currently impossible to predict with a high degree of certainty the long-term outcome in a case of newly diagnosed ITP. Furthermore, whether the pathophysiologic mechanisms behind the spontaneously resolving and the chronic variant differ is also largely unknown. In the present study, we investigated T-cell gene expression profiles from children with newly diagnosed and chronic ITP and verified some of the differently expressed genes with real-time PCR and ELISA.
Study design
Patient characteristics and methods are provided in detail in supplemental Methods. All studies were approved by the regional ethics committee in Gothenburg or the Medical Ethical Committee of Qilu Hospital, Shandong University, and all participants provided informed consent in accordance with the Declaration of Helsinki.
Isolation of T-cells, RNA, and hybridization to the DNA microarrays have previously been described in detail.2,9 Real-time PCR was performed as previously described.10 IL-4 and IL-16 were analyzed using commercial ELISAs from R&D systems (Minneapolis, MN). TNF-related weak inducer of apoptosis (TWEAK) was analyzed by a commercial ELISA from eBioscience (San Diego, CA). All data are presented as mean ± SEM, and P values ≤ .05 were considered significant.
Results and discussion
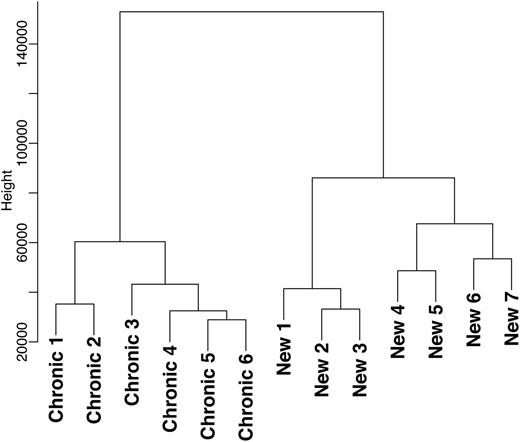
To investigate whether the different phases of the disease are molecularly similar or separate entities, we performed DNA microarray analysis (GEO accession number: GSE46922) of T-cells from newly diagnosed children with ITP and children with chronic ITP. In the newly diagnosed ITP children, blood collection was undertaken within 1 week after presentation and before any specific ITP therapy was administered. The genome-wide expression profiles were clustered in a dendrogram plot, and no overlap was found between the newly diagnosed and chronic cohorts (Figure 1). This observation shows that the newly diagnosed and chronic phases are molecularly separate entities. These data are in line with findings from Wright et al who found differences in T-cell reactivity against platelets between acute and chronic ITP.11
The genome-wide expression profiles were clustered in a dendrogram plot that represents the hierarchical clustering analysis of differentially expressed genes from the T-cell genome-wide expression profiles of children with newly diagnosed (n = 7) and chronic ITP (n = 6). No overlap was found between these 2 phases of the disease.
The genome-wide expression profiles were clustered in a dendrogram plot that represents the hierarchical clustering analysis of differentially expressed genes from the T-cell genome-wide expression profiles of children with newly diagnosed (n = 7) and chronic ITP (n = 6). No overlap was found between these 2 phases of the disease.
We identified 6309 differently expressed transcripts (supplemental Table 3) between the 2 phases of the disease and clustered them according to Gene Ontology (GO) annotations (supplemental Figure 1). Genes involved in, for example, T-cell activation, lymphocyte proliferation, and chemotaxis were overrepresented in newly diagnosed compared with chronic ITP. In contrast, genes involved in B-cell differentiation and CD8+ α:β T-cell lineage commitment were significantly overrepresented in chronic compared with newly diagnosed ITP (supplemental Figure 1). These findings suggest that newly diagnosed ITP is associated with an unspecific and wider inflammatory gene activation profile and that the chronic form is associated with pathways traditionally associated with chronic ITP, that is, a Th1 immune response with autoantibody production and CD8+ cytotoxic T-cells.1,2
To verify some of the differences observed in the DNA microarray analysis, we performed real-time PCR. We analyzed the chemokine CX3C motif receptor 1 (CX3CR1) which we previously showed is involved in T-cell trafficking in chronic ITP.12 Interestingly, the T-cell expression of CX3CR1 was significantly increased in newly diagnosed compared with chronic ITP in both analyses, suggesting involvement of T-cell trafficking in both phases of the disease (Figure 2A-B).
mRNA and plasma levels of cytokines in newly diagnosed and chronic ITP. DNA microarray expression is presented in (A) CX3CR1 (relative expression of newly diagnosed 5765 ± 988 vs chronic 2907 ± 283), (C) IL-16 (relative expression of newly diagnosed 1312 ± 124 vs chronic 812 ± 161), (F) TNF-related weak inducer of apoptosis (TWEAK) (relative expression of newly diagnosed 649 ± 75 vs chronic 397 ± 36), and (H) IL-4 (relative expression of newly diagnosed 16.3 ± 5.8 vs chronic 48.0 ± 6.2). Real-time PCR analysis CX3CR1 is presented in (B) (relative expression of newly diagnosed 0.019 ± 0.0086 vs chronic 0.0050 ± 0.0016). Plasma analysis is presented in (D) IL-16 in the Swedish cohort (relative expression of newly diagnosed 160 ± 18.7 vs chronic 96 ± 11.2), (E) IL-16 in the Chinese cohort (relative expression of newly diagnosed 114 ± 16.6 vs chronic 70 ± 4.2), (G) TWEAK in the Chinese cohort (relative expression of newly diagnosed 881 ± 57.7 vs chronic 632 ± 54.3) and (I) IL-4 in the Chinese cohort (relative expression of newly diagnosed 2.8 ± 0.30 vs chronic 6.4 ± 0.40).
mRNA and plasma levels of cytokines in newly diagnosed and chronic ITP. DNA microarray expression is presented in (A) CX3CR1 (relative expression of newly diagnosed 5765 ± 988 vs chronic 2907 ± 283), (C) IL-16 (relative expression of newly diagnosed 1312 ± 124 vs chronic 812 ± 161), (F) TNF-related weak inducer of apoptosis (TWEAK) (relative expression of newly diagnosed 649 ± 75 vs chronic 397 ± 36), and (H) IL-4 (relative expression of newly diagnosed 16.3 ± 5.8 vs chronic 48.0 ± 6.2). Real-time PCR analysis CX3CR1 is presented in (B) (relative expression of newly diagnosed 0.019 ± 0.0086 vs chronic 0.0050 ± 0.0016). Plasma analysis is presented in (D) IL-16 in the Swedish cohort (relative expression of newly diagnosed 160 ± 18.7 vs chronic 96 ± 11.2), (E) IL-16 in the Chinese cohort (relative expression of newly diagnosed 114 ± 16.6 vs chronic 70 ± 4.2), (G) TWEAK in the Chinese cohort (relative expression of newly diagnosed 881 ± 57.7 vs chronic 632 ± 54.3) and (I) IL-4 in the Chinese cohort (relative expression of newly diagnosed 2.8 ± 0.30 vs chronic 6.4 ± 0.40).
We also analyzed cytokine levels in the 2 phases. In the Swedish cohort of pediatric ITP patients, we found that the T-cell mRNA expression of IL-16 was elevated in newly diagnosed compared with chronic patients (Figure 2C). This was also reflected in plasma with significantly higher levels in newly diagnosed than in chronic ITP (Figure 2D). We could also verify this finding in a second Chinese cohort of newly diagnosed ITP children and children with chronic ITP (Figure 2E). IL-16 is an inflammatory cytokine, and deficiency in mice leads to suppression of chronic rejection in a cardiac transplant model.13 Furthermore, antibody-mediated neutralization of IL-16 leads to protection of NOD mice from type I diabetes.14
Another cytokine with increased mRNA expression in T-cells from newly diagnosed compared with chronic ITP was TWEAK (Figure 2F). The differential expression of this cytokine was also reflected at the protein level in plasma (Figure 2G). TWEAK is a member of the tumor necrosis family (TNFSF12) and is a weak apoptosis inducer.15 Serum levels of TWEAK are increased in collagen-induced arthritis and experimental autoimmune encephalomyelitis, and a neutralizing antibody reduces the severity of both diseases.16,17 These data are in line with our previous findings of decreased activation-induced cell death of T-cells in chronic ITP.18
The third cytokine, IL-4, had increased mRNA expression in chronic compared with newly diagnosed ITP (Figure 2H). This was also observed in plasma (Figure 2I). IL-4 is also termed B-cell stimulatory factor 1 and is, as the name implies, involved in B-cell activation and antibody production but also in T-cell activation.19,20 IL-4 is also known for its role in Th2 response. However, we and others have previously shown that chronic ITP is associated with a Th1 response, for example, secretion of interferon-γ.2,6,21 Therefore, the low levels of IL-4 recorded are most likely involved in B-cell and T-cell stimulation and activation, but this remains to be demonstrated in future studies. This finding contrasts with those of Semple et al who did not find any differences in serum levels of IL-4 between acute and chronic ITP.6 The nomenclature of the different phases of ITP has changed, which might explain the divergent results. However, the most likely explanation is that the assay we used is more sensitive than the method used by Semple et al; they did not find any acute or chronic patients with detectable levels of IL-4.
The differences between newly diagnosed and chronic ITP are not likely to be a result of treatment, because only 1 chronic ITP patient on treatment was included. Furthermore, this patient did not stand out in any of the analyses. However, from our findings we cannot determine whether these changes between newly diagnosed and chronic ITP are present at diagnosis or whether the changes occur in chronic ITP throughout time.
Recently Zhang et al found increased mRNA levels of vanin-1 in whole blood from chronic compared with acute ITP.8 In our study, there was no significant difference in vanin-1 expression between the 2 ITP phases in the DNA microarray analysis. This discrepancy is likely a result of a difference in the cell composition analyzed; we used isolated T-cells whereas Zhang et al used whole blood, which is a broader mix of different cell types. Indeed, the expression of vanin-1 in their study was highest in granulocytes, monocytes, and platelets with only low levels in T-cells.
In conclusion, our data show that there are clear molecular differences between newly diagnosed and chronic pediatric ITP. The data emphasize how crucial the patient selection is in clinical trials, and it can be questioned if early therapeutic intervention will have any effect on the natural course of a newly diagnosed ITP disease, as previously hoped for.22
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work in the authors’ laboratory was supported by grants from the Swedish Research Council (K2009-65X-15424-05-3, K2011-X-20401-05-6), the Swedish federal government under the LUA/ALF agreement, the Foundations of the National Board of Health and Welfare, Torsten and Ragnar Söderberg Foundation, Clas Groschinsky Foundation, the Arosenius Foundation, Åke Wiberg Foundation, Jeansson Foundation, Tore Nilsson Foundation for Medical Research, Magnus Bergvall Foundation, Wilhelm and Martina Lundgren Science Foundation, the Knut and Alice Wallenberg Foundation, Samariten Foundation, Chalmers foundation, Bioinformatics Infrastructure for Life Sciences (BIL), Tai Shan Scholar Foundation, National Natural Science Foundation of China (No. 81070396, No. 81070408, No. 81070407, No. 81070411, No. 81100334, No. 81100335, No. 81100336, No. 81100348, No. 81101869, No. 81170475, No. 81200344, No. 81270578), National Science Fund for Distinguished Young Scholars (No. 81125002), 973 Program (No. 2009CB521904, No. 2011CB503906), State Program of National Natural Science Foundation of China for Innovative Research Group 2011-2013 (No. 81021001), National Public Health Grand Research Foundation (No. 201202017), Key Clinical Research Project of Public Health Ministry of China 2010-2012, Natural Science Foundation of Shandong Province (No. ZR2010HQ002, No. ZR2010CQ040, No. Y2008C121), National Key Vocational School About Clinical Specialty for Blood Disorders, Clinical Medicine Center Foundation of Shandong Province, Leading Medical Professionals Foundation of Shandong Province, Outstanding Young Scientist Research Award Foundation of Shandong Province (No. BS2010YY024, No. BS2010YY039, No. BS2011SW013, No. BS2011YY021), the Research Fund for the Doctoral Program of Higher Education of China (No. 20100131120058), and Independent Innovation Foundation of Shandong University (No. 26010172611135).
Authorship
Contribution: M.J. and Y.H. performed and analyzed the experiments, analyzed data, and wrote the paper; L.S., F.S.C., Q.W., X.J. and K.M. recruited patients, collected patient material, and analyzed data; I.N. analyzed microarray data and reviewed the manuscript; H.W., M.H., and B.O. coordinated the study, analyzed and interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bob Olsson, Department of Psychiatry and Neurochemistry, University of Gothenburg, V-house, Sahlgrenska University Hospital Mölndal, SE-431 80 Mölndal, Sweden; e-mail: bob.olsson@medic.gu.se; and Ming Hou, Department of Haematology, Qilu Hospital, Shandong University, Jinan, China; e-mail: houming@medmail.com.cn.
References
Author notes
M.J. and Y.H. shared first authorship.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal