Key Points
Anti–IFN-γ autoantibodies are associated with HLA-DRB1*16:02 and DQB1*05:02.
Abstract
Adult patients with disseminated nontuberculous mycobacterial (dNTM) infections usually have severe immune system defects. Recently, several studies have shown that anti–IFN-γ autoantibodies may play an important role in the pathogenicity of dNTM infections. A considerable proportion of reported cases of anti–IFN-γ autoantibodies show either clinical or laboratory evidence of autoimmune disease. In the present study, we identified 19 formerly healthy adults who later developed dNTM infections, of whom 17 were further investigated immunologically. High-titer anti–IFN-γ autoantibodies capable of inhibiting IL-12 production in vitro were found in the plasma of all of these patients. In addition to dNTM infection, 35% and 71% of our patients also suffered from salmonellosis and herpes zoster, respectively. This observation suggests that IFN-γ may be crucial in controlling salmonella infection and reactivating latent varicella-zoster virus infection in humans. 2 HLA alleles, DRB1*16:02 DQB1*05:02 (odds ratio = 8.68; 95% confidence interval, 3.47-21.90; P = 1.1 × 10−6; Pc = 3.08 × 10−5 and odds ratio = 7.16; 95% confidence interval, 3.02-17.05; P = 1 × 10−7; Pc = 1.4 × 10−6, respectively), were found in 82% (14 of 17) of our patients. In conclusion, our data suggest that anti–IFN-γ autoantibodies may play a critical role in the pathogenesis of dNTM infections and reactivation of latent varicella-zoster virus infection and are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02.
Introduction
The nontuberculous mycobacteria (NTM) include a variety of species that are widely distributed in the environment. These mycobacteria are usually less virulent in humans than Mycobacterium tuberculosis (tuberculosis). Recently, infections caused by NTM have been increasingly recognized, particularly in persons with malignancy, chronic renal disease, or AIDS.1-3 Except for localized infections, diseases caused by NTM, particularly those manifested as disseminated infections, are rarely encountered in immunocompetent subjects.1,2
Anticytokine autoantibodies have recently been demonstrated to be important in disease pathogenesis and to be associated with various microorganisms.4-9 In cases with anti–IFN-γ autoantibody expression, disseminated NTM (dNTM) infection is the most common clinical presentation.7-13 The majority of patients with anticytokine autoantibodies have associated comorbidities, either neoplastic or autoimmune.4,9,14,15 These comorbidities and their associated therapies may render patients more susceptible to infectious diseases. Nevertheless, the role of anticytokine autoantibodies in the pathogenesis of infectious diseases in healthy subjects remains unclear.
Since their discovery in the 1970s, the HLA genes have been linked to more than 100 diseases, most of which are autoimmune diseases.16,17 Increased susceptibility to infectious diseases has also been positively associated with certain HLA alleles.17 The majority of patients reported to have anti–IFN-γ autoantibodies are of Asian descent, which may suggest the involvement of a common genetic factor.9 However, no linked HLA gene(s) has been identified among these patients to date.
In the present study, we describe several important findings. First, a high prevalence (100%) of anti–IFN-γ autoantibodies was observed among previously healthy Chinese adults who later suffered from dNTM infections. Second, one-third of the patients were coinfected with salmonellosis and three-fourths suffered from reactivated latent varicella-zoster virus (VZV) infection. Third, 2 HLA alleles, DRB1*16:02 and DQB1*05:02, were found at an unexpectedly high frequency among our patients.
Methods
Patients and definitions
All of the participants were adults (age more than 18 years) and were followed regularly at medical centers in Taiwan. Disseminated mycobacterial infection was diagnosed in patients presenting with 2 or more noncontiguous sites and positive blood or BM cultures. The diagnosis of pulmonary NTM infection was based on the criteria proposed by the American Thoracic Society and Infectious Diseases Society of America.1 Patients with one or more of the following conditions were excluded: pulmonary coinfection with NTM and tuberculosis, HIV infection, systemic lupus erythematosus, rheumatoid arthritis, malignancy, a serum creatinine level greater than 1.5 mg/dL, diabetes mellitus, liver cirrhosis, congenital immunodeficiency, and treatment with immunosuppressive or immunomodulation therapy, including steroids. The study was approved (DMR98-IRB-261) by the Institutional Review Board of China Medical University Hospital and informed written consent was obtained from all of the patients in accordance with the Declaration of Helsinki.
Microbiologic methods
All of the clinical specimens were collected and processed according to the methods recommended by Pfyffer et al.18 In brief, after collection and treatment, the specimens were incubated in Middlebrook 7H9 broth in the Mycobacteria Growth Indicator Tube system (Becton Dickinson Microbiology Systems) at 35°C under an atmosphere containing 5% CO2 for 2-3 weeks. Mycobacterial growth was detected by a fluorescence quenching–based oxygen sensor. Mycobacterial species identification was based on criteria proposed by Brown-Elliott and Wallace19 and Vincent and Gutierrez.20 Live Mycobacterium bovis Bacillus Calmette-Guérin (BCG; Pasteur substrain) were cultivated under similar conditions; later, these microorganisms were used in experiments to stimulate whole blood (WB) and PBMCs from patients and healthy donors.
Preparation and activation of WB and PBMCs
Blood samples from patients and from healthy donors were collected into sterile heparinized tubes and mixed with an equal volume of RPMI 1640 (Gibco-BRL) supplemented with 100 U/mL of penicillin (Invitrogen) and 100 μg/mL of streptomycin (Invitrogen). The mixture was dispensed into 6 wells (1 mL/well) of a 24-well plate and incubated for 48 hours at 37°C under an atmosphere containing 5% CO2. PBMCs were obtained by Ficoll-Paque (GE Healthcare) density-gradient centrifugation of heparinized WB and then cultured at 1 × 106 cells/mL in a complete RPMI 1640 medium containing 10% heat-inactivated FCS. Four different conditions were used for the WB and PBMC activation: (1) medium only, (2) live BCG alone, (3) live BCG and recombinant IFN-γ (250 ng/mL; R&D Systems), and (4) live BCG and recombinant IL-12 (20 ng/mL; R&D Systems). After a 48-hour incubation, 500 μL of supernatant was collected from each well and stored at −80°C for cytokine determination.
Detection of WB and PBMC cytokine production
Cytokine concentration was measured with an ELISA using human IL-12p40, human IL-6, and human IFN-γ kits from BD Biosciences according to the manufacturer's recommendations. The optical density was determined using a Spectra Max M2 ELISA reader (Molecular Devices). All experiments were performed in duplicate.
Detection and titration of IFN-γ–neutralizing factors
Plasma from patients and donors was serially diluted (10−1 to 10−6) and incubated with recombinant human IFN-γ at a final concentration of 100 pg/mL for 1 hour at 37°C under an atmosphere containing 5% CO2. The IFN-γ level was measured with a human IFN-γ ELISA kit from BD Biosciences according to the manufacturer's recommendations. All experiments were performed in duplicate.
Identification of anti–IFN-γ autoantibodies in patient plasma
A 96-well microplate was precoated with 100 μL of anti–human IFN-γ Ab/well (51-26131E; BD Biosciences) and incubated overnight at 4°C. The plate was washed 3 times with PBS-Tween 0.05% and then blocked by incubation with PBS-5% human serum albumin for 2 hours. The IFN-γ–binding sites were saturated with 5 μg/mL of recombinant IFN-γ during an overnight incubation at 4°C. The plate was washed again, serially diluted serum from patients and healthy donors (dilutions: 1:32, 1:128, and 1:1024) was added, and the plate was incubated overnight at 4°C. The plate was thoroughly washed and Fc-specific peroxidase-conjugated AffiniPure mouse anti–human IgG (Jackson ImmunoResearch Laboratories) was added to a final concentration of 40 ng/mL. The plate was incubated for 1 hour at room temperature and then washed 5 times with PBS-Tween 0.05%. The substrate solution (100 μL/well) was added and the plate was incubated for 30 minutes at room temperature.
HLAs: typing for HLA-A, HLA-B, HLA-DRB1, and HLA-DQB1
HLA-A and HLA-B polymorphisms in exon 2, exon 3, and exon 4 of the target genes were determined using a sequence-based typing (SBT) method.21,22 Genotype ambiguity was resolved by amplifying the specific alleles with group-specific primers.23,24 The amplification mixture (12 μL) contained 30-100 ng of genomic DNA, 1 × PCR buffer (67mM Tris and 16mM ammonium sulfate, pH 8.8), 200μM of each dNTP, 5% DMSO, 0.1 mg/mL of Cresol red, and 0.3 U of Taq DNA polymerase (ABgene House). The PCR cycling conditions consisted of a pre-PCR denaturing step at 94°C for 2 minutes, followed by 10 touchdown cycles (94°C for 30 seconds, followed by a decreasing temperature cycle of 0.4°C per second ranging from 65°C to 61°C with final annealing for 30 seconds and 72°C for 90 seconds), and then 28 extension cycles (94°C for 30 seconds, 61°C for 30 seconds, and 72°C for 90 seconds), and a final extension of 5 minutes at 72°C. DRB1 and DQB1 polymorphisms were evaluated using the SBT method. Briefly, the second exons of DRB1 and DQB1 were amplified by group-specific primers located in the flanking introns.25,26 The same PCR conditions were the same as those used for HLA-A and HLA-B except that the 90-second PCR extension times were reduced to 45 seconds.
The PCR products were visualized using a 2% agarose gel electrophoresis. Positive PCR products were subsequently treated with exonuclease I and shrimp alkaline phosphatase (PCR product presequencing kit; USB Corporation) to remove excess dNTPs and leftover primers. Sequencing reactions were conducted using the Big Dye Terminator Version 3.1 cycle sequencing kit (Applied Biosystems) in the forward and reverse directions with exon-specific sequencing primers. The sequencing was run on an automated ABI 3730 sequencer (Applied Biosystems) and analyzed using Assign-SBT 3.5.1 software (Connexio Genomics). A 4-digit designation using the new naming format was used to assign HLA types.27 The present patients were compared with a control population of 102 Minnan patients (the results of this comparison were presented at the 13th International Histocompatibility Workshop28 ). The Minnan population is the major group of Han Chinese in Taiwan. They are the descendants of early settlers who emigrated to Taiwan 400 years ago from the FuJian province of East China. All patients were unrelated and self-identified as Minnan.
Flow cytometry
For cell-surface marker staining, 200 μL of peripheral blood was incubated with FITC-, PE-, or PerCP-conjugated antibodies for 30 minutes at room temperature in the dark. The stained samples were treated with 1× FACS Lysis Solution (BD Biosciences) and then washed 3 times with PBS. The cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences) and data analysis was performed using the standard Cell Quest 4.0.2 software. Specific antibodies used in flow cytometric experiments included mouse anti–human CD3 FITC or PerCP (SK7; BD Biosciences), mouse anti–human CD4 FITC or PerCP (ski3; BD Biosciences), mouse anti–human CD5 FITC (UCHT2; BD Pharmingen), mouse anti–human CD8 PE (SK1; BD Biosciences), mouse anti–human CD14 PE (MψP9; BD Biosciences), mouse anti–human CD19 PE (4G7; BD Biosciences), mouse anti–human CD3/CD56 + 16 (SK7/B73.1/MY31; BD Biosciences), mouse anti–human CD56 FITC (NCAM16.2; BD Biosciences), mouse IgG1/IgG2a isotype control (X39/X40; BD Simultest), and anti–mouse IgG1 control PerCP (X40; BD Biosciences). All of the antibodies were used at the concentrations suggested by the manufacturers.
Statistical analysis
HLA allele frequencies were calculated by direct counting. The allelic odds ratio (OR), 95% confidence interval (CI) and 2-tailed P values were obtained using StatCalc (EpiInfo Version 6.0; Centers for Disease Control and Prevention). The corrected P values (Pc) were calculated by multiplying the P values by the number of alleles represented in the samples (according to the Bonferroni inequality method). A value of Pc < .05 was considered significant.
Results
Clinical presentations and outcomes of patients with dNTM infections
Nineteen dNTM patients were identified, 2 of whom died before further investigation was possible; therefore, 17 patients were included in the present study. The patient demographics, laboratory data, clinical diagnoses, and outcomes are summarized in Table 1. Nine patients (53%) were male and the average age was 60 years (range, 47-87). During the course of the disease, most of the patients presented with clinical manifestations 1-5 years before receiving a diagnosis of dNTM. In an extreme case, patient 3 had suffered from recurrent pneumonia of unknown etiology since age 18. Lymphadenopathy, particularly of the cervical nodes, was the most common clinical presentation and was observed in 15 patients. Twelve patients (71%) had osteomyelitis and 10 also had lymphadenopathy. Eight patients had associated pulmonary infections and only one patient had muscular involvement. More than one-third of patients (6 of 17) had various cutaneous lesions ranging from nonspecific sterile pustules to cellulitis to erythema induratum. Both rapid-growing and slow-growing mycobacteria were isolated from our patients (Table 1); furthermore, 10 patients had concomitant infections with Salmonella enteritidis (6 patients), Staphylococcus aureus (1 patient), Penicillium marneffei (2 patients), Cryptococcus neoformans (1 patient), and legionellosis (1 patient). Intriguingly, during the course of the disease, more than half of our patients (12 cases) suffered from reactivation of latent VZV infection. Five patients (patients 1, 5, 7, 8, and 9) were free from active disease and did not receive any antimicrobial agents, but the remainder had persistent infections and received long-term antibiotic suppression therapy.
Demographic data and clinical manifestations of patients with disseminated nontuberculous mycobacterial infections
| Patient . | Age, y/sex . | Onset . | Affected sites . | Pathogen(s) . | Laboratory data . | Diagnosis . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 55/M | 50 y | Lymph node, multiple bone sites | Mycobacterium abscessus, S enteritidis B, P marneffei | WBC:9450 (64.6/24.3/7.9/3.1/0.1), HIV (−), ANA (−), RA < 20, SSA (−), SSB (−), C3/C4:199/74.5, C-ANCA (−), P-ANCA (−), Coomb test (−), IgG/A/M: 1980/159/65.8, HBsAg (−), HCV (−), HLA-B27 (−) no chromosome abnormality, thyroid function (−) | Chronic noncaseating granulomatous lymphadenitis, osteomyelitis, Salmonella sepsis, herpes zoster | Survival with no active disease |
| 2 | 51/F | 47 y | Cervical, mediastinal, and axillary lymph nodes, multiple bone sites, skin | Mycobacterium scrofulaceum | WBC: 7390 (74.9/16.4/6.6/1.8/0.3), IgG/A/M/E: 1310/458/137/27.4, C3/C4: 203/36, ANA (−), RA < 20, HIV (−) AFP: 27.3, CEA:3.26, CA-125: 70, CA-199:3.72, normal adrenal function | Osteomyelitis, chronic cervical lymphadenitis, herpes zoster | Chronic infection under therapy |
| 3 | 69/M | 18 y | Cervical lymph node, lung, bone | Mycobacterium avium complex, S enteritidis B | WBC: 8560 (59.6/28.4/6.5/4.2/1.3), ANA (−), anti-nDNA: 1.9, RA: 88.7, C3/C4: 202/25.1, IgG/A/M/E: 2980/968/94/17.3, C-ANCA (+), P-ANCA (−), CEA: 0.46, SCC: 12.8, PSA: 1.03, HBsAg (−), HCV (−), HIV (−), normal adrenal and thyroid function | Pneumonia, lymphadenitis, osteomyelitis, Salmonella sepsis, herpes zoster | Persistent infection under therapy |
| 4 | 47/F | 44 y | Bone, cervical lymph nodes | S enteritidis D Mycobacterium gordonae | WBC: 7420 (45.5/43.8/5.9/4.4/0.4) ANA (−), C3/C4: 255/29.2, SSA/SSB (−), IgG/A/M/E: 1770/3.9/89.2/10.34, BM(−), IEP (−), PEP of blood: increase of polyclonal gamma globulin normal adrenal function | Osteomyelitis, cervical lymphadenitis, Salmonella sepsis, herpes zoster | Chronic infection under therapy |
| 5 | 55/M | 53 y | Multiple bone sites, muscles | Mycobacterium terrae | WBC: 9070 (65.8/18.4/13.1/2.4/0.3), HBsAg (−), HCV (−), C3/C4: 210/27, IgG/A/M/E: 1870/312/116/11.52, RF < 20, ANA-1:40, HIV (−), AFP: 1.7, CEA:1.93, PSA:1.037, CA19-9:15.9, SCC: 0.9 | Osteomyelitis, psoas and back muscle abscesses | Survival without active disease |
| 6 | 81/M | 81 y | Cervical and mediastinal lymph nodes, skin | Nontuberculous mycobacterium | WBC: 9730 (72.4/13.6/6.2/7.5/0.3), ANA (−), RA < 20, IgG/A/M: 1780/432/92.6, IgE:521.70, C3/C4: 131/36.1, HIV (−), AFP:2.73, CEA: 0.84, PSA: 17.899, CA199: 32, IEP: no monoclonal gammopathy, thyroid (−) | Cutaneous infection, lymphadenitis | Persistent infection under therapy |
| 7 | 66/M | 61 y | Cervical lymph nodes, lung | Nontuberculous mycobacterium | WBC: 7510 (69.7/18.8/5.9/5.5/0.1), HIV (−), RF < 20, ANA (−), Ig G/A/M-1340/367/55.6, C3/C4-115/27.2, IgE-388.91, CEA-1.22, CA199-10.2, PSA-1.23, CA125-117.9 | NTM pneumonia, cervical lymphadenitis | Survival without active disease |
| 8 | 87/M | 84 y | Multiple bone sites, skin, lymph nodes | M scrofulaceum, S aureus | WBC: 9950 (57.7/28.9/5.8/7/0.6), ANA-1:40, APF-3.61, CEA-1.72, PSA-3.14, CA199-12.8, HBV(−), HCV(−) | Osteomyelitis, lymphadenitis, pneumonia, herpes zoster, MRSA cellulitis | Survival without active disease |
| 9 | 48/M | 44 y | Bone, lung, mediastinal lymph nodes | M avium complex, S enteritidis | WBC: 7120 (54.4/29.2/4.9/10.5/1), HBsAg (−), HCV (+), AFP: 3.92 | Mycotic aneurysm, pneumonia, osteomyelitis, herpes zoster | Survival without active disease |
| 10 | 61/F | 59 y | Lymph nodes (neck, abdomen, mediastinum, retroperitoneum), skin, bone | Nontuberculous mycobacterium | WBC: 33 750 (51/9/2/25), IgG/A/M: 1550/206/108, RA < 20, C3/C4: 171/64, ANA: 1:40, AFP: 1.92, CEA: 1.44, CA-125: 9.5, CA19-9: 4.1, colonoscopy and UGI: polyps, gastritis, HIV (−), HBV(−), HCV (−) | Multiple site lymphadenopathy, osteomyelitis | Persistent infection under therapy |
| 11 | 49/M | 48 y | Lung, lymph nodes (mediastinum, neck) | Mycobacterium kansasii | WBC: 16 980 (77.9/15.6/5.1/1.2), HIV (−), colonoscopy: polyps, AFP: 1.45, CEA: 1.14, PSA: 5.671, CA19-9: 9.4; ANA: 1:40, IgG/A/M: 1990/191/182, RA < 20, C3/C4: 166/27.9 | Lymphadenitis, pneumonia, colitis, herpes zoster | Persistent infection under therapy |
| 12 | 56/F | 48 y | Bone, mediastinal lymph nodes, skin | Nontuberculous mycobacterium | WBC: 10 640 (74.7/17.1/3.8/3.3), AFP: 1.7, CEA < 0.45, SCC:0.5, IgG/A/M: 2340/639/124, RA < 20, ANA: negative, HIV (−), normal thyroid function | Osteomyelitis, erythema induratum, mediastinal lymphadenitis, herpes zoster | Persistent infection under therapy |
| 13 | 46/F | 45 y | Lung, lymph nodes | Nontuberculous mycobacterium, S enteritidis | WBC: 17 700 (81.7/7.7/9.3/1.2), CEA: 0.38, CA-124: 20.6, CA19-9:7.8, normal level of IgG/A/M | Pneumonia, neck lymphadenitis | Persistent infection under therapy |
| 14 | 72/F | 72 y | Bone (multiple sites) | M avium complex | WBC:10 750(86.2/7.6/3.2/2.5), AFP:0.79, CEA: 1.67, CA-125: 13.8, CA-153: 4.4, CA-19-9: 17.6, RA: 585, ANA (−), C3: 86.5, C4: 11.2, anti-ENA(−), Scl-70(−) IgG/A/M/E:1880/354/185/388.55, HIV(−) | Osteomyelitis (multiple sites) | Expired due to traumatic intracranial hemorrhage |
| 15 | 49/F | 47 y | Lung, lymph node, pericardium, bone | Nontuberculous mycobacterium, Legionellosis, P marneffei, | ANA(−), IgG/A/M: 2490/673/185, C3/C4: 178/36.4, RF: 22.4, ds-DNA (−), HIV(−) | Pneumonia, lymphadenitis (neck, mediastinum) | Unknown |
| 16 | 47/F | 47 y | Lung, bone, lymph nodes, skin | Nontuberculous mycobacterium, S enteritidis | WBC: 11 590(64.2/25.9/3.5/6.0), AFP: 1.43, CEA: 0.27 CA15-3: 6.8, CA-125: 6.2, CA19-9: 1.6, ANA: 1:40, RA < 20, C3/C4: 186/70.6, anti-nDNA (−), anti-microsomal antibody (−), anti-thyroglobulin antibody (−), anti-smooth muscle antibody: 1:20, C-ANCA (−), P-ANCA (−), anti-cardiolipin IgM/G: −/+, SS-A: 96.79, SS-B: 12.2, RNP: 3.1, HIV(−) | Arthritis, lymphadenitis (neck, mediastinum, abdomen, inguinal area) | Persistent infection under therapy |
| 17 | 76/M | 60 y | Lung, lymph nodes | M abscessus, M chelonae | WBC: 11 790(76.6/12.3/5.4./5.4), RA: 52.7, ANA:1:40, HIV(−), IgG/A/M: 3160/542/111, C3/C4: 84.1/31.7, AFP: 1.41, CEA: 1.14, PSA: 1.3, CA19-9: 8.7 | Lymphadenitis (neck, mediastinum, axilla) | Persistent infection under therapy |
| Patient . | Age, y/sex . | Onset . | Affected sites . | Pathogen(s) . | Laboratory data . | Diagnosis . | Outcome . |
|---|---|---|---|---|---|---|---|
| 1 | 55/M | 50 y | Lymph node, multiple bone sites | Mycobacterium abscessus, S enteritidis B, P marneffei | WBC:9450 (64.6/24.3/7.9/3.1/0.1), HIV (−), ANA (−), RA < 20, SSA (−), SSB (−), C3/C4:199/74.5, C-ANCA (−), P-ANCA (−), Coomb test (−), IgG/A/M: 1980/159/65.8, HBsAg (−), HCV (−), HLA-B27 (−) no chromosome abnormality, thyroid function (−) | Chronic noncaseating granulomatous lymphadenitis, osteomyelitis, Salmonella sepsis, herpes zoster | Survival with no active disease |
| 2 | 51/F | 47 y | Cervical, mediastinal, and axillary lymph nodes, multiple bone sites, skin | Mycobacterium scrofulaceum | WBC: 7390 (74.9/16.4/6.6/1.8/0.3), IgG/A/M/E: 1310/458/137/27.4, C3/C4: 203/36, ANA (−), RA < 20, HIV (−) AFP: 27.3, CEA:3.26, CA-125: 70, CA-199:3.72, normal adrenal function | Osteomyelitis, chronic cervical lymphadenitis, herpes zoster | Chronic infection under therapy |
| 3 | 69/M | 18 y | Cervical lymph node, lung, bone | Mycobacterium avium complex, S enteritidis B | WBC: 8560 (59.6/28.4/6.5/4.2/1.3), ANA (−), anti-nDNA: 1.9, RA: 88.7, C3/C4: 202/25.1, IgG/A/M/E: 2980/968/94/17.3, C-ANCA (+), P-ANCA (−), CEA: 0.46, SCC: 12.8, PSA: 1.03, HBsAg (−), HCV (−), HIV (−), normal adrenal and thyroid function | Pneumonia, lymphadenitis, osteomyelitis, Salmonella sepsis, herpes zoster | Persistent infection under therapy |
| 4 | 47/F | 44 y | Bone, cervical lymph nodes | S enteritidis D Mycobacterium gordonae | WBC: 7420 (45.5/43.8/5.9/4.4/0.4) ANA (−), C3/C4: 255/29.2, SSA/SSB (−), IgG/A/M/E: 1770/3.9/89.2/10.34, BM(−), IEP (−), PEP of blood: increase of polyclonal gamma globulin normal adrenal function | Osteomyelitis, cervical lymphadenitis, Salmonella sepsis, herpes zoster | Chronic infection under therapy |
| 5 | 55/M | 53 y | Multiple bone sites, muscles | Mycobacterium terrae | WBC: 9070 (65.8/18.4/13.1/2.4/0.3), HBsAg (−), HCV (−), C3/C4: 210/27, IgG/A/M/E: 1870/312/116/11.52, RF < 20, ANA-1:40, HIV (−), AFP: 1.7, CEA:1.93, PSA:1.037, CA19-9:15.9, SCC: 0.9 | Osteomyelitis, psoas and back muscle abscesses | Survival without active disease |
| 6 | 81/M | 81 y | Cervical and mediastinal lymph nodes, skin | Nontuberculous mycobacterium | WBC: 9730 (72.4/13.6/6.2/7.5/0.3), ANA (−), RA < 20, IgG/A/M: 1780/432/92.6, IgE:521.70, C3/C4: 131/36.1, HIV (−), AFP:2.73, CEA: 0.84, PSA: 17.899, CA199: 32, IEP: no monoclonal gammopathy, thyroid (−) | Cutaneous infection, lymphadenitis | Persistent infection under therapy |
| 7 | 66/M | 61 y | Cervical lymph nodes, lung | Nontuberculous mycobacterium | WBC: 7510 (69.7/18.8/5.9/5.5/0.1), HIV (−), RF < 20, ANA (−), Ig G/A/M-1340/367/55.6, C3/C4-115/27.2, IgE-388.91, CEA-1.22, CA199-10.2, PSA-1.23, CA125-117.9 | NTM pneumonia, cervical lymphadenitis | Survival without active disease |
| 8 | 87/M | 84 y | Multiple bone sites, skin, lymph nodes | M scrofulaceum, S aureus | WBC: 9950 (57.7/28.9/5.8/7/0.6), ANA-1:40, APF-3.61, CEA-1.72, PSA-3.14, CA199-12.8, HBV(−), HCV(−) | Osteomyelitis, lymphadenitis, pneumonia, herpes zoster, MRSA cellulitis | Survival without active disease |
| 9 | 48/M | 44 y | Bone, lung, mediastinal lymph nodes | M avium complex, S enteritidis | WBC: 7120 (54.4/29.2/4.9/10.5/1), HBsAg (−), HCV (+), AFP: 3.92 | Mycotic aneurysm, pneumonia, osteomyelitis, herpes zoster | Survival without active disease |
| 10 | 61/F | 59 y | Lymph nodes (neck, abdomen, mediastinum, retroperitoneum), skin, bone | Nontuberculous mycobacterium | WBC: 33 750 (51/9/2/25), IgG/A/M: 1550/206/108, RA < 20, C3/C4: 171/64, ANA: 1:40, AFP: 1.92, CEA: 1.44, CA-125: 9.5, CA19-9: 4.1, colonoscopy and UGI: polyps, gastritis, HIV (−), HBV(−), HCV (−) | Multiple site lymphadenopathy, osteomyelitis | Persistent infection under therapy |
| 11 | 49/M | 48 y | Lung, lymph nodes (mediastinum, neck) | Mycobacterium kansasii | WBC: 16 980 (77.9/15.6/5.1/1.2), HIV (−), colonoscopy: polyps, AFP: 1.45, CEA: 1.14, PSA: 5.671, CA19-9: 9.4; ANA: 1:40, IgG/A/M: 1990/191/182, RA < 20, C3/C4: 166/27.9 | Lymphadenitis, pneumonia, colitis, herpes zoster | Persistent infection under therapy |
| 12 | 56/F | 48 y | Bone, mediastinal lymph nodes, skin | Nontuberculous mycobacterium | WBC: 10 640 (74.7/17.1/3.8/3.3), AFP: 1.7, CEA < 0.45, SCC:0.5, IgG/A/M: 2340/639/124, RA < 20, ANA: negative, HIV (−), normal thyroid function | Osteomyelitis, erythema induratum, mediastinal lymphadenitis, herpes zoster | Persistent infection under therapy |
| 13 | 46/F | 45 y | Lung, lymph nodes | Nontuberculous mycobacterium, S enteritidis | WBC: 17 700 (81.7/7.7/9.3/1.2), CEA: 0.38, CA-124: 20.6, CA19-9:7.8, normal level of IgG/A/M | Pneumonia, neck lymphadenitis | Persistent infection under therapy |
| 14 | 72/F | 72 y | Bone (multiple sites) | M avium complex | WBC:10 750(86.2/7.6/3.2/2.5), AFP:0.79, CEA: 1.67, CA-125: 13.8, CA-153: 4.4, CA-19-9: 17.6, RA: 585, ANA (−), C3: 86.5, C4: 11.2, anti-ENA(−), Scl-70(−) IgG/A/M/E:1880/354/185/388.55, HIV(−) | Osteomyelitis (multiple sites) | Expired due to traumatic intracranial hemorrhage |
| 15 | 49/F | 47 y | Lung, lymph node, pericardium, bone | Nontuberculous mycobacterium, Legionellosis, P marneffei, | ANA(−), IgG/A/M: 2490/673/185, C3/C4: 178/36.4, RF: 22.4, ds-DNA (−), HIV(−) | Pneumonia, lymphadenitis (neck, mediastinum) | Unknown |
| 16 | 47/F | 47 y | Lung, bone, lymph nodes, skin | Nontuberculous mycobacterium, S enteritidis | WBC: 11 590(64.2/25.9/3.5/6.0), AFP: 1.43, CEA: 0.27 CA15-3: 6.8, CA-125: 6.2, CA19-9: 1.6, ANA: 1:40, RA < 20, C3/C4: 186/70.6, anti-nDNA (−), anti-microsomal antibody (−), anti-thyroglobulin antibody (−), anti-smooth muscle antibody: 1:20, C-ANCA (−), P-ANCA (−), anti-cardiolipin IgM/G: −/+, SS-A: 96.79, SS-B: 12.2, RNP: 3.1, HIV(−) | Arthritis, lymphadenitis (neck, mediastinum, abdomen, inguinal area) | Persistent infection under therapy |
| 17 | 76/M | 60 y | Lung, lymph nodes | M abscessus, M chelonae | WBC: 11 790(76.6/12.3/5.4./5.4), RA: 52.7, ANA:1:40, HIV(−), IgG/A/M: 3160/542/111, C3/C4: 84.1/31.7, AFP: 1.41, CEA: 1.14, PSA: 1.3, CA19-9: 8.7 | Lymphadenitis (neck, mediastinum, axilla) | Persistent infection under therapy |
AFP indicates alpha-fetoprotein; ANA, antinuclear antibody; CEA, carcinoembryonic antigen; IEP, immunoelectrophoresis; MRSA, methicillin-resistant S aureus; PSA, prostate-specific antigen; and RF, rheumatoid factor.
Inhibition of IFN-γ detection in the presence of patient WB
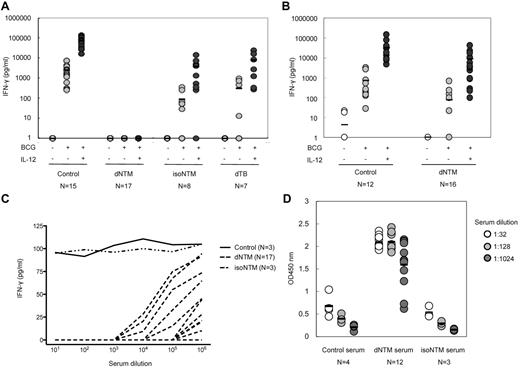
We also assessed the production of IFN-γ in WB after stimulation with BCG or BCG + IL-12. Under either condition, IFN-γ was barely detectable in the plasma of the 17 patients with dNTM infections (Figure 1A). However, after the removal of the PBMCs from the plasma, these cells were able to produce IFN-γ in response to BCG or BCG + IL-12 stimulation (Figure 1B). Conversely, the production of IFN-γ from WB after activation did not differ significantly between healthy donors and patients with isolated NTM and disseminated tuberculosis infections (Figure 1A). This finding suggested that some plasma factor(s) inhibited the detection of IFN-γ in the patients with dNTM infections.
Anti–IFN-γ autoantibodies with high inhibitory activity in patients with dNTM infections. (A) IFN-γ was barely detected in the WB of patients with dNTM infections when stimulated with live BCG or BCG + IL-12. (B) In the absence of patient plasma, the production of IFN-γ was detectable. (C) Patient plasma was serially diluted and incubated with 100 pg/mL of IFN-γ. Plasma from healthy donors (control) and patients with isolated pulmonary NTM infection (isoNTM) showed minimal blocking activity for the detection of IFN-γ. However, plasma from patients with dNTM infections at dilutions of up to 1/106 inhibited the detection of IFN-γ. The data from 2 independent experiments with similar results were combined. (D) Anti–IFN-γ autoantibodies were identified as IgG antibodies. IFN-γ was immobilized by capture antibodies on an ELISA plate and plasma samples from patients and controls were added to the wells. Antibodies to human IgG were added to measure the binding of human IgG autoantibodies to human IFN-γ.
Anti–IFN-γ autoantibodies with high inhibitory activity in patients with dNTM infections. (A) IFN-γ was barely detected in the WB of patients with dNTM infections when stimulated with live BCG or BCG + IL-12. (B) In the absence of patient plasma, the production of IFN-γ was detectable. (C) Patient plasma was serially diluted and incubated with 100 pg/mL of IFN-γ. Plasma from healthy donors (control) and patients with isolated pulmonary NTM infection (isoNTM) showed minimal blocking activity for the detection of IFN-γ. However, plasma from patients with dNTM infections at dilutions of up to 1/106 inhibited the detection of IFN-γ. The data from 2 independent experiments with similar results were combined. (D) Anti–IFN-γ autoantibodies were identified as IgG antibodies. IFN-γ was immobilized by capture antibodies on an ELISA plate and plasma samples from patients and controls were added to the wells. Antibodies to human IgG were added to measure the binding of human IgG autoantibodies to human IFN-γ.
Demonstration of anti–IFN-γ IgG
To confirm the presence of a factor(s) that could interfere with the detection of IFN-γ by ELISA, we incubated a fixed-concentration (100 pg/mL) of recombinant IFN-γ with serially diluted plasma from the study patients and from healthy controls. In the presence of patient plasma, recombinant human IFN-γ was barely detected, even at the weakest plasma dilution (10−6 dilution in 7 patients). This suggested that some plasma factor was interfering with IFN-γ detection by ELISA. This blocking activity varied widely among the patients and was not related to disease activity (Figure 1C). Serially diluted plasma from healthy donors and patients was added to IFN-γ–coated wells (100 μL/well), incubated, and then treated with mouse anti–human IgG antibodies. Low optical density values were observed in the specimens from healthy donors, but high values were observed in specimens from patients with dNTM infections (Figure 1D). These results indicated that IgG was the IFN-γ–binding factor in the patients' plasma. We also tested the inhibitory activity of the patients' plasma against other cytokines (IFN-α, TNF-α, IL-6, IL-12p70, IL-17, IL-18, GM-CSF, and erythropoietin), but no inhibitory activity could be observed (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These data suggested that the autoantibodies were specific for IFN-γ.
Anti–IFN-γ autoantibodies interfere with the production of IL-12
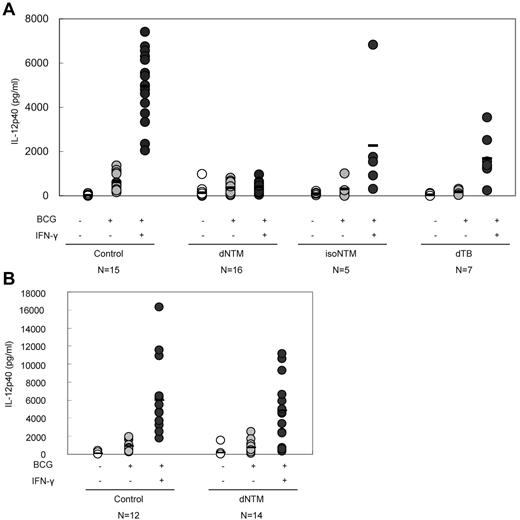
IFN-γ enhances IL-12 production. In WB after stimulation with BCG or BCG + IFN-γ (particularly the latter), there was a marked elevation of the IL-12p40 concentration in the plasma of healthy subjects. Conversely, a blunted IL-12p40 response, even on stimulation with BCG + IFN-γ, was observed in samples from patients with dNTM infections (Figure 2A). After the removal of the plasma, the response of IL-12p40 to BCG + IFN-γ stimulation was recovered (Figure 2B). This phenomenon suggested that the anti–IFN-γ autoantibodies from patients with dNTM infections had a biologic effect and functionally interfered with IL-12p40 production.
Inhibition of IL-12 production in patients with dNTM infections. (A) In the presence of patient plasma, the IFN-γ–dependent up-regulation of IL-12 production was significantly inhibited. (B) When the plasma was removed (PMBCs only), the IFN-γ–dependent up-regulation of IL-12 production recovered.
Inhibition of IL-12 production in patients with dNTM infections. (A) In the presence of patient plasma, the IFN-γ–dependent up-regulation of IL-12 production was significantly inhibited. (B) When the plasma was removed (PMBCs only), the IFN-γ–dependent up-regulation of IL-12 production recovered.
No difference in the distribution of immune cells between cohort patients and healthy subjects
We also analyzed and compared the proportions of immune cells, including monocytes (CD14), T-lymphocytes (CD4 and CD8), and natural killer cells (CD16 and CD56), between our patients and healthy donors (supplemental Table 1). No major differences were observed between these 2 groups. Furthermore, we evaluated the autoimmune disease profiles in our patients, including the presence of antinuclear antibodies; rheumatoid factor; serum IgG, IgA, and IgM; complement C3 and C4; and antineutrophil cytoplasmic antibodies, and none of these results supported a diagnosis of autoimmune disease (Table 1). These findings suggest that the presence of anti–IFN-γ autoantibodies is not correlated with the presence of autoimmune disease.
DRB1*16:02 and DQB1*05:02 are associated with anti–IFN-γ autoantibodies
The HLA typing results from 17 dNTM patients with anti–IFN-γ autoantibodies are shown in Table 2. Intriguingly, limited allele polymorphism was observed. HLA-A*11:01, HLA-B*40:01, HLA-DRB1*16:02, and HLA-DQB1*05:02 were the most frequently observed alleles among the patients in the study. Compared with previously published data,28 among the HLA class I alleles, the frequencies of A*02:03 and B*40:01 were increased in patients, with ORs of 4.52 (95% CI, 1.54-13.14, P = .0040) and 2.46 (95% CI, 1.06-5.68, P = .0196), respectively; however, these increases were not statistically significant after Bonferroni correction (Table 3). Similarly, HLA class II DRB1*04:05, DRB1*15:02, DRB1*16:02, DQB1*04:01, and DQB1*05:02 were more frequent in the dNTM patients than in the controls, whereas DRB1*08:03, DRB1*09:01, and DQB1*03:03 were less frequent in the dNTM patient group (Table 3). Nevertheless, after Bonferroni correction, only the differences in DRB1*16:02 and DQB1*05:02 remained statistically significant, with ORs of 8.68 (95% CI, 3.47-21.90, P = 1.1 × 10−6, Pc = 3.08 × 10−5) and 7.16 (95% CI, 3.02-17.05, P = 1 × 10−7, Pc = 1.4 × 10−6), respectively (Table 3). The similar significance of DRB1*16:02 and DQB1*05:02 is reasonable because the 2 alleles are in strong linkage disequilibrium (LD) throughout Asia29 and in our dataset (LD = 0.0677, D′ or normalized LD = 1 and r2 = 0.64).
HLA-A, HLA-B, HLA-DRB1, and HLA-DQB1 allele typing in 17 patients with disseminated mycobacterial infection and autoantibodies to IFN-γ
| Patient no. . | HLA-A . | HLA-B . | HLA-DRB1 . | HLA-DQB1 . | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 02:03 | 11:02 | 40:01 | 54:01 | 04:05 | 15:02 | 04:01 | 05:01 |
| 2 | 02:03 | 11:02 | 38:02 | 54:01 | 04:05 | 16:02 | 04:01 | 05:02 |
| 3 | 11:01 | 24:02 | 40:01 | 54:01 | 14:01 | 16:02 | 05:02 | 05:02 |
| 4 | 11:01 | 11:01 | 15:25 | 54:01 | 04:05 | 16:02 | 04:01 | 05:02 |
| 5 | 11:01 | 24:02 | 40:01 | 40:01 | 15:01 | 16:02 | 05:02 | 06:02 |
| 6 | 11:01 | 11:01 | 27:04 | 40:01 | 04:05 | 16:02 | 04:01 | 05:02 |
| 7 | 11:01 | 33:03 | 13:01 | 40:01 | 15:01 | 16:02 | 05:02 | 06:01 |
| 8 | 24:02 | 24:02 | 40:01 | 40:01 | 15:02 | 16:02 | 05:01 | 05:02 |
| 9 | 02:03 | 11:02 | 27:04 | 38:02 | 14:01 | 16:02 | 05:02 | 05:02 |
| 10 | 02:03 | 11:01 | 38:02 | 40:01 | 11:01 | 16:02 | 03:01 | 05:02 |
| 11 | 11:01 | 24:02 | 40:01 | 40:02 | 04:05 | 04:05 | 04:01 | 04:01 |
| 12 | 02:03 | 24:02 | 40:01 | 40:01 | 12:02 | 16:02 | 03:01 | 05:02 |
| 13 | 11:01 | 24:10 | 13:01 | 56:04 | 15:02 | 16:02 | 05:01 | 05:02 |
| 14 | 02:03 | 33:03 | 15:02 | 58:01 | 03:01 | 15:02 | 02:01 | 05:01 |
| 15 | 11:01 | 24:02 | 13:01 | 40:01 | 16:02 | 16:02 | 05:02 | 05:02 |
| 16 | 02:03 | 11:01 | 15:01 | 38:02 | 12:02 | 16:02 | 03:01 | 05:02 |
| 17 | 02:03 | 02:07 | 38:02 | 46:01 | 09:01 | 16:02 | 03:03 | 05:02 |
| Patient no. . | HLA-A . | HLA-B . | HLA-DRB1 . | HLA-DQB1 . | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 02:03 | 11:02 | 40:01 | 54:01 | 04:05 | 15:02 | 04:01 | 05:01 |
| 2 | 02:03 | 11:02 | 38:02 | 54:01 | 04:05 | 16:02 | 04:01 | 05:02 |
| 3 | 11:01 | 24:02 | 40:01 | 54:01 | 14:01 | 16:02 | 05:02 | 05:02 |
| 4 | 11:01 | 11:01 | 15:25 | 54:01 | 04:05 | 16:02 | 04:01 | 05:02 |
| 5 | 11:01 | 24:02 | 40:01 | 40:01 | 15:01 | 16:02 | 05:02 | 06:02 |
| 6 | 11:01 | 11:01 | 27:04 | 40:01 | 04:05 | 16:02 | 04:01 | 05:02 |
| 7 | 11:01 | 33:03 | 13:01 | 40:01 | 15:01 | 16:02 | 05:02 | 06:01 |
| 8 | 24:02 | 24:02 | 40:01 | 40:01 | 15:02 | 16:02 | 05:01 | 05:02 |
| 9 | 02:03 | 11:02 | 27:04 | 38:02 | 14:01 | 16:02 | 05:02 | 05:02 |
| 10 | 02:03 | 11:01 | 38:02 | 40:01 | 11:01 | 16:02 | 03:01 | 05:02 |
| 11 | 11:01 | 24:02 | 40:01 | 40:02 | 04:05 | 04:05 | 04:01 | 04:01 |
| 12 | 02:03 | 24:02 | 40:01 | 40:01 | 12:02 | 16:02 | 03:01 | 05:02 |
| 13 | 11:01 | 24:10 | 13:01 | 56:04 | 15:02 | 16:02 | 05:01 | 05:02 |
| 14 | 02:03 | 33:03 | 15:02 | 58:01 | 03:01 | 15:02 | 02:01 | 05:01 |
| 15 | 11:01 | 24:02 | 13:01 | 40:01 | 16:02 | 16:02 | 05:02 | 05:02 |
| 16 | 02:03 | 11:01 | 15:01 | 38:02 | 12:02 | 16:02 | 03:01 | 05:02 |
| 17 | 02:03 | 02:07 | 38:02 | 46:01 | 09:01 | 16:02 | 03:03 | 05:02 |
Comparison of the frequencies of HLA-DRB1*1602 and HLA-DQB1*0502 between patients and controls
| HLA . | Allele frequency . | OR . | 95% CI . | P . | Pc . | |
|---|---|---|---|---|---|---|
| Patient (n = 17) . | Control (n = 102) . | |||||
| A*02:03 | 0.235 | 0.064 | 4.52 | (1.54-13.14) | .0040 | .0645 |
| B*40:01 | 0.382 | 0.201 | 2.46 | (1.06-5.68) | .0196 | .6664 |
| DRB1*04:05 | 0.176 | 0.069 | 2.91 | (0.91-9.00) | .0472 | 1.3216 |
| DRB1*08:03 | 0.000 | 0.118 | 0.00 | (0-1.15) | .0306 | .8568 |
| DRB1*09:01 | 0.029 | 0.172 | 0.15 | (0.01-1.05) | .0326 | .9128 |
| DRB1*15:02 | 0.118 | 0.010 | 13.47 | (2.00-111.44) | .0043 | .1204 |
| DRB1*16:02 | 0.441 | 0.083 | 8.68 | (3.47-21.90) | 1.1 × 10−6 | 3.08 × 10−5 |
| DQB1*03:03 | 0.029 | 0.172 | 0.15 | (0.01-1.05) | .0325 | .4550 |
| DQB1*04:01 | 0.176 | 0.059 | 3.43 | (1.05-10.91) | .0282 | .3948 |
| DQB1*05:02 | 0.500 | 0.123 | 7.16 | (3.02-17.05) | 1 × 10−7 | 1.4 × 10−6 |
| HLA . | Allele frequency . | OR . | 95% CI . | P . | Pc . | |
|---|---|---|---|---|---|---|
| Patient (n = 17) . | Control (n = 102) . | |||||
| A*02:03 | 0.235 | 0.064 | 4.52 | (1.54-13.14) | .0040 | .0645 |
| B*40:01 | 0.382 | 0.201 | 2.46 | (1.06-5.68) | .0196 | .6664 |
| DRB1*04:05 | 0.176 | 0.069 | 2.91 | (0.91-9.00) | .0472 | 1.3216 |
| DRB1*08:03 | 0.000 | 0.118 | 0.00 | (0-1.15) | .0306 | .8568 |
| DRB1*09:01 | 0.029 | 0.172 | 0.15 | (0.01-1.05) | .0326 | .9128 |
| DRB1*15:02 | 0.118 | 0.010 | 13.47 | (2.00-111.44) | .0043 | .1204 |
| DRB1*16:02 | 0.441 | 0.083 | 8.68 | (3.47-21.90) | 1.1 × 10−6 | 3.08 × 10−5 |
| DQB1*03:03 | 0.029 | 0.172 | 0.15 | (0.01-1.05) | .0325 | .4550 |
| DQB1*04:01 | 0.176 | 0.059 | 3.43 | (1.05-10.91) | .0282 | .3948 |
| DQB1*05:02 | 0.500 | 0.123 | 7.16 | (3.02-17.05) | 1 × 10−7 | 1.4 × 10−6 |
P values were estimated by the Mantel-Haenszel χ2 or Fisher exact test. Alleles are listed as P < .05. Pc values were obtained by multiplying the P values by the number of alleles at each locus represented in the samples (HLA-A: 16 alleles, HLA-B :34 alleles, HLA-DRB1: 28 alleles, and HLA-DQB1: 14 alleles).
IFN-γ is critical for controlling latent VZV infection but is dispensable for other chronic viral infections.
With the exception of the 2 deceased patients, all of our patients had serologic and/or clinical evidence of VZV infection during their lifetimes and 12 suffered from the reactivation of this virus either before or after the diagnosis of dNTM infection (supplemental Table 2). Cutaneous herpes zoster was the only clinical manifestation, and no severe sequelae were reported in any of these patients. We also evaluated the status of other chronic viral infections by measuring specific antiviral antibodies, including hepatitis B and C, CMV, HSV, and EBV (supplemental Table 2). In the patients chronically infected with these viruses (other than VZV), no obvious clinical manifestations or laboratory findings indicating the reactivation of these viruses were observed (data not shown). These clinical observations suggested that the presence of anti–IFN-γ autoantibodies in these patients was specifically associated with the restriction of VZV reactivation and not with the reactivation of other chronic viral infections.
Discussion
In the present study, our patients had several distinct characteristics. First, a high prevalence (100%) of anti–IFN-γ autoantibodies was observed among previously healthy Chinese adults who later suffered from dNTM infections. This particular phenotype was predictive for the presence of anti–IFN-γ autoantibodies. Second, 35% of our patients were coinfected with salmonellosis, a phenotype that is mainly observed in patients who have Mendelian susceptibility to mycobacterial disease (MSMD),30 but is not typically observed in patients with anti–IFN-γ autoantibodies.7,9,11 Third, a high proportion (71%) of our patients suffered from the reactivation of latent VZV infection, a phenotype rarely reported in either MSMD patients or in patients with anti–IFN-γ autoantibodies.7-13,30 Finally and most importantly, 2 HLA alleles, DRB1*16:02 and DQB1*05:02, occurred with unexpectedly high frequencies among our patients.
In recent years, cases of bioactive autoantibodies against IFN-γ have been described.7-13 In the presence of these autoantibodies, the “physiologic concentration” of IFN-γ in response to mycobacterial infections is markedly suppressed, and the affected subjects are susceptible to disseminated mycobacterial infections, a phenotype unique to MSMD patients.30-32 Patients with dNTM infections due to anti–IFN-γ autoantibodies were first reported in 2004.7,8 Patel et al reported that, of 23 patients with dNTM infections, only 6 (26%) had high-titer anti–IFN-γ autoantibodies in their plasma.9 Several subsequent case series and reports also identified small groups of patients with unusual autoantibodies of this type.10-13 Unlike our patients, nearly half of the reported patients7-13 had either clinical or laboratory evidence of autoimmune disease or had received steroid therapy, which can disrupt patient immunity and promote the formation of autoantibodies or impair the IFN-γ response.33 Such immunologic perturbations may interfere with the detection of IFN-γ in vitro or render patients more susceptible to infectious diseases. In our cohort, a clinically specific phenotype (formerly healthy Chinese adults later developing dNTM infections) was highly predictive of the presence of anti–IFN-γ autoantibodies. A similar finding was recently reported by Browne et al, who found a high prevalence of anti–IFN-γ autoantibodies in dNTM patients who lived in Thailand or Taiwan,34 observations that are consistent with our findings. The delineation of the molecular mechanism(s) that explains the extraordinarily high prevalence of anti–IFN-γ autoantibodies in the Taiwanese and Thai populations will be an important medical issue in the future.
Genetic factors may be involved in the production of anti–IFN-γ autoantibodies.9 In cases of autoimmune disease, numerous studies have demonstrated that HLA genes, particularly those belonging to class II, play an important role in disease pathogenesis.16,35 A similar linkage between HLA genes and infectious diseases has also been described previously.17,36 In the present study, we found high prevalence rates of 2 alleles, DRB1*16:02 and DQB1*05:02, among our patients. Eighty-two percent (14 of 17) had both the DRB1*16:02 and DQB1*05:02 alleles, with ORs of 8.68 (95% CI, 3.47-21.90, P = 1.1 × 10−6, Pc = 3.08 × 10−5) and 7.16 (95% CI, 3.02-17.05, P = 1 × 10−7, Pc = 1.4 × 10−6), respectively. However, strong LD between DRB1*16:02 and DQB1*05:02 has been reported in Asian populations,29 including Taiwanese. It is therefore difficult to conclude which allele, DRB1*16:02 or DQB1*05:02, is more significantly associated with anti–IFN-γ autoantibodies. DRB1*16:02 is common in Southeast Asians, South Americans, and Pacific Islanders but rare in whites,37 whereas DRB1*16:01 is common in white populations. A similar strong LD between DRB1*16:01 and DQB1*05:02 has been reported in white populations.29,37 However, patients with dNTM infections and anti–IFN-γ autoantibodies7-9,34 are mainly observed in east Asia. Therefore, it is likely that DRB1*16:02, rather than DQB1*05:02, is the causal factor associated with this particular disease (represented by dNTM infections and anti–IFN-γ autoantibodies). Both the DRB1 and DQB1 loci belong to the HLA class II gene system, and the proteins encoded by these genes are expressed on the surfaces of APCs and recognized by the receptors of Th cells. Interactions between APCs and Th cells may encourage the development of anti–IFN-γ autoantibodies. A better understanding of the association between DRB1*16:02 or DQB1*05:02 and the production of anti–IFN-γ autoantibodies will help us to determine the pathogenesis of dNTM infection or perhaps even the pathogenesis of autoimmune diseases. To the best of our knowledge, this is the first report of an association between HLA polymorphism and an anticytokine autoantibody disease.
IFNs play an important role in controlling viral replication. At least 2 types of IFNs, type I and type II, have been identified. Type I IFNs (IFN-α and IFN-β) exhibit a wide range of biologic activities and are involved in controlling viral infections.38 Based on in vitro studies and knockout mouse models, IFN-γ, the only type II IFN, was initially thought to play an important role in immunity to viral infections.39 However, studies of MSMD patients suggested that IFN-γ is essential for controlling infections that are caused by intracellular bacteria, such as mycobacteria and salmonella, and it is considered dispensable or even redundant for viral immunity. In contrast to this observation, an earlier report by Dorman et al found that 4 patients with IFN-γ receptor deficiency, including 2 patients with VZV, had increased susceptibility to viral pathogens.40 Among our patients, 12 of 17 (71%) suffered from the reactivation of latent VZV infection, herpes zoster (HZ), but not of other latent viruses. The incidence of HZ increases with age. In the general population, the estimated lifetime risk for development of HZ is approximately 30%, and the risk can reach up to 50% in patients age 85.41-43 With respect to the reference rates published by Lin et al,43 the calculated standardized incidence ratio for our patients was 82.2 (95% CI, 35.7-128.8). The high prevalence (71%) of HZ in our patients suggests that IFN-γ might play an important or even critical role in controlling the reactivation of latent VZV infection. It has been documented that VZV can block type I IFN production.44 Therefore, we hypothesize that in patients infected with VZV, the production of type I IFNs is impaired and IFN-γ substitutes for type I IFNs in controlling VZV reactivation. Consistent with our hypothesis, Roesler et al also reported a dominant partial IFN-γ receptor-1 deficiency in a child with meningoencephalitis that was caused by VZV reactivation.45 Browne et al found that a considerable proportion of patients suffering from dNTM infections with or without opportunistic infections also suffered from severe VZV infections.34 Anti–IFN-γ autoantibodies were detected in 88% of the patients in that study, similar to the findings in our cohort. Therefore, the impaired biologic function of both IFN pathways (suppression by the virus itself and by anti–IFN-γ autoantibodies) may explain the high prevalence of HZ among our patients. Further investigations are needed to clarify the role and mechanism of IFN-γ in maintaining the latency of VZV infection.
In conclusion, the results of the present study show a high prevalence (100%) of anti–IFN-γ autoantibodies in previously healthy Chinese adults suffering from dNTM infections. These autoantibodies might represent the most important cause of severe NTM infections in Southeast Asian populations, at least in the nonaboriginal Chinese population in Taiwan. Just as there is a well-known correlation between ankylosing spondylitis and HLA-B27,46 our data showed an association between specific HLA alleles (DRB1*16:02 and DQB1*05:02) and a particular phenotype (previously healthy adults suffering from dNTM infections and expressing anti–IFN-γ autoantibodies). Based on these observations, we strongly suggest that anti–IFN-γ autoantibodies could provide a novel model for the study of autoimmunity, particularly with respect to molecular mimicry between self-antigens and infectious agents. Further investigation is required to determine the types of epitopes, whether from microorganisms or from the environment, that bind to specific HLA alleles and induce anti–IFN-γ autoantibody production.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the clinicians who referred their patients and the patients who agreed to participate in the study.
This study was supported by grants from the National Health Research Institutes (NHRI-EX100-10028SC), the National Science Council (100-2314-B-182-050), and the China Medical University (CMU97-329 and CMU99-NTU-07).
Authorship
Contribution: C.-Y.C. and C.-L.K. designed the research, analyzed the data, and wrote the manuscript; C.-C.C. performed the research, analyzed the data, and wrote the manuscript; J.-P.L. and C.-H.L. performed the research and analyzed the data; M.-W.H., W.-J.L., P.-C.L., H.-J.C., C.-H.C., J.-Y.F., C.-P.F. referred the patients; and Y.-P.S., C.-Y.L., and J.-H.W. analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cheng-Lung Ku, Graduate Institute of Clinical Medical Sciences, Chang Gung University, 259 Wen-Hwa 1st Road, Kwei-Shan Tao-Yuan, Taiwan 333, Republic of China; e-mail: clku@mail.cgu.edu.tw.
References
Author notes
C.-C.C., J.-P.L., and C.-H.L. contributed equally to this work.