In this issue of Blood, Astermark et al have identified novel genetic markers of inhibitory antibody formation in hemophilia patients that may ultimately lead to prediction and even prevention of this severe complication of hemophilia treatment.1
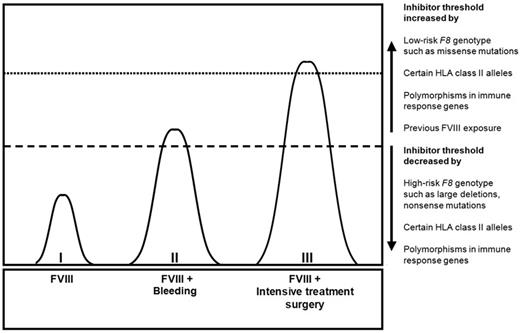
The combined action of genetic and environmental determinants on the risk of inhibitor development.10
The combined action of genetic and environmental determinants on the risk of inhibitor development.10
Hemophilia treatment has improved dramatically over the past decades. Before the 1960s, hemophilia was a crippling disease as the bleeding tendency led to irreversible arthropathy and increased mortality.2 Improvements in concentration and purification techniques gave patients in the Western world access to clotting factor concentrates, warding off the sequelae of repeated joint bleeds. These advances were overshadowed by the viral infections that occurred in the 1980s, when many hemophilia patients tragically became infected with HIV and the hepatitis C virus.3 Nowadays, major advances in safety of clotting factor products and institution of regular prophylactic clotting factor infusions have made a normal life with hemophilia possible.
Unfortunately, these achievements of modern hemophilia care are off the map for patients that develop inhibitory antibodies to factor VIII (FVIII). These so-called inhibitors form a severe complication of FVIII therapy in approximately 25% to 30% of young patients with severe hemophilia A. They preclude treatment with FVIII products by neutralizing FVIII activity. Although immune tolerance therapy and alternative hemostatic treatments that bypass FVIII are available, it is generally more difficult to prevent bleeding and arthropathy. As a matter of fact, hemophilia patients with inhibitors are jolted back to an era when hemophilia was associated with severe impairments in daily life.
So, why is it that a minority of patients develops inhibitors and that the majority of patients is seemingly tolerant to the foreign FVIII protein? What we know is that inhibitor development is caused by an intricate interplay of both genetic and environmental factors (see figure).4,5 Astermark and colleagues have demonstrated that the F8 genotype and other markers in immune response genes are major players in the field.6 These genetic markers of inhibitor development include HLA class II alleles and several single nucleotide polymorphisms (SNPs) in immune response genes.7-9
Still, further insight into the etiology of inhibitor development is urgently needed. If it is possible to predict a specific patient's individual risk of developing inhibitors, individualized treatment regimens or modification of immunologic factors in high-risk patients could possibly prevent inhibitors. Moreover, identification of immunologic pathways to inhibitor development may provide novel therapeutic targets to prevent inhibitors. So, how to push forward in the quest toward the prediction and prevention of inhibitor development?
In the current study, Astermark et al endeavored to unravel the genetic susceptibility for inhibitor development. This enormous challenge required a substantial number of patients. The authors joined hemophilia researchers worldwide and succeeded in studying 833 patients by combining cohorts from 3 different studies. An evaluation of 13 331 SNPs in primarily immune response and immune modifier genes yielded 53 SNPs that predicted inhibitor status in all cohorts. Of these, 13 markers were statistically significantly associated with inhibitor development in the combined cohort (meta P values < .001). In addition, 8 of the 53 SNPs were significant predictors among the discordant brother pairs. The identified genetic markers are known to be involved in various B and T cell–mediated mechanisms and intracellular signaling pathways.
In the field of hemophilia this study is unprecedented in its size, both in the number of patients and the number of genetic markers that were investigated. Refraining from a genome-wide association study, the authors took a more efficient approach and focused on genes likely to be involved in the immune response. They have strengthened the external validity of their findings by replicating their findings in 3 different cohorts and a subgroup of brother pairs discordant in inhibitor status. This makes the results of this study more persuasive.
Although Astermark and colleagues took great care to validate their results, the scientific and clinical importance of the identified associations needs further clarification. Chance findings among the large number of studied SNPs are not unimaginable and the results are at variance with earlier studies.8 In addition, SNPs beyond the pathways that are traditionally associated with immune-related responses were not evaluated.
The genetic markers that were identified in this study may be regarded as an important departure point for future studies. These novel genetic markers may be used to construct a clinical prediction score for inhibitor development. Ideally, this score would include all important predictors. Before the first treatment with FVIII products it would consist exclusively of genetic predictors. Once treatment with FVIII products is initiated, the risk score would encompass treatment-related factors as well, directing clinicians to take funded treatment decisions. The future will tell whether the predictive value of such a score is sufficient to be clinically relevant. Moreover, it may be challenging to determine genetic markers in young patients before the first hemorrhage occurs.
The next step toward prevention of inhibitors is the characterization of immunologic pathways that are involved in inhibitor development. They may provide potential therapeutic targets for future prevention or treatment of inhibitor development.
Even though the clinical implications of the findings of this study are not yet imminent, they set the stage for further study. Moreover, unraveling the immunologic process of anti-FVIII antibody formation in patients with hemophilia may also provide insight into basic immunologic (patho)physiology and could ultimately not only benefit those with hemophilia, but possibly also a wider group of patients with auto-immune diseases.
Conflict-of-interest disclosure: S.C.G. has reported receiving unrestricted research support from ZLB Behring, Novo Nordisk, Wyeth, Baxter, and Bayer. K.F. is a member of the European Hemophilia Treatment and Standardization Board sponsored by Baxter, has received unrestricted research grants from CSL Behring and Bayer, and has given lectures at educational symposiums organized by Pfizer and Bayer. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal