Key Points
T cells from patients with CLL exhibit features of T-cell exhaustion.
These findings exclude CMV as the sole cause of T-cell defects in CLL.
Abstract
T-cell exhaustion, originally described in chronic viral infections, was recently reported in solid and hematologic cancers. It is not defined whether exhaustion contributes to T-cell dysfunction observed in chronic lymphocytic leukemia (CLL). We investigated the phenotype and function of T cells from CLL patients and age-matched controls. CD8+ and CD4+ T cells from CLL patients had increased expression of exhaustion markers CD244, CD160, and PD1, with expansion of a PD1+BLIMP1HI subset. These molecules were most highly expressed in the expanded population of effector T cells in CLL. CLL CD8+ T cells showed functional defects in proliferation and cytotoxicity, with the cytolytic defect caused by impaired granzyme packaging into vesicles and nonpolarized degranulation. In contrast to virally induced exhaustion, CLL T cells showed increased production of interferon-γ and TNFα and increased expression of TBET, and normal IL2 production. These defects were not restricted to expanded populations of cytomegalovirus (CMV)–specific cells, although CMV seropositivity modulated the distribution of lymphocyte subsets, the functional defects were present irrespective of CMV serostatus. Therefore, although CLL CD8+ T cells exhibit features of T-cell exhaustion, they retain the ability to produce cytokines. These findings also exclude CMV as the sole cause of T-cell defects in CLL.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is associated with profound defects in T-cell function, resulting in failure of antitumor immunity and increased susceptibility to infections. We previously demonstrated global alterations in gene expression profiles of T cells from CLL patients compared with healthy controls, with down-regulation of genes involved in vesicle transport and cytoskeletal regulation.1 These changes in expression of cytoskeletal genes in T cells from CLL patients translate into a functional defect in immunologic synapse formation with antigen presenting cells (APCs).2 Furthermore, T cells from the Eμ-TCL1 transgenic CLL mouse model exhibit comparable changes in gene and protein expression, and T-cell function, to that seen in human CLL patients.3,4 A further feature of both the human disease and the mouse model is that there is an expansion of the number of circulating CD8+ T cells, which show evidence of chronic activation.3,5-7
T-cell exhaustion, a state of acquired T-cell dysfunction initially described in the context of chronic viral infections, was recently reported in hematologic malignancies, including adult T-cell leukemia/lymphoma, chronic myeloid leukemia, and acute myeloid leukemia.8-10 Gene expression profiling of exhausted CD8+ T cells reveals a distinct transcriptional state with similarities to the alterations in gene expression that we observed in CD8+ T cells in CLL patients, with down-regulation of cytoskeletal genes leading to impaired immunologic synapse formation and vesicle trafficking.11,12 In addition to the gene expression changes, the persistent stimulation by viral antigens leads to a hierarchical loss of effector CD8+ T-cell function, resulting in loss of proliferative capacity, impaired cytotoxicity, and reduced cytokine production. This exhausted state is also associated with increased expression of inhibitory receptors including programmed death-1 (PD1, CD279), CD160 (BY55), and CD244 (2B4).13
We hypothesized that chronic stimulation may result in T cells from patients with CLL becoming functionally “exhausted,“ similar to that reported in chronic viral infections. A major potential confounding factor is cytomegalovirus (CMV) seropositivity, known to influence the major lymphoid subsets in healthy individuals, with expanded populations of CMV-specific CD4+ and CD8+ T cells reported in CMV-seropositive (CMV+) CLL patients.14-17 Here we show that CD8+ T cells from patients with CLL exhibit defects in proliferation, cytotoxicity, and increased expression of inhibitory receptors, irrespective of CMV serostatus. These functional and phenotypic changes are also seen in CMV seronegative (CMV−) patients, thereby excluding CMV as the sole cause of the T-cell defect seen in CLL.
Methods
Patients
Peripheral blood samples were obtained from 39 CLL patients from the tissue bank maintained by the Department of Hemato-Oncology of St Bartholomew's Hospital, London, United Kingdom. Ethical approval was confirmed by the East London and The City Health Authority Local Research Ethics Committee, and written informed consent was obtained in accordance with the Declaration of Helsinki. All of the patients were untreated at time of blood withdrawal, and had a median age of 59 years (range 43-86). The patients had predominantly early stage CLL with 31/39 (79.5%) classed as having Binet stage A disease. Peripheral blood samples were also obtained from a control group of 20 healthy volunteers, who were age-matched with a median age of 61 years (range 49-72). The CMV serostatus of patients and healthy donors was determined by the Virology Department at the Royal London Hospital. 22/39 (56%) of patients and 13/20 (65%) of healthy donors were found to be CMV+.
Monoclonal antibodies
The following directly conjugated monoclonal antibodies (mAbs) were used in this study: CD3-Pacific Blue, CD3-PECy7, CD4-PECy7, CD4-eFluor780, CD8-PerCPCy5.5, CD107a-AlexaFluor647, CD127-FITC, CD160-AlexaFluor647, CD197-PE, CD197-APC, CD244-PE, CD244-APC, TBET-PE, IFNγ-FITC, CTLA4-PE, and TIM3-APC were all obtained from eBioscience. CD19-AlexaFluor700, CD45RA-FITC, CD122-PE, PD1-FITC, PD1-APC, IL2-PE, IL4-PE, and TNFα-FITC were all obtained from BD Bioscience. Blimp1-PE was obtained from Santa Cruz Biotechnology and LAG3-APC was obtained from R&D Systems. For confocal microscopy, unconjugated primary antibodies specific for CD107a and granzyme B were from Abcam, and Alexa Fluor 488 and 647–labeled goat anti–mouse IgG were from Life Technologies.
Isolation of PBMCs and lymphocyte subsets
Peripheral blood samples were diluted 1:1 with phosphate buffered saline (PBS) before separation of peripheral blood mononuclear cells (PBMCs) by density gradient centrifugation. Cells were washed in RPMI1640 supplemented with 10% fetal calf serum (FCS; PAA Laboratories) and 25 mg of gentamicin (Gibco), and either used immediately or frozen and stored in liquid nitrogen. CD8+ T cells were negatively selected using the CD8+ T-cell isolation kit (Miltenyi Biotec).
Immunofluorescence staining and flow cytometric analysis
For surface staining, PBMCs were washed twice in PBS containing 2% FCS (staining buffer). Cells were then incubated with directly conjugated mAbs for 30 minutes at 4°C. The cells were then washed and resuspended in staining buffer with 250 ng/mL DAPI (Invitrogen), and kept at 4°C until analysis. For staining of intracellular proteins, cells were washed in serum-free PBS before incubation with fixable viability dye eFluor780 (eBioscience) for 30 minutes at 4°C. Staining of surface antigens was performed as described. The cells were then fixed using IC fixation buffer (eBioscience) for intracytoplasmic staining or fixation/permeabilization solution (eBioscience) for intranuclear staining at room temperature for 20 to 30 minutes. The cells were then permeabilized using permeabilization buffer (eBioscience) before incubation with anti-cytokine or anti–transcription factor mAbs at room temperature for 20 to 30 minutes. Flow cytometry was performed on a BD Fortessa flow cytometer with subsequent analysis using FlowJo Version 8.8.7 software (TreeStar). Analysis was performed after gating on live singlet cells.
T-cell stimulation
For assessment of cytokine production, cells were stimulated with 50 ng/mL phorbol myristate acetate (PMA) and 1μM ionomycin (Sigma-Aldrich). For assessment of degranulation, cells were stimulated with 1 μg/mL staphylococcal enterotoxin B (SEB; Sigma-Aldrich) with the addition of 10 μL/mL anti–CD107a-AlexaFluor647.18 For both experiments the cell cultures were incubated for 1 hour before addition of 0.66 μL/mL Golgistop (BD Bioscience), before 4 hours further incubation at 37°C; 5% CO2. For assessment of proliferation, 1 × 107cells/mL were washed and stained with 5μM carboxyfluorescein succinimidyl ester (CFSE, eBioscience) for 10 minutes at 37°C. After quenching and further washing, the cells were stimulated with 1 μg/mL soluble anti-CD3 (clone HIT3a, BD Bioscience) and 5 μg/mL soluble anti-CD28 (clone CD28.2, eBioscience) for 72 hours.
Cytotoxicity assay
HLA-A*0201–expressing CD8+ cells were stimulated in vitro with dendritic cells pulsed with a CLL IgVH-derived peptide TLYLQMNSL weekly for 4 weeks, and killing of peptide-pulsed H2 cells was assessed. Target cells were labeled with 100μCi 51Cr (3.7MBq; NEN) and seeded in 96-well U-bottom microtiter plates at a concentration of 2.5 × 103/mL in triplicates. Effector cells were added in a ratio of 1:3, 1:10, and 1:30 and cocultured for 4 hours at 37°C; 5% CO2. After 4 hours the supernatants were harvested and the released 51Cr was measured in a γ-Counter (Wallac). Spontaneous release was determined by incubation of treated target cells in medium alone and maximum release was determined by resuspending the wells with 2% Triton X-100. Specific lysis was determined as previously described.2
Cell conjugation assays and confocal microscopy
Healthy B cells or CLL cells (1 × 106 cells) were stained with 7-amino-4-chloromethylcoumarin CellTracker Blue and pulsed with 2μg/mL of staphylococcal superantigen cocktail (Sigma-Aldrich) for 30 minutes at 37°C. B cells were centrifuged with an equal number of CD8+ T cells and incubated at 37°C for 10 minutes, plated onto poly-l-lysine–coated slides, and fixed for 15 minutes at room temperature with 3% formaldehyde. After fixation, the cells were permeabilized in 0.3% Triton (Sigma-Aldrich) for 5 minutes and blocked with 0.1% bovine serum albumin (Miltenyi Biotec) in PBS for 10 minutes. Primary and secondary antibodies were applied sequentially for 30 minutes at 4°C in 5% goat serum (Sigma-Aldrich). Filamentous actin (F-actin) was stained with rhodamine phalloidin (Invitrogen). Confocal microscopy was performed using a Zeiss LSM510 confocal laser-scanning microscope using a 63× objective.
Statistical analysis
All datasets were subject to normality testing using the Shapiro-Wilk normality test. Where all datasets could be accurately modeled by a Gaussian distribution an unpaired t test was used for analysis of differences between groups; where this was not the case the 2-sided Mann Witney U test was used. For comparison of 4 groups the Kruskal-Wallis test was used with Dunn posttest for multiple comparisons. P values of less than .05 were considered statistically significant.
Results
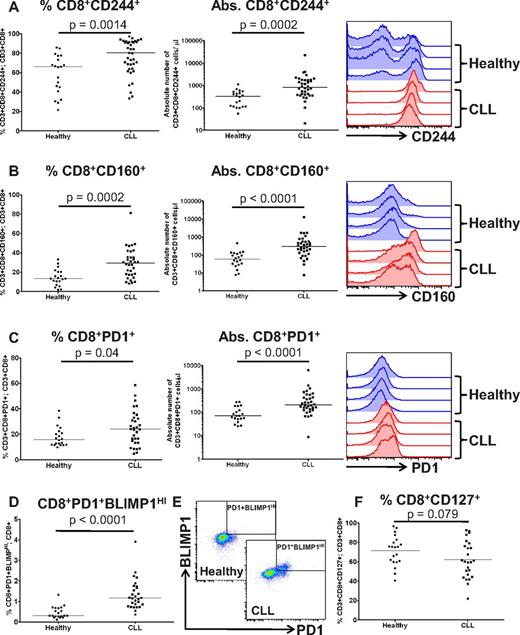
Increased expression of PD1, CD160, and CD244 on T cells from patients with CLL
Increased expression of CD160, CD244, and PD1 are key features of T-cell exhaustion.13 These molecules can act as negative regulators of lymphocyte activation when bound by their respective ligands, herpes virus entry mediator (HVEM), CD48, and programmed death ligand-1 (PDL1).19-21 We performed immunophenotyping of peripheral blood CD3+ T cells from patients with CLL compared with healthy controls, to determine whether they expressed these exhaustion markers. Compared with controls, there was a significant increase in both the percentage and absolute numbers of T cells from CLL patients expressing CD244, CD160 and PD1 (Figure 1A-C, supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). BLIMP1 has been shown to be important in driving CD8+ T-cell exhaustion in chronic viral infections.22 Expression of PD1 identified a subset of T cells with high expression of BLIMP1 (PD1+BLIMP1HI), that were expanded in both the CD3+CD8+ (P < .0001) and CD3+CD4+ (P < .0001) compartments in CLL (Figure 1D-E, supplemental Figure 1). Of note, we did not observe increased expression of CTLA4, TIM3, or LAG3 on T cells from CLL patients. A further feature of exhausted T cells in many chronic viral infections is down-regulation of CD127 (IL-7R), as the T cells lose responsiveness to homeostatic cytokines and become dependent on antigen for continued survival.23,24 However, we saw no statistically significant decrease in expression of CD127 on CLL CD8+ T cells compared with healthy controls (P = .079; Figure 1F).
Increased expression of PD1, CD160, and CD244 on CD8+ T cells from patients with CLL. The expression of (A) CD244, (B) CD160, and (C) PD1 were assessed on CD8+ T cells from patients with CLL and healthy age-matched controls. Significant increases in both the proportion, and the absolute number, of T cells expressing these molecules were observed. (D) PD1 expression identified a population of CD3+CD8+ T cells with high expression of BLIMP1, which are expanded in CLL. (E) Flow cytometric plots showing the presence of this CD3+CD8+PD1+BLIMP1HI population that is increased in CLL patients. (F) Expression of CD127 is not significantly down-regulated on CLL T cells.
Increased expression of PD1, CD160, and CD244 on CD8+ T cells from patients with CLL. The expression of (A) CD244, (B) CD160, and (C) PD1 were assessed on CD8+ T cells from patients with CLL and healthy age-matched controls. Significant increases in both the proportion, and the absolute number, of T cells expressing these molecules were observed. (D) PD1 expression identified a population of CD3+CD8+ T cells with high expression of BLIMP1, which are expanded in CLL. (E) Flow cytometric plots showing the presence of this CD3+CD8+PD1+BLIMP1HI population that is increased in CLL patients. (F) Expression of CD127 is not significantly down-regulated on CLL T cells.
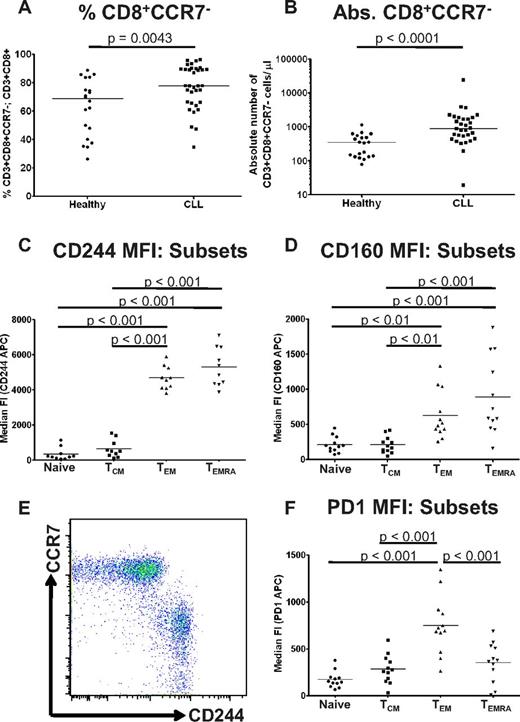
CD244, CD160, and PD1 are preferentially expressed on CD3+CD8+CCR7− effector T cells which are increased in CLL
Previous studies have shown skewing of the T-cell compartment toward activated T-cell subsets in CLL patients and in the Eμ-TCL1 mouse model.3,7 We therefore reasoned that the increased expression of CD244, CD160, and PD1 on CLL CD8+ T cells would be because of an increased proportion of exhausted effector T cells. Consistent with these previous reports,3,7 we found both an increased proportion and increased absolute numbers of CD3+CD8+CCR7− T cells in CLL patients compared with healthy controls (Figure 2A-B). We therefore investigated expression of CD160, CD244, and PD1 on T-cell subsets from CLL patients as defined by their expression of CCR7 and CD45RA,25 and defined cells as naive (CCR7+CD45RA+), central memory (TCM: CCR7+CD45RA−), effector memory (TEM: CCR7−CD45RA−) and CD45RA+ TEM cells (TEMRA). Expression of CD160 and CD244 increased as cells moved from naive to TEM phenotype (Figure 2C-E). In particular, expression of CD244 was inversely related to CCR7 expression (Figure 2E). In contrast, expression of PD1 was highest on TEM cells (Figure 2F) and decreased on TEMRA cells. Similar patterns of expression were seen on lymphocyte subsets from healthy individuals (supplemental Figure 2). These results indicate that the circulating T-cell compartment in CLL is shifted toward an increased proportion of phenotypically exhausted antigen-experienced T cells.
CD244, CD160, and PD1 are preferentially expressed on CD3+CD8+CCR7− effector T cells which are increased in CLL. (A) CLL patients had an increased proportion (A) and increased numbers (B) of CD3+CD8+CCR7− effector T cells. The relative expression of CD160, CD244, and PD1 was analyzed on CD8+ lymphocyte subsets from CLL patients as defined by coexpression of CCR7 and CD45RA. Quadrant gates were used to compare the MFI of CD160, CD244, and PD1 on CCR7+CD45RA+ naive cells, CCR7+CD45RA− TCM cells, CCR7−CD45RA− TEM cells, and CCR7−CD45RA+ TEMRA cells. Expression of CD244 (C) and CD160 (D) was increased on TEM cells, and was highest on TEMRA cells. (E) Expression of CD244 was virtually specific for a CCR7− phenotype. (F) In contrast, expression of PD1 was highest on TEM cells.
CD244, CD160, and PD1 are preferentially expressed on CD3+CD8+CCR7− effector T cells which are increased in CLL. (A) CLL patients had an increased proportion (A) and increased numbers (B) of CD3+CD8+CCR7− effector T cells. The relative expression of CD160, CD244, and PD1 was analyzed on CD8+ lymphocyte subsets from CLL patients as defined by coexpression of CCR7 and CD45RA. Quadrant gates were used to compare the MFI of CD160, CD244, and PD1 on CCR7+CD45RA+ naive cells, CCR7+CD45RA− TCM cells, CCR7−CD45RA− TEM cells, and CCR7−CD45RA+ TEMRA cells. Expression of CD244 (C) and CD160 (D) was increased on TEM cells, and was highest on TEMRA cells. (E) Expression of CD244 was virtually specific for a CCR7− phenotype. (F) In contrast, expression of PD1 was highest on TEM cells.
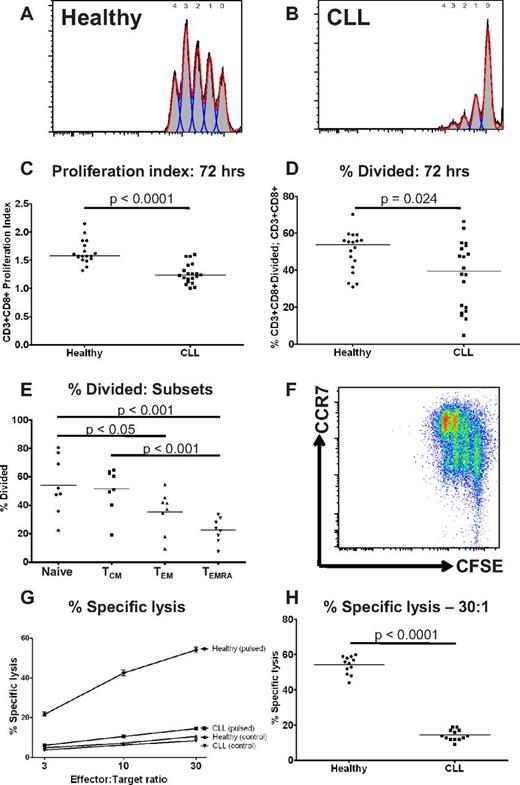
CD8+ T cells from patients with CLL show functional defects in proliferation and cytoxicity
T-cell exhaustion also results in progressive loss of T-cell function, including loss of proliferative and cytotoxic capacity.13 Therefore, we assessed the ability of CD8+ T cells from both CLL patients and controls to proliferate in response to stimulation by anti-CD3 and anti-CD28 for 72 hours, using a CFSE based assay.26 The proliferation index of CD3+CD8+ T cells from CLL patients was significantly lower than healthy controls (P < .0001; Figure 3A-C). There was also a reduction in the proportion of CD8+ T cells that had entered cell division (P = .024; Figure 3D). These findings were consistent with a skew toward CCR7− effector cells (TEM and TEMRA) which had lower proliferative capacity than naive and TCM cells (Figure 3E-F). This suggests that the proliferative defect seen in CLL CD8+ T cells is because of a combination of a reduction in the proportion of cells able to divide on polyclonal activation, and prolongation of the division time of the proliferating cells. Another feature of functional T-cell exhaustion is impairment of ex vivo target cell killing.27 Therefore, the ability of CD8+ T cells from CLL patients and healthy donors to lyse idiotype-pulsed target cells was assessed by a 51chromium release assay. In keeping with our previous observations, we observed impaired ability to induce idiotype-specific CD8+ T cells capable of killing idiotype-pulsed target cells compared with healthy donors.2,28 This defect in target cell lysis was observed at all effector-target cell ratios. (P < .0001 at effector-target ratio of 30:1; Figure 3G-H).
CD8+ T cells from patients with CLL show functional defects in proliferation and cytoxicity. The proliferative potential of CD8+ T cells from CLL patients and healthy controls was assessed using a CFSE based assay. Representative histograms are shown of (A) a healthy donor and (B) a CLL patient. (C) The proliferation index was significantly lower for CD8+ T cells from CLL patients compared with healthy controls. (D) There was also a reduction in the proportion of CD8+ T cells that had entered cell division (percentage divided) in CLL patients compared with healthy controls. The proliferative capacity of the various CD8+ lymphocyte subsets as defined by coexpression of CCR7 and CD45RA was assessed. (E-F) CD8+CCR7− T cells (TEM and TEMRA) had reduced proliferative capacity compared with CD8+CCR7− T cells (Naive and TCM). The cytotoxic activity of CD8+ T cells from patients and controls was assessed. CD8+ T cells from CLL patients fail to lyse idiotype-pulsed target cells in contrast to CD8+ T cells from healthy controls: percentage specific lysis at all effector target ratios (G), and at a effector-target ratio of 30:1 (H).
CD8+ T cells from patients with CLL show functional defects in proliferation and cytoxicity. The proliferative potential of CD8+ T cells from CLL patients and healthy controls was assessed using a CFSE based assay. Representative histograms are shown of (A) a healthy donor and (B) a CLL patient. (C) The proliferation index was significantly lower for CD8+ T cells from CLL patients compared with healthy controls. (D) There was also a reduction in the proportion of CD8+ T cells that had entered cell division (percentage divided) in CLL patients compared with healthy controls. The proliferative capacity of the various CD8+ lymphocyte subsets as defined by coexpression of CCR7 and CD45RA was assessed. (E-F) CD8+CCR7− T cells (TEM and TEMRA) had reduced proliferative capacity compared with CD8+CCR7− T cells (Naive and TCM). The cytotoxic activity of CD8+ T cells from patients and controls was assessed. CD8+ T cells from CLL patients fail to lyse idiotype-pulsed target cells in contrast to CD8+ T cells from healthy controls: percentage specific lysis at all effector target ratios (G), and at a effector-target ratio of 30:1 (H).
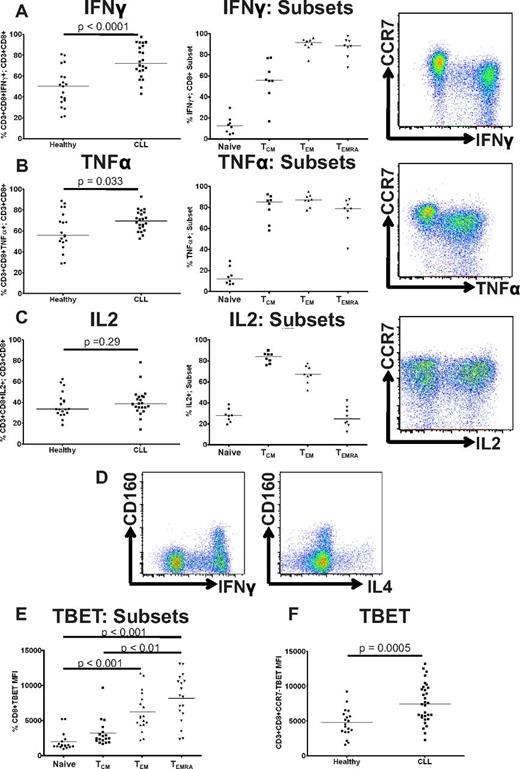
CD8+ T cells from CLL patients show increased production of IFNγ and TNFα, and increased expression of TBET, but normal production of IL2
A further feature of functional T-cell exhaustion during chronic viral infections is failure to produce effector cytokines. This happens in a hierarchical manner, with the ability to produce IL2 being lost at early stages of exhaustion, followed by loss of TNFα production and finally IFNγ.29 We therefore analyzed cytokine production after stimulation with PMA and ionomycin, and demonstrate that in contrast to the pattern observed in chronic viral infections, CD3+CD8+ T cells from CLL patients had increased production of IFNγ (P < .0001) and TNFα (P = .033; Figure 4A-B), without any reduction in IL2 production (P = .29; Figure 4C) compared with healthy controls. Once again this reflected the increased numbers of TEM and TEMRA cells, which produced more IFNγ and TNFα than naive and TCM cells (Figure 4A-C). CD160 expression is usually rapidly down-regulated after PMA stimulation in vitro.30 However, we noted relative maintenance of CD160 expression on CD3+CD8+ cells in some CLL patients, which occurred on IFNγ-producing rather than IL4-producing cells (Figure 4D). The transcription factor TBET plays a crucial role in T-cell development, acting as a master controller of Th1/Tc1 differentiation and as a driver of effector memory differentiation.31,32 TBET expression was higher in TEM and TEMRA cells than in naive or TCM cells (Figure 4E). To avoid the confounding effect of the skew toward effector cell subsets, we examined TBET expression in CD3+CD8+CCR7− T cells from patients with CLL compared with healthy controls, and noted increased TBET expression in CD3+CD8+CCR7− T cells from CLL patients (P = .0005; Figure 4F). Taken together, these findings are consistent with up-regulation of TBET driving CLL CD8+ T cells to differentiate into effector cells with a type-1 cytokine profile.
CD8+ T cells from CLL patients show increased production of IFNγ and TNFα, and increased expression of TBET, but normal production of IL2. Cytokine production by CD8+ T cells was assessed by intracytoplasmic staining for IFNγ, TNFα, and IL2 after stimulation with PMA and ionomycin. CD8+ T cells from CLL patients showed increased production of IFNγ (A) and TNFα (B) reflecting the global skew toward CD8+CCR7− subsets (TEM and TEMRA). (C) In contrast, there was no significant difference in the production of IL2 by CD8+ T cells from patients and controls. (D) CD160 was expressed on CD8+ T cells that produced IFNγ but not on cells producing IL4, consistent with its expression being restricted to Tc1 cells. (E) The expression of TBET in CD8+ T-cell subsets from CLL patients was analyzed as defined by coexpression of CCR7 and CD45RA as before. The expression of TBET was increased in TEM cells, with the highest expression levels in the TEMRA subset. (F) CD3+CD8+CCR7− T cells from patients with CLL show significantly increased TBET expression compared with controls.
CD8+ T cells from CLL patients show increased production of IFNγ and TNFα, and increased expression of TBET, but normal production of IL2. Cytokine production by CD8+ T cells was assessed by intracytoplasmic staining for IFNγ, TNFα, and IL2 after stimulation with PMA and ionomycin. CD8+ T cells from CLL patients showed increased production of IFNγ (A) and TNFα (B) reflecting the global skew toward CD8+CCR7− subsets (TEM and TEMRA). (C) In contrast, there was no significant difference in the production of IL2 by CD8+ T cells from patients and controls. (D) CD160 was expressed on CD8+ T cells that produced IFNγ but not on cells producing IL4, consistent with its expression being restricted to Tc1 cells. (E) The expression of TBET in CD8+ T-cell subsets from CLL patients was analyzed as defined by coexpression of CCR7 and CD45RA as before. The expression of TBET was increased in TEM cells, with the highest expression levels in the TEMRA subset. (F) CD3+CD8+CCR7− T cells from patients with CLL show significantly increased TBET expression compared with controls.
The impact of CMV serostatus on the distribution of subsets and expression of CD244, CD160, and PD1
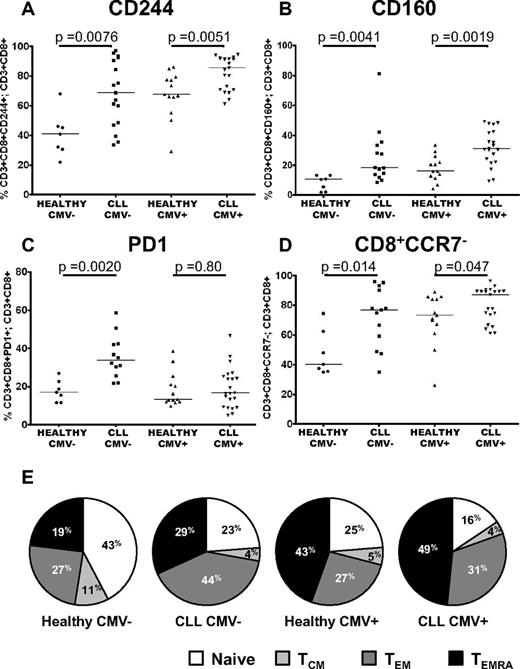
CMV seropositivity is known to have a profound influence on the major lymphoid subsets in healthy individuals, with expanded populations of CMV specific CD4+ and CD8+ T cells reported in CMV+ CLL patients.14-17 Because T-cell exhaustion has been extensively described in chronic viral infections, it was important to assess the effect of this in CLL. Expression of CD244 and CD160 was increased in CLL patients compared with healthy controls irrespective of CMV-serostatus (Figure 5A-B). In contrast, as shown in Figure 5C, PD1 expression was increased in CLL patients only in CMV− individuals (P = .002). Further investigation of the distribution of lymphocyte subsets provided an explanation for these findings. Consistent with previous studies we show that CCR7− effector cells are expanded in CMV+ CLL patients and healthy donors. However we also noted that these cells were increased in CMV− CLL patients (Figure 5D). To examine this in more detail, we compared naive, TCM, TEM and TEMRA cells in CLL patients and controls separately for CMV+ and CMV− individuals. Importantly, CMV serostatus affected the balance of the TEM/TEMRA subsets, with CMV seropositivity skewing CD8+ T-cell differentiation from a naive to a TEMRA phenotype in both patients and healthy controls. In contrast, in CMV− CLL patients, the increased proportion of CD8+CCR7− effector cells primarily reflected an expansion of TEM cells (Figure 5E). These alterations in the distribution of lymphocyte subsets account for the observation that PD1 expression was only significantly increased in CMV− CLL patients, as the expanded TEM cells seen in these patients had increased expression of PD1. In contrast, in CMV+ CLL patients there was no global increase in PD1 expression, because there was expansion of TEMRA cells that have lower expression of PD1. Expression of PD1 was increased on CD3+CD4+ T cells from CLL patients irrespective of CMV serostatus, as the equivalent TEMRA subset is not present in the CD3+CD4+ compartment (supplemental Figure 4).
The impact of CMV serostatus on the distribution of subsets and expression of CD244, CD160, and PD1. The proportion of T cells expressing PD1, CD160, and CD244 were measured by flow cytometry in patients with CLL and healthy controls matched for CMV serostatus. Significant increases in the expression of CD244 (A) and CD160 (B) were observed independently of CMV serostatus. (C) In contrast, expression of PD1 was only increased comparing CMV− patients and controls. (D) CD3+CD8+CCR7− cells are expanded in CLL irrespective of CMV serostatus. (E) There is a reduction in naive and central memory cells because of both CLL and CMV seropositivity. The presence of CLL skews the T-cell repertoire toward a TEM phenotype, whereas the presence of CMV leads to an expansion of TEMRA cells.
The impact of CMV serostatus on the distribution of subsets and expression of CD244, CD160, and PD1. The proportion of T cells expressing PD1, CD160, and CD244 were measured by flow cytometry in patients with CLL and healthy controls matched for CMV serostatus. Significant increases in the expression of CD244 (A) and CD160 (B) were observed independently of CMV serostatus. (C) In contrast, expression of PD1 was only increased comparing CMV− patients and controls. (D) CD3+CD8+CCR7− cells are expanded in CLL irrespective of CMV serostatus. (E) There is a reduction in naive and central memory cells because of both CLL and CMV seropositivity. The presence of CLL skews the T-cell repertoire toward a TEM phenotype, whereas the presence of CMV leads to an expansion of TEMRA cells.
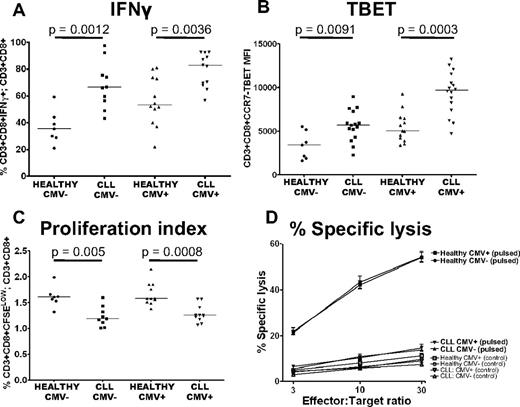
The defects in T-cell function seen in CLL are present irrespective of CMV serostatus
The description of expanded populations of CMV-specific T cells in CLL raised the question as to whether the observed defects in T-cell function are because of CMV.33 We therefore examined the impact of CMV serostatus on proliferation, cytolytic activity, and cytokine production of CD8+ T cells from CLL patients. The proportion of CD3+CD8+ T cells that were positive for IFNγ was increased in CLL patients irrespective of CMV serostatus (Figure 6A). TBET expression in CD3+CD8+CCR7− T cells was also increased in both CMV− (P = .0091) and CMV+ (P = .0003) patients compared with controls (Figure 6B). The defects in proliferative capacity and cytotoxic activity of CD8+ T cells from CLL patients were also observed irrespective of CMV serostatus (Figure 6C-D). These findings exclude CMV seropositivity as the sole cause of functional defects in CLL T cells.
The defects in T-cell function seen in CLL are present irrespective of CMV serostatus. The function of CLL CD8+ T cells was compared with CD8+ T cells from healthy controls matched for CMV serostatus. Increased production of IFNγ (A), increased expression of TBET (B), decreased proliferative capacity (C), and decreased cytolytic ability (D) of CLL T cells were all found irrespective of CMV serostatus. Pulsed target cells (closed symbols); unpulsed control target cells (open symbols). The graph representing percentage-specific lysis shows the mean and standard error of results obtained from 13 CLL patients, (5 CMV− and 8 CMV+), and 12 healthy controls (5 CMV− and 7 CMV+).
The defects in T-cell function seen in CLL are present irrespective of CMV serostatus. The function of CLL CD8+ T cells was compared with CD8+ T cells from healthy controls matched for CMV serostatus. Increased production of IFNγ (A), increased expression of TBET (B), decreased proliferative capacity (C), and decreased cytolytic ability (D) of CLL T cells were all found irrespective of CMV serostatus. Pulsed target cells (closed symbols); unpulsed control target cells (open symbols). The graph representing percentage-specific lysis shows the mean and standard error of results obtained from 13 CLL patients, (5 CMV− and 8 CMV+), and 12 healthy controls (5 CMV− and 7 CMV+).
CD8+ T cells from CLL patients show defective cytotoxicity because of failure of granzyme localization to the immunologic synapse
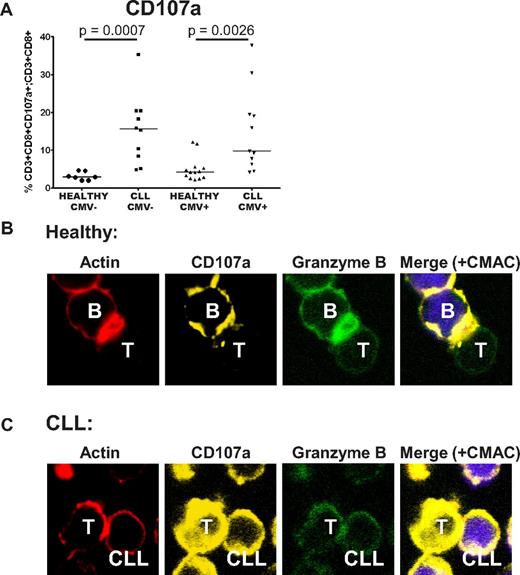
We previously demonstrated that CLL T cells exhibit impaired actin polymerization and defective immunologic synapse formation with APCs. Therefore, we sought to examine the impact of dysfunctional immunologic synapse formation on the observed cytolytic defects. The ability of the CD8+ T cells to degranulate in response to SEB was assessed by analysis of CD107a (LAMP1) transfer to the cell surface.18 CD8+ T cells from CLL patients retained the ability to degranulate, and actually showed enhanced transfer of CD107a to the cell surface, again irrespective of CMV serostatus (Figure 7A). Confocal microscopic analysis revealed that although normal T cells were able to localize F-actin, CD107a and granzyme B to the immunologic synapse (polarization), there was failure of transport of these molecules within CLL T cells, irrespective of their level of expression (Figure 7B-C). These data suggest that the cytotoxic defect seen in CLL T cells is because of a combination of disordered, nonpolarized degranulation, and is consistent with failure of effective granzyme B packaging into the cytolytic vesicles.
CD8+ T cells from CLL patients show defective cytotoxicity because of failure of granzyme localization to the immunologic synapse. The cytotoxic activity of CD8+ T cells from CLL patients was assessed further. (A) CD8+ T cells from CLL patients retain the ability to degranulate in response to SEB, as shown by their increased ability to transfer CD107a to the cell surface. (B) Healthy CD8+ T cells showed effective F-actin (red, rhodamine phalloidin) immunologic synapse formation with colocalization of CD107a (yellow, Alexa Fluor 647) and granzyme B (green, Alexa Fluor 488) at the synapse contact site with healthy B cells (+sAg). (C) In contrast, CD8+ T cells from CLL patients fail to form effective F-actin immune synapses with autologous CLL cells (+sAg) that is associated with strong, nonpolarized expression of CD107a and a lack of granzyme B polarization to the contact site.
CD8+ T cells from CLL patients show defective cytotoxicity because of failure of granzyme localization to the immunologic synapse. The cytotoxic activity of CD8+ T cells from CLL patients was assessed further. (A) CD8+ T cells from CLL patients retain the ability to degranulate in response to SEB, as shown by their increased ability to transfer CD107a to the cell surface. (B) Healthy CD8+ T cells showed effective F-actin (red, rhodamine phalloidin) immunologic synapse formation with colocalization of CD107a (yellow, Alexa Fluor 647) and granzyme B (green, Alexa Fluor 488) at the synapse contact site with healthy B cells (+sAg). (C) In contrast, CD8+ T cells from CLL patients fail to form effective F-actin immune synapses with autologous CLL cells (+sAg) that is associated with strong, nonpolarized expression of CD107a and a lack of granzyme B polarization to the contact site.
Discussion
In this study we investigated the nature of the T-cell defect in CLL. We demonstrate that T cells from CLL patients exhibit features of T-cell exhaustion, an acquired state of T-cell dysfunction first described in the context of chronic viral infections.29 CLL CD4+ and CD8+ T cells show increased expression of the exhaustion markers, CD244, CD160, and PD1, with expansion of a PD1+ T-cell subset with high expression of Blimp1, a transcription factor implicated in the development of exhaustion.22 These molecules are more highly expressed on effector T cells, and the increase in their expression in CLL correlates with a skewing of the T-cell subsets toward effector differentiation. CD8+ T cells from CLL patients also show functional evidence of exhaustion, with impaired proliferative capacity and reduced ability to lyse target cells. The principal way in which CLL T cells differ from the exhausted T-cells described in chronic viral infections is that they retain the capacity to produce cytokines, such as IFNγ and TNFα. This may reflect differences in antigen affinity, with chronic stimulation by high affinity viral antigens leading to a spectrum of functional disturbances distinct to that seen after chronic stimulation by low-affinity self-antigens. It is also possible that the exhaustion pathways are co-opted by CLL cells to inhibit immune responses, but to maintain production of cytokines, such as IFNγ and TNFα, which have been shown to be protumoral.34,35 Interestingly, the phenotypic and functional profile reported here mirrors that of senescent T cells induced by regulatory T cells.36 Regulatory T cells are expanded in CLL, with numbers correlating with disease stage and prognosis, so it is probable that this T-cell subgroup regulates antitumor immune responses in CLL.37-39
Since the first description of expanded populations of CMV specific T cells in CLL, it has been unclear as to the contribution of these populations to the T cell defects observed in this disease.16,17 It has been suggested that these CMV specific T cells inhibit the function of other antigen-specific T-cell populations, either by a direct effect, or by constriction of the total T-cell repertoire.33 In this report, we show that although CMV positivity modulated the distribution of lymphocyte subsets, the functional defects were present irrespective of CMV serostatus, thereby excluding CMV as the sole cause of T-cell defects in CLL. The modulation of lymphocyte subset distribution accounts for some previously reported differences regarding the expression of PD1 on CLL T cells.40,41 Using both CMV serostatus and expression of CCR7/CD45RA we were able to disentangle the impact of CLL and CMV on the distribution of CD8+ T-cell subsets more clearly. In agreement with previous studies we found that TEMRA cells were only significantly expanded in CMV+ patients and controls16,41 ; however, we also observed that the presence of CLL alone skews the T-cell repertoire toward a TEM phenotype. Interestingly, TEMRA cells are “classically exhausted,” with the highest expression of CD244 and CD160, the lowest proliferative capacity, and loss of IL2 production. Both CLL and CMV exposure lead to a reduction in naive and TCM cell numbers compared with healthy CMV− controls.
Given the importance of BCR-signaling in CLL, there has been a great deal of speculation as to the identity of other antigens that may drive this disease.42 However a recent landmark paper has provided evidence that a particular feature of CLL BCRs is that they are able to induce autonomous signaling independent of antigen, in a manner dependent on the heavy-chain complementarity-determining region (HCDR3) and an internal epitope of the BCR.43 A further finding was that BCRs derived from leukemic cells in the TCL1 mouse model of CLL were also autonomously active. The development of leukemia in this mouse model is also associated with an expansion of CD8+ effector T cells, despite being dependent on the single TCL1 transgene. It is therefore possible that the “pseudoexhausted” state of CLL T cells described in this report is because of their chronic stimulation by autonomously active CLL cells.
The increased expression of inhibitory receptors on CLL T cells could also be expected to contribute to impaired T-cell function. The up-regulation of CD160 and PD1 is of particular interest, given recent reports documenting mutations/gene fusions involving their respective ligands PDL1 and HVEM in other B-cell malignancies.44,45 We recently demonstrated that these ligands are overexpressed and mediate T-cell synapse dysfunction in CLL, and these pathways are also important in blocking CD8+ cytotoxic function, as CLL CD8+ T cells retain the ability to degranulate, but lack cytolytic function.46 However, these cytoskeletal defects result in disordered and nonpolarized degranulation, and failure of granzyme B colocalization with CD107a, consistent with defective packaging of cytolytic molecules into secretory vesicles.
In conclusion, we identified a novel phenotypic and functional profile of terminally differentiated CD8+ T cells in patients with CLL. These pseudoexhausted T cells may be a result of chronic stimulation by low-affinity self-antigens, producing a state that is distinct to the exhaustion seen after chronic stimulation by high affinity viral antigens. They retain some functions, such as the ability to produce protumoral cytokines and the ability to degranulate, but their effector cytotoxic function is blocked by cytoskeletal defects. These are probably because of a combination of their pseudoexhausted state, and increased interaction with inhibitory ligands expressed on the surface of the CLL cells.11,46 The up-regulation of CD244, CD160, and PD1 is of translational relevance, as these molecules may be useful biomarkers of immune reconstitution, and be potential targets for therapeutic strategies aimed at improving T-cell immunity. Although CMV seropositivity has profound effects on the T-cell repertoire of both healthy individuals and CLL patients, these alterations in T-cell phenotype and function are also apparent in CMV− patients.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from CR-UK (to J.C.R. and J.G.G.), the European Hematology Association (A.G.R.), MRC (J.K.D.), and by funding from the National Cancer Institute (P01 CA95426; J.G.G.) for the CLL Research Consortium and from Goldman Sachs (to J.C.R. and J.G.G.).
National Institutes of Health
Authorship
Contribution: J.C.R. designed, performed, and analyzed the experiments, collected the control samples, and wrote the paper; J.K.D. designed and analyzed the experiments and wrote the paper; F.M. and R.F. performed and analyzed the experiments; S.I. and S.A. cared for the patients, coordinated the collection of the patients samples, interpreted the data, and wrote the paper; A.G.R. designed and analyzed the experiments, wrote and edited the paper, and supervised the study; and J.G.G. designed the experiments, interpreted the data, wrote and edited the paper, and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John C. Riches, Barts Cancer Institute, Queen Mary University of London, 3rd Floor John Vane Science Centre, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: johnriches@doctors.org.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal