Key Points
HIV-1 transmission is influenced by the compatibility of sexual partners for two immune system genes, KIR and HLA.
KIR/HLA incompatibility likely allows alloreactive NK cells from the exposed partner to reject incoming cells from the HIV-1–infected partner.
Abstract
Killer immunoglobulin-like receptors (KIRs) regulate natural killer (NK) cells in a human leukocyte antigen (HLA)–dependent manner. KIR/HLA mismatched hematopoietic stem cell transplants induce alloreactive NK cells, which prevent leukemia relapse. Certain KIR/HLA combinations protect against HIV-1 infection, but the effect of KIR/HLA mismatches between sexual partners has never been investigated. In this study, we analyzed the effect of allogeneic KIR/HLA combinations on HIV-1 transmission in a West African population of HIV-1–discordant and concordant couples. HIV-1–discordant couples were characterized by recipient partners with homozygous KIR2DL2, and by a mismatched recipient partner KIR2DL1/HLA-C2 with index partner HLA-C1/C1 combination expected to allow licensed missing self NK cell killing of index partners' cells. HIV-1–concordant couples on the other hand were characterized by KIR2DL3 homozygous recipient partners with HLA-C1/C2 bearing index partners, resulting in a matched KIR/HLA combination expected to inhibit NK cell killing. In vitro cocultures of healthy donor-derived NK cells and HIV-1 patient-derived CD4+ T cells confirmed the involvement of these allogeneic KIR/HLA combinations in NK cell–mediated CD4+ T-cell killing. Our data suggest that KIR/HLA incompatibility between sexual partners confers protection against HIV-1 transmission and that this may be due to alloreactive NK cell killing of the HIV-1–infected partner's cells.
Introduction
Natural killer (NK) cells are innate immune cells that form the first line of defense against viruses and tumors.1 NK cells are regulated by various activating and inhibitory receptors, and integration of all signals determines whether the NK cell will be activated or not.2 An important family of NK receptors are the killer immunoglobulin-like receptors (KIRs), which contain 14 different functional members encoded head-to-tail on chromosome 19q13.4.3 Several inhibitory KIRs interact specifically with human leukocyte antigen (HLA) class I molecules expressed on potential target cells.4 KIR2DL1 recognizes HLA-C allotypes with a lysine at position 80 (named HLA-C2), KIR2DL2/L3 recognize HLA-C allotypes with asparagine at position 80 (named HLA-C1), and KIR3DL1 recognizes HLA-B allotypes containing the Bw4 epitope (named HLA-Bw4). Interaction of inhibitory KIRs with HLA ensures NK cell tolerance of normal autologous cells, and triggers NK cell cytotoxicity toward cells which are HLA compromised (eg, after tumor transformation or viral infection) or HLA incompatible (eg, in transplantation).5 In addition, inhibitory KIR/HLA interactions are required for NK cells to mature and gain functional competence to be triggered through their activation receptors, a process referred to as licensing or education.5
Both KIR and HLA loci show extreme population diversity and rapid evolution, suggesting that they are under pathogen-mediated selection and that they influence disease outcome at the individual level.6 Indeed, several epidemiologic studies have associated KIR/HLA compound genotypes with susceptibility to infectious diseases, autoimmune and inflammatory conditions, and cancer.7 KIR/HLA polymorphisms were also found to act under inter-individual or allogeneic conditions, for example, by influencing reproductive success and transplantation outcome. Notably, in the setting of hematopoietic stem cell transplantation as a treatment for acute myeloid leukemia, allogeneic donor/recipient KIR/HLA combinations were associated with leukemia eradication, protection from graft-versus-host disease, and lower relapse rates.8-11 Beneficial KIR/HLA combinations specifically consisted of donor inhibitory KIR that recognized HLA allotypes present in the donor but that were lacking in the recipient. Functional studies showed that such “missing self” combinations correlated with the expansion of a donor-derived alloreactive NK cell subset in the recipient with strong alloreactivity toward residual immune and leukemia cells in a donor-to-recipient direction.8,10 Similar allogeneic KIR/HLA mismatches but in the recipient-to-donor direction were found associated with reduced graft survival after HLA-C incompatible kidney transplantation.12
HIV-1 acquisition and transmission efficiency is highly variable between individuals and depends on host, viral, as well as environmental factors.13 Host genetics can have an important impact on HIV-1 acquisition, with polymorphisms in CCR5 and HLA as most replicated examples.14 Notably, the level of HLA class I allele discordance between transmission pairs correlates with a decreased risk of sexual or perinatal HIV-1 transmission.15-19 KIR/HLA combinations influence the rate of HIV-1 disease progression.20-27 Studies of HIV-exposed seronegative (HESN) individuals also showed an effect of KIR/HLA genetic variation on susceptibility to HIV-1 acquisition,28-34 with several of those reporting KIR/HLA combinations with an NK cell activating profile to be characteristic of HESN subjects.28-30,32 To date, no study has investigated the impact of allogeneic KIR/HLA combinations across sexual partners on HIV-1 transmission.
In this study, we investigated whether specific allogeneic KIR/HLA combinations, such as those applied in hematopoietic stem cell transplantation, influence HIV-1 transmission and lack thereof in a West African population of HIV-1–discordant (one partner HIV-1–positive, other partner HIV-1–negative) and HIV-1–concordant (both partners HIV-1 positive) couples. In agreement with our previous study of HESN female sex workers,28 we found that HIV-1 transmission in heterosexual couples depends on the KIR/HLA genotype of the recipient partners. In addition, HIV-1 transmission and lack thereof correlated with the presence of specific matched and mismatched allogeneic KIR/HLA combinations across partners which we found were predictive of NK cell–mediated killing of allogeneic target cells.
Methods
Study populations
HIV-1–discordant, HIV-1–concordant, and HIV-negative couples were recruited at the outpatient clinic of Fann Hospital in Dakar, Senegal. Blood samples and standard questionnaires with information on socio-demographics and sexual behavior were collected. Index and recipient partners in HIV-1–discordant couples were the HIV-1–infected and uninfected partners, respectively, with all uninfected partners considered as HESN. All HIV-1–concordant couples included in this study showed confirmed phylogenetic linkage of transmitted viruses and directionality of intracouple transmission,35 and index and recipient partners were assigned accordingly. For the functional studies, blood samples were obtained from healthy blood donors and HIV-1 patients at the Antwerp Blood Transfusion Center and Institute of Tropical Medicine, respectively. All HIV-1 patients had CD4 counts > 500 cells/μL, one-half of them were under antiretroviral therapy. The studies were approved by the Internal Review Board of the Institute of Tropical Medicine and by the Ethical Committees of the Senegalese Ministry of Health and the University Hospital of Antwerp. All study subjects gave written informed consent before enrollment in accordance with the Declaration of Helsinki.
Sample collection and HIV testing
Whole blood was drawn into EDTA tubes (Becton Dickinson). Plasma and peripheral blood mononuclear cells (PBMCs) were separated from whole blood by density gradient centrifugation. Plasma and dry PBMC pellets were stored at −80°C. HIV status was evaluated in plasma by current serologic testing combining ELISAs and Western blots. The HIV-negative status of HESN partners in HIV-1–discordant couples was confirmed by HIV-1 and HIV-2–diagnostic PCR on genomic DNA extracted from PBMCs.
KIR and HLA genotyping
Genomic DNA was extracted from PBMCs using a QIAamp DNA blood mini kit (QIAGEN). The presence or absence of 5 inhibitory KIR genes (KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL5, and KIR3DL1), 6 activating KIR genes (KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5, and KIR3DS1) and 1 framework inhibitory KIR gene (KIR3DL2) was determined by PCR with sequence specific oligonucleotides using Lifecodes KIR genotyping kits (Gen-Probe). KIR genes segregate in distinctive group A and group B haplotypes at the centromeric, as well as at the telomeric, part of the KIR locus.36 Centromeric and telomeric KIR genotypes were assigned according to the definition applied by Cooley et al.37 In brief, centromeric AA genotypes contain KIR2DL3 but no KIR2DL2 or KIR2DS2, centromeric AB genotypes contain KIR2DL3 with KIR2DL2 and/or KIR2DS2, and centromeric BB genotypes contain KIR2DL2 and/or KIR2DS2 but no KIR2DL3. Telomeric AA genotypes contain KIR3DL1 and KIR2DS4 but no KIR3DS1 or KIR2DS1, telomeric AB genotypes contain KIR3DL1 and KIR2DS4 with KIR3DS1 and/or KIR2DS1, and telomeric BB genotypes lack KIR3DL1 and/or KIR2DS4. HLA-B and HLA-C typing was performed by PCR with sequence specific oligonucleotides using LABType SSO genotyping kits (One Lambda). The 2-digit typing results permit to distinguish Bw4-80I and Bw4-80T alleles from Bw6 alleles, and C1 alleles from C2 alleles.
NK cell killing assay
PBMCs were separated from fresh whole blood and buffy coat samples obtained from HIV-1 patients and healthy blood donors, respectively. NK cells were magnetically purified from blood donor PBMCs by negative selection according to the manufacturer's instructions (Miltenyi Biotec), and incubated at 37°C and 5% CO2 during 2 days in R10 (RPMI containing 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 10% FBS) supplemented with 200 units/mL IL-2 (Gentaur). CD4+ T cells were magnetically purified from HIV-1 patient PBMCs by positive selection according to the manufacturer's instructions (Miltenyi Biotec), and incubated overnight in R10. NK cells were cocultured with CD4+ T cells at an effector-target ratio of 10:1 in R10 during 4 hours. As a positive control, NK-susceptible K562 target cells were labeled with PKH67 (Sigma-Aldrich; according to the manufacturer's instructions) and cocultured with NK cells at an effector-target ratio of 2.5:1. As negative controls, target cells were cultured with medium alone. After incubation, cells were stained with CD3-FITC and CD4-PE (Becton Dickinson; CD4+ T cell–containing cultures only), and with 7-AAD and annexin V–APC (Becton Dickinson; all cultures) and acquired on a FACSCalibur flow cytometer (Becton Dickinson).
Statistical analyses
Demographic and clinical characteristics of the different couple groups were compared with χ2 tests or Fisher exact tests (when cells in the contingency table had expected counts less than 5) for categorical data and with Mann-Whitney U tests for continuous data. KIR and HLA allele frequencies were compared between 2 groups with χ2 tests or Fisher exact tests. Multivariate analysis of selected KIR/HLA combinations was done with stepwise logistic regression. Model selection was guided by the Akaike information criterion (AIC), which assesses the fit between the data and the model with a penalty for the number of parameters (ie, favoring the more parsimonious model). Levels of in vitro target cell killing were compared between selected KIR/HLA combinations with Kruskal-Wallis or Mann-Whitney U tests. Statistical analyses were performed with SPSS Version 16.0, R Version 2.15.0, or GraphPad Prism Version 5.
Results
Characteristics of the study population
Thirty-five HIV-1–discordant, 35 HIV-1–concordant, and 38 HIV-negative couples were included in the study. Demographic, clinical and behavioral characteristics of the couples are summarized in Table 1. The different couple groups showed comparable age distributions, they were all predominantly of Senegalese origin, and they reported comparable relationship durations and sexual contact frequencies. HIV-1–discordant couples reported more frequent condom use than HIV-negative and HIV-1–concordant couples. Index partners in HIV-1–concordant couples were more frequently the male partner compared with those in HIV-1–discordant couples.
Demographic and clinical characteristics of the study population
| Characteristic . | DC (n = 35) . | NC (n = 38) . | CC (n = 35) . | DC vs NC P‡ . | DC vs CC P‡ . |
|---|---|---|---|---|---|
| Age, y | |||||
| Male partners | 46 (38-52) | 42 (35-49) | 47 (41-53) | .194 | .502 |
| Female partners | 34 (29-40) | 36 (30-41) | 33 (29-40) | .510 | .606 |
| Senegalese origin, n (%)* | |||||
| Male partners | 31 (89) | 36 (95) | 33 (94) | .418 | .673 |
| Female partners | 32 (91) | 36 (95) | 34 (97) | .666 | .614 |
| Duration of sexual relation, y† | 8 (4-15) | 8 (4-16) | 10 (6-13) | .782 | .259 |
| Sexual contacts/mo† | 5 (4-8) | 8 (3-12) | 8 (4-12) | .536 | .199 |
| Condom use, n (%)† | |||||
| Never | 5 (15) | 29 (78) | 10 (29) | < .001 | .010 |
| < 50% of the time | 4 (12) | 7 (19) | 13 (37) | ||
| > 50% of the time | 1 (3) | 0 (0) | 1 (3) | ||
| Always | 24 (71) | 1 (3) | 11 (31) | ||
| Male index partners, n (%) | 16 (46) | NA | 33 (94) | NA | < .001 |
| Time since HIV diagnosis, y | |||||
| Index partners | 2.0 (0.7-4.9) | NA | 3.0 (0.8-5.1) | NA | .386 |
| Recipient partners | NA | NA | 2.3 (0.6-3.5) | NA | NA |
| Antiretroviral therapy, n (%) | |||||
| Index partners | 27 (77) | NA | 27 (79) | NA | .819 |
| Recipient partners | NA | NA | 16 (46) | NA | NA |
| CD4 count, cells/μL | |||||
| Index partners | 227 (70-366) | NA | 239 (184-345) | NA | .247 |
| Recipient partners | NA | NA | 309 (242-522) | NA | NA |
| Viral load, log10 copies/mL | |||||
| Index partners | 1.8 (1.7-4.3) | NA | 1.7 (1.7-4.0) | NA | .714 |
| Recipient partners | NA | NA | 3.4 (1.7-4.9) | NA | NA |
| Characteristic . | DC (n = 35) . | NC (n = 38) . | CC (n = 35) . | DC vs NC P‡ . | DC vs CC P‡ . |
|---|---|---|---|---|---|
| Age, y | |||||
| Male partners | 46 (38-52) | 42 (35-49) | 47 (41-53) | .194 | .502 |
| Female partners | 34 (29-40) | 36 (30-41) | 33 (29-40) | .510 | .606 |
| Senegalese origin, n (%)* | |||||
| Male partners | 31 (89) | 36 (95) | 33 (94) | .418 | .673 |
| Female partners | 32 (91) | 36 (95) | 34 (97) | .666 | .614 |
| Duration of sexual relation, y† | 8 (4-15) | 8 (4-16) | 10 (6-13) | .782 | .259 |
| Sexual contacts/mo† | 5 (4-8) | 8 (3-12) | 8 (4-12) | .536 | .199 |
| Condom use, n (%)† | |||||
| Never | 5 (15) | 29 (78) | 10 (29) | < .001 | .010 |
| < 50% of the time | 4 (12) | 7 (19) | 13 (37) | ||
| > 50% of the time | 1 (3) | 0 (0) | 1 (3) | ||
| Always | 24 (71) | 1 (3) | 11 (31) | ||
| Male index partners, n (%) | 16 (46) | NA | 33 (94) | NA | < .001 |
| Time since HIV diagnosis, y | |||||
| Index partners | 2.0 (0.7-4.9) | NA | 3.0 (0.8-5.1) | NA | .386 |
| Recipient partners | NA | NA | 2.3 (0.6-3.5) | NA | NA |
| Antiretroviral therapy, n (%) | |||||
| Index partners | 27 (77) | NA | 27 (79) | NA | .819 |
| Recipient partners | NA | NA | 16 (46) | NA | NA |
| CD4 count, cells/μL | |||||
| Index partners | 227 (70-366) | NA | 239 (184-345) | NA | .247 |
| Recipient partners | NA | NA | 309 (242-522) | NA | NA |
| Viral load, log10 copies/mL | |||||
| Index partners | 1.8 (1.7-4.3) | NA | 1.7 (1.7-4.0) | NA | .714 |
| Recipient partners | NA | NA | 3.4 (1.7-4.9) | NA | NA |
Data are obtained at enrollment of the couples and are representative of that moment. Data are median values (interquartile range) or n (%) when indicated.
DC indicates HIV-1–discordant couples; NC, HIV-negative couples; CC, HIV-1–concordant couples; and NA, not applicable.
Other countries of origin were Guinea, Sierra Leone, Mali, Togo, Guinea Bissau, and Mauretania.
Based on female partners' data; comparable results were obtained when male partners' data were analyzed.
Categorical data were analyzed with χ2 tests or Fisher exact tests when cells had expected counts less than 5. Continuous data were analyzed with Mann-Whitney U tests. P values < .05 are in bold.
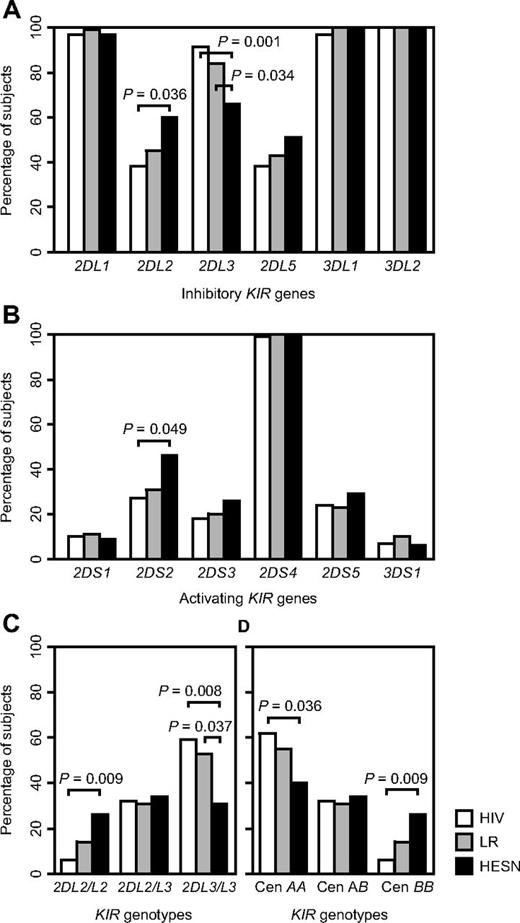
HESN subjects show a higher frequency of KIR2DL2 and a lower frequency of KIR2DL3 than HIV-1–infected controls
Previous studies observed specific KIR profiles in HESN subjects.28,30,32 To confirm these findings, we compared frequencies of KIR genes and KIR genotypes between HESN partners (HIV uninfected partners in HIV-1–discordant couples), HIV-1–infected controls (both partners of HIV-1–concordant couples), and low risk controls (both partners of HIV-negative couples). HESN subjects displayed significantly higher frequencies of KIR2DL2 and KIR2DS2 and a significantly lower frequency of KIR2DL3 than HIV-1–infected controls (Figure 1A-B). KIR2DL2 and KIR2DL3, alleles of the same gene locus, are characteristic of centromeric group B and group A haplotypes, respectively.36,37 HESN subjects were more frequently KIR2DL2 homozygous and less frequently KIR2DL3 homozygous than HIV-1–infected controls, consistent with higher frequencies of centromeric KIR BB and lower frequencies of KIR AA genotypes among HESN subjects than among HIV-1–infected controls (Figure 1C-D). No such differences were noted at the telomeric end of the KIR locus, marked by KIR3DL1/S1, KIR2DS4, and KIR2DS1 (data not shown). Although not always reaching statistical significance, low-risk controls showed KIR frequencies in between those from HESN subjects and HIV-1–infected controls (Figure 1A-D).
Frequencies of KIR genes and KIR genotypes in HIV-exposed seronegative subjects compared with those in low risk controls and HIV-1–infected subjects. Differences between groups are compared by χ2 tests or Fisher exact tests when cells have expected counts less than 5. HESN, HIV-exposed seronegative subjects, n = 35; LR, low risk controls, n = 74; HIV, HIV-1–infected controls, n = 68; Cen, centromeric; P values < .05 are shown. (A) Inhibitory KIR genes. (B) Activating KIR genes. (C) KIR2DL2/KIR2DL3 genotypes. (D) Centromeric KIR genotypes.
Frequencies of KIR genes and KIR genotypes in HIV-exposed seronegative subjects compared with those in low risk controls and HIV-1–infected subjects. Differences between groups are compared by χ2 tests or Fisher exact tests when cells have expected counts less than 5. HESN, HIV-exposed seronegative subjects, n = 35; LR, low risk controls, n = 74; HIV, HIV-1–infected controls, n = 68; Cen, centromeric; P values < .05 are shown. (A) Inhibitory KIR genes. (B) Activating KIR genes. (C) KIR2DL2/KIR2DL3 genotypes. (D) Centromeric KIR genotypes.
HESN subjects show a higher frequency of KIR2DL2 in the absence of HLA-C1 than HIV-1–infected controls
KIR2DL2 and KIR2DL3 regulate NK cell function in an HLA-C–dependent manner. Therefore, we investigated whether the observed differences in KIR2DL2/KIR2DL3 frequencies between the subject groups were influenced by concurrent carriage of cognate HLA-C alleles, first within the same individuals. On their own, there were no differences in C1 or C2 allele frequencies between the 3 subject groups (data not shown). The higher frequency of homozygous KIR2DL3 among HIV-1–infected controls did not preferably occur together with C1 or C2 (data not shown). However, the higher frequency of homozygous KIR2DL2 among HESN subjects appeared statistically significant only in the absence of C1 (ie, together with homozygous C2; 9% of HESN versus 0% of HIV-1–infected controls, P = .037). We found no differences in the frequencies of Bw4-80I or Bw4-80T, alone or in combination with KIR3DS1 or KIR3DL1, between the 3 groups (data not shown).
HIV-1–discordant and concordant couples are characterized by distinct allogeneic KIR/HLA combinations
HIV-1 transmission occurs in part through incoming HIV-1–infected cells (cell-associated virus) present in genital secretions,38,39 which could be efficiently targeted by allogeneic NK cell responses. We hypothesized that such responses are dependent on the level of allogeneic KIR/HLA incompatibility between sexual partners, similar to what is observed in haploidentical hematopoietic stem cell transplantation with missing self or matched KIR/HLA combinations determining whether NK cells will be activated or not.11 To test this, we compared frequencies of missing self and matched KIR/HLA combinations between partners of HIV-1–discordant and concordant couples included in our study (Table 2). We found that HIV-1–discordant couples were characterized by a recipient partner KIR2DL1/HLA-C2 with index partner HLA-C1/C1 combination (OR = 10.1, P = .028), allowing licensed missing self NK cell killing of index cells through KIR2DL1. HIV-1–concordant couples on the other hand were characterized by KIR2DL3 homozygous recipient partners with HLA-C1/C2 bearing index partners (OR = 0.06, P = .001), resulting in a matched KIR/HLA combination expected to inhibit NK cell killing through KIR2DL3 (and KIR2DL1, which is present in most subjects of our study population). The higher frequency of homozygous KIR2DL2 among HESN recipient partners in HIV-1–discordant couples did not significantly correlate with a specific index partner HLA-C genotype. We found no differences in allogeneic combinations of KIR3DS1 or KIR3DL1 with HLA-Bw4 between HIV-1–discordant and concordant couples (data not shown).
Frequencies of matched and missing self allogeneic KIR/HLA combinations across recipient and index partners in HIV-1–discordant and concordant couples
| Type* . | Recipient . | Index . | DC (n = 35) . | CC (n = 35) . | DC vs CC . | |||
|---|---|---|---|---|---|---|---|---|
| KIR . | HLA . | HLA . | OR . | 95% CI . | P† . | |||
| Matched | 2DL1 | Any | C1/C2 | 32 | 54 | 0.40 | 0.15-1.07 | .066 |
| Matched | 2DL1 | Any | C2/C2 | 24 | 23 | 1.04 | 0.34-3.18 | .947 |
| Missing self | 2DL1 | C1/C2 | C1/C1 | 23 | 3 | 10.1 | 1.19-85.6 | .028 |
| Missing self | 2DL1 | C2/C2 | C1/C1 | 9 | 11 | 0.75 | 0.16-3.63 | 1.000 |
| Matched | 2DL2/2DL2 | Any | C1/C1 | 6 | 0 | NA | NA | .239 |
| Matched | 2DL2/2DL2 | Any | C1/C2 | 18 | 6 | 3.54 | 0.66-18.9 | .151 |
| Missing self | 2DL2/2DL2 | C1/C1 | C2/C2 | 0 | 0 | NA | NA | NA |
| Missing self | 2DL2/2DL2 | C1/C2 | C2/C2 | 0 | 0 | NA | NA | NA |
| Matched | 2DL2/2DL3 | Any | C1/C1 | 17 | 11 | 1.60 | 0.41-6.26 | .495 |
| Matched | 2DL2/2DL3 | Any | C1/C2 | 11 | 14 | 0.77 | 0.19-3.16 | 1.000 |
| Missing self | 2DL2/2DL3 | C1/C1 | C2/C2 | 3 | 0 | NA | NA | 1.000 |
| Missing self | 2DL2/2DL3 | C1/C2 | C2/C2 | 0 | 0 | NA | NA | NA |
| Matched | 2DL3/2DL3 | Any | C1/C1 | 14 | 9 | 1.78 | 0.39-8.09 | .710 |
| Matched | 2DL3/2DL3 | Any | C1/C2 | 3 | 34 | 0.06 | 0.01-0.46 | .001 |
| Missing self | 2DL3/2DL3 | C1/C1 | C2/C2 | 6 | 9 | 0.65 | 0.10-4.13 | 1.000 |
| Missing self | 2DL3/2DL3 | C1/C2 | C2/C2 | 9 | 6 | 1.55 | 0.24-9.88 | 1.000 |
| Type* . | Recipient . | Index . | DC (n = 35) . | CC (n = 35) . | DC vs CC . | |||
|---|---|---|---|---|---|---|---|---|
| KIR . | HLA . | HLA . | OR . | 95% CI . | P† . | |||
| Matched | 2DL1 | Any | C1/C2 | 32 | 54 | 0.40 | 0.15-1.07 | .066 |
| Matched | 2DL1 | Any | C2/C2 | 24 | 23 | 1.04 | 0.34-3.18 | .947 |
| Missing self | 2DL1 | C1/C2 | C1/C1 | 23 | 3 | 10.1 | 1.19-85.6 | .028 |
| Missing self | 2DL1 | C2/C2 | C1/C1 | 9 | 11 | 0.75 | 0.16-3.63 | 1.000 |
| Matched | 2DL2/2DL2 | Any | C1/C1 | 6 | 0 | NA | NA | .239 |
| Matched | 2DL2/2DL2 | Any | C1/C2 | 18 | 6 | 3.54 | 0.66-18.9 | .151 |
| Missing self | 2DL2/2DL2 | C1/C1 | C2/C2 | 0 | 0 | NA | NA | NA |
| Missing self | 2DL2/2DL2 | C1/C2 | C2/C2 | 0 | 0 | NA | NA | NA |
| Matched | 2DL2/2DL3 | Any | C1/C1 | 17 | 11 | 1.60 | 0.41-6.26 | .495 |
| Matched | 2DL2/2DL3 | Any | C1/C2 | 11 | 14 | 0.77 | 0.19-3.16 | 1.000 |
| Missing self | 2DL2/2DL3 | C1/C1 | C2/C2 | 3 | 0 | NA | NA | 1.000 |
| Missing self | 2DL2/2DL3 | C1/C2 | C2/C2 | 0 | 0 | NA | NA | NA |
| Matched | 2DL3/2DL3 | Any | C1/C1 | 14 | 9 | 1.78 | 0.39-8.09 | .710 |
| Matched | 2DL3/2DL3 | Any | C1/C2 | 3 | 34 | 0.06 | 0.01-0.46 | .001 |
| Missing self | 2DL3/2DL3 | C1/C1 | C2/C2 | 6 | 9 | 0.65 | 0.10-4.13 | 1.000 |
| Missing self | 2DL3/2DL3 | C1/C2 | C2/C2 | 9 | 6 | 1.55 | 0.24-9.88 | 1.000 |
Data are percentages. P values < .05 are in bold.
DC indicates HIV-1 discordant couples; CC, HIV-1 concordant couples; OR, odd's ratio; CI, confidence interval; and NA, not applicable.
According to models described by Moretta et al.11 “Matched” occurs when the specified inhibitory KIR of the recipient recognizes an HLA-C allotype present in the index (regardless of recipient HLA). “Missing self” occurs when the specified inhibitory KIR of the recipient recognizes an HLA-C allotype present in the recipient that is lacking in the index.
χ2 tests or Fisher exact tests when cells have expected counts less than 5.
Allogeneic KIR/HLA combinations independently predict couple status
Next, we investigated whether the identified KIR/HLA combinations correlated independently with the HIV-1–discordant or concordant status of the couples by use of stepwise multivariate logistic regression (Table 3). All models were controlled for index partner sex, which was significantly different between HIV-1–discordant and concordant couples. The missing self KIR2DL1/HLA-C2 with HLA-C1/C1 combination and the matched homozygous KIR2DL3 with HLA-C1/C2 combination independently predicted couple status and together they made up the best model (Table 3 model 3). Recipient homozygous KIR2DL3 and to a large extent also recipient homozygous KIR2DL2 did not independently contribute to the couple status (Table 3 models 1-2). This suggests that within the context of a stable heterosexual relationship, recipient KIR genotype does not independently influence HIV-1 acquisition but depends on the HLA genotype of the index partner.
Multivariate analysis of KIR/HLA effects on HIV-1 discordant and concordant couple status
| Predictor variables . | OR . | 95% CI . | P . | AIC . |
|---|---|---|---|---|
| Model 1 | ||||
| Index male sex | 0.02 | 0.003-0.19 | < .001 | 63.9 |
| Recipient KIR2DL2/KIR2DL2 | 3.48 | 0.39-31.1 | .264 | |
| Recipient KIR2DL1/C1/C2 + index C1/C1 | 17.6 | 1.80-174 | .014 | |
| Recipient KIR2DL3/KIR2DL3 | 1.21 | 0.26-5.71 | .809 | |
| Recipient KIR2DL3/KIR2DL3 + index C1/C2 | 0.04 | 0.002-0.86 | .039 | |
| Model 2 | ||||
| Index male sex | 0.02 | 0.003-0.20 | < .001 | 62.0 |
| Recipient KIR2DL2/KIR2DL2 | 3.17 | 0.41-24.5 | .269 | |
| Recipient KIR2DL1/C1/C2 + index C1/C1 | 17.3 | 1.80-170 | .014 | |
| Recipient KIR2DL3/KIR2DL3 + index C1/C2 | 0.05 | 0.003-0.82 | .036 | |
| Model 3 | ||||
| Index male sex | 0.02 | 0.003-0.18 | < .001 | 61.2 |
| Recipient KIR2DL1/C1/C2 + index C1/C1 | 14.5 | 1.50-138 | .020 | |
| Recipient KIR2DL3/KIR2DL3 + index C1/C2 | 0.04 | 0.002-0.66 | .025 |
| Predictor variables . | OR . | 95% CI . | P . | AIC . |
|---|---|---|---|---|
| Model 1 | ||||
| Index male sex | 0.02 | 0.003-0.19 | < .001 | 63.9 |
| Recipient KIR2DL2/KIR2DL2 | 3.48 | 0.39-31.1 | .264 | |
| Recipient KIR2DL1/C1/C2 + index C1/C1 | 17.6 | 1.80-174 | .014 | |
| Recipient KIR2DL3/KIR2DL3 | 1.21 | 0.26-5.71 | .809 | |
| Recipient KIR2DL3/KIR2DL3 + index C1/C2 | 0.04 | 0.002-0.86 | .039 | |
| Model 2 | ||||
| Index male sex | 0.02 | 0.003-0.20 | < .001 | 62.0 |
| Recipient KIR2DL2/KIR2DL2 | 3.17 | 0.41-24.5 | .269 | |
| Recipient KIR2DL1/C1/C2 + index C1/C1 | 17.3 | 1.80-170 | .014 | |
| Recipient KIR2DL3/KIR2DL3 + index C1/C2 | 0.05 | 0.003-0.82 | .036 | |
| Model 3 | ||||
| Index male sex | 0.02 | 0.003-0.18 | < .001 | 61.2 |
| Recipient KIR2DL1/C1/C2 + index C1/C1 | 14.5 | 1.50-138 | .020 | |
| Recipient KIR2DL3/KIR2DL3 + index C1/C2 | 0.04 | 0.002-0.66 | .025 |
Data are calculated by binomial logistic regression analysis. P values < .05 are in bold. OR values > 1 are predictive of HIV-1–discordant status, OR values < 1 are predictive of HIV-1–concordant status. Models 2 and 3 are obtained from model 1 by backwards stepwise selection based on the AIC. The model with the lowest AIC shows the best fit.
OR indicates odds ratio; CI, confidence interval; and AIC, Akaike Information Criterion.
Allogeneic KIR/HLA combinations determine NK cell killing of HIV-1 patient-derived CD4+ T cells
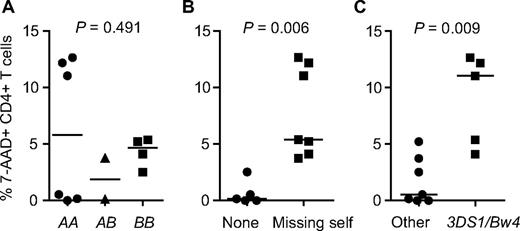
Finally, we investigated whether the observed KIR/HLA combinations correlated with in vitro allogeneic NK cell killing of HIV-1 patient-derived CD4+ T cells (Table 4, Figure 2). Healthy blood donor–derived NK cells were activated during 2 days with IL-2, cocultured with HIV-1 patient-derived CD4+ T cells for 4 hours, and assessed for levels of CD4+ T-cell killing by flow cytometry. Six blood donors and 12 HIV-1 patients were tested, with each blood donor being assessed against 2 different HIV-1 patients (1 therapy naive and 1 therapy treated patient). All blood donors displayed high levels of NK cell killing of K562 cells, which were added as a positive control (data not shown). Levels of CD4+ T-cell killing varied extensively between donor/patient pairs but did not correlate with the viral load or therapy status of the HIV-1 patients (Table 4). Of the 12 donor/patient pairs tested, 7 were characterized by a licensed missing self KIR/HLA combination (1 of which had the exact donor KIR2DL1/HLA-C2 with patient HLA-C1/C1 combination characteristic of HIV-1–discordant couples) and these showed the highest levels of CD4+ T-cell killing (Table 4, Figure 2). Five donor/patient pairs lacked a missing self KIR/HLA combination (3 of which had the homozygous KIR2DL3 with HLA-C1/C2 combination characteristic of HIV-1–concordant couples) and these showed the lowest levels of CD4 killing (Table 4, Figure 2). The KIR3DS1/HLA-Bw4 combination, previously associated with protection against HIV-1 disease progression and in vitro inhibition of viral replication,20,27,40 but tested here in allogeneic constellation, also showed high levels of CD4+ T-cell killing (Table 4, Figure 2). Possession of a centromeric KIR BB genotype (ie, homozygous KIR2DL2) did not associate with increased levels of NK cell–mediated killing.
In vitro allogeneic NK cell responses against HIV-1 patient-derived CD4+ T cells as a function of KIR/HLA genotype
| NK cells . | CD4+ T cells . | Donor KIR/HLA . | Patient HLA . | Donor/patient KIR/HLA . | CD4+ T-cell killing . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood donor . | Patient . | HIV-1 load* . | KIR† . | KIR2DL . | HLA-C . | HLA-C . | Missing self‡ . | 3DS1/Bw4 . | 7-AAD (%) . | Annexin V (%) . |
| BFC01 | ITM01 | 4.52 | AA | 2DL1 + 2DL3/3 | C2/C2 | C1/C1 | 2DL1 | Yes | 12.65 | 3.85 |
| BFC08 | ITM21 | < 1.30 | AA | 2DL1 + 2DL3/3 | C1/C1 | C2/C2 | 2DL3 | Yes | 12.17 | 12.87 |
| BFC01 | ITM03 | < 1.30 | AA | 2DL1 + 2DL3/3 | C2/C2 | C1/C1 | 2DL1 | Yes | 11.04 | 4.87 |
| BFC05 | ITM13 | 5.43 | BB | 2DL1 + 2DL2/2 | C1/C2 | C2/C2 | 2DL2 | Yes | 5.38 | 7.05 |
| BFC06 | ITM15 | < 1.30 | BB | 2DL1 + 2DL2/2 | C1/C2 | C1/C1 | 2DL1 | No | 5.21 | 6.41 |
| BFC05 | ITM17 | < 1.30 | BB | 2DL1 + 2DL2/2 | C1/C2 | C2/C2 | 2DL2 | Yes | 4.10 | 4.93 |
| BFC07 | ITM19 | < 1.30 | AB | 2DL1 + 2DL2/3 | C1/C2 | C2/C2 | 2DL2/3 | No | 3.73 | 4.48 |
| BFC06 | ITM18 | < 1.30 | BB | 2DL1 + 2DL2/2 | C1/C2 | C1/C2 | None | No | 2.52 | 2.69 |
| BFC03 | ITM12 | < 1.30 | AA | 2DL1 + 2DL3/3 | C2/C2 | C1/C2 | None | No | 0.53 | 1.22 |
| BFC03 | ITM09 | 5.53 | AA | 2DL1 + 2DL3/3 | C2/C2 | C1/C2 | None | No | 0.16 | 0 |
| BFC08 | ITM24 | 4.64 | AA | 2DL1 + 2DL3/3 | C1/C1 | C1/C2 | None | No | 0 | 0 |
| BFC07 | ITM23 | 4.71 | AB | 2DL1 + 2DL2/3 | C1/C2 | C1/C2 | None | No | 0 | 0 |
| NK cells . | CD4+ T cells . | Donor KIR/HLA . | Patient HLA . | Donor/patient KIR/HLA . | CD4+ T-cell killing . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood donor . | Patient . | HIV-1 load* . | KIR† . | KIR2DL . | HLA-C . | HLA-C . | Missing self‡ . | 3DS1/Bw4 . | 7-AAD (%) . | Annexin V (%) . |
| BFC01 | ITM01 | 4.52 | AA | 2DL1 + 2DL3/3 | C2/C2 | C1/C1 | 2DL1 | Yes | 12.65 | 3.85 |
| BFC08 | ITM21 | < 1.30 | AA | 2DL1 + 2DL3/3 | C1/C1 | C2/C2 | 2DL3 | Yes | 12.17 | 12.87 |
| BFC01 | ITM03 | < 1.30 | AA | 2DL1 + 2DL3/3 | C2/C2 | C1/C1 | 2DL1 | Yes | 11.04 | 4.87 |
| BFC05 | ITM13 | 5.43 | BB | 2DL1 + 2DL2/2 | C1/C2 | C2/C2 | 2DL2 | Yes | 5.38 | 7.05 |
| BFC06 | ITM15 | < 1.30 | BB | 2DL1 + 2DL2/2 | C1/C2 | C1/C1 | 2DL1 | No | 5.21 | 6.41 |
| BFC05 | ITM17 | < 1.30 | BB | 2DL1 + 2DL2/2 | C1/C2 | C2/C2 | 2DL2 | Yes | 4.10 | 4.93 |
| BFC07 | ITM19 | < 1.30 | AB | 2DL1 + 2DL2/3 | C1/C2 | C2/C2 | 2DL2/3 | No | 3.73 | 4.48 |
| BFC06 | ITM18 | < 1.30 | BB | 2DL1 + 2DL2/2 | C1/C2 | C1/C2 | None | No | 2.52 | 2.69 |
| BFC03 | ITM12 | < 1.30 | AA | 2DL1 + 2DL3/3 | C2/C2 | C1/C2 | None | No | 0.53 | 1.22 |
| BFC03 | ITM09 | 5.53 | AA | 2DL1 + 2DL3/3 | C2/C2 | C1/C2 | None | No | 0.16 | 0 |
| BFC08 | ITM24 | 4.64 | AA | 2DL1 + 2DL3/3 | C1/C1 | C1/C2 | None | No | 0 | 0 |
| BFC07 | ITM23 | 4.71 | AB | 2DL1 + 2DL2/3 | C1/C2 | C1/C2 | None | No | 0 | 0 |
Every healthy blood donor is assessed against 2 different HIV-1 patients. The data are sorted in descending order based on CD4+ T cell killing (% 7-AAD). There were no missing self combinations at the level of KIR3DL1/HLA-Bw4.
Data are log10 HIV-1 RNA copies/mL. All patients with undetectable (< 1.30 log10 copies/mL) are under antiretroviral therapy, all patients with detectable (> 1.30 log10 copies/mL) are therapy-naive.
Centromeric KIR genotypes.
Defined as the blood donor inhibitory KIRs that recognized an HLA allotype present in the blood donor but that was lacking in the HIV-1 patient.11
In vitro allogeneic NK cell killing of HIV-1 patient-derived CD4+ T cells as a function of KIR/HLA genotype. Differences between groups were analyzed by Kruskal-Wallis or Mann-Whitney U tests, horizontal lines represent median values. (A) Effect of centromeric KIR genotypes of NK cell donor. (B) Effect of missing self NK cell donor/HIV-1 patient KIR/HLA combinations. Missing self combinations are defined as NK cell donor inhibitory KIRs that recognize an HLA allotype present in the NK cell donor but that is lacking in the HIV-1 patient.11 See “Results” and Table 4 for details. (C) Effect of allogeneic NK cell donor KIR3DS1 in combination with HIV-1 patient HLA-Bw4.
In vitro allogeneic NK cell killing of HIV-1 patient-derived CD4+ T cells as a function of KIR/HLA genotype. Differences between groups were analyzed by Kruskal-Wallis or Mann-Whitney U tests, horizontal lines represent median values. (A) Effect of centromeric KIR genotypes of NK cell donor. (B) Effect of missing self NK cell donor/HIV-1 patient KIR/HLA combinations. Missing self combinations are defined as NK cell donor inhibitory KIRs that recognize an HLA allotype present in the NK cell donor but that is lacking in the HIV-1 patient.11 See “Results” and Table 4 for details. (C) Effect of allogeneic NK cell donor KIR3DS1 in combination with HIV-1 patient HLA-Bw4.
Discussion
In this study, we investigated whether allogeneic KIR/HLA combinations are associated with HIV-1 transmission and lack thereof in a Senegalese population of HIV-1–discordant and concordant couples. Allogeneic KIR/HLA combinations allowing licensed NK cell killing of KIR ligand-mismatched target cells were found to improve survival in leukemia patients undergoing hematopoietic stem cell transplantation.8-11 Certain KIR/HLA genotypes are associated with acquisition and/or transmission of HIV-1,28-34 but the effect of allogeneic recipient/index KIR/HLA combinations has never been investigated. We found that HIV-1 acquisition was associated with the KIR/HLA genotype of the recipient partners, confirming previous studies. In addition, transmission correlated with recipient/index allogeneic KIR/HLA combinations predictive of recipient NK cell alloreactivity toward index target cells.
HESN recipient partners in HIV-1–discordant couples showed increased frequencies of centromeric KIR BB genotypes consisting of homozygous KIR2DL2 with or without KIR2DS2, whereas HIV-1–infected controls more frequently had centromeric AA genotypes consisting of homozygous KIR2DL3. Interestingly, the increased frequency of homozygous KIR2DL2 among HESN recipient partners was only statistically significant in the absence of its HLA-C1 ligand. These data corroborate our previous findings in HESN female sex workers from Côte d'Ivoire who were also characterized by group B KIR haplotypes and specific autologous inhibitory KIR/HLA mismatches.28 Other studies of European and Asian HESN populations found higher frequencies of KIR3DS1,30 lower frequencies of KIR3DL1 in the presence of its HLA-Bw4 ligand,32 and increased KIR3DS1/KIR3DL1 transcript ratios,29 together supporting the conclusion that KIR/HLA genotypes with an activating profile are protective against HIV-1 acquisition.
HIV-1 transmission occurs at least in part through incoming HIV-1–infected cells (cell-associated virus) present in genital secretions.38,39 We hypothesized that NK cells from the recipient partner capable of directly eliminating incoming HIV-infected cells from the index partner before any viral replication in the recipient can occur could offer strong protection against HIV-1 acquisition. According to the principles of missing self and NK cell education,5 such NK cell responses would be regulated by KIR expressed on recipient NK cells sensing the lack of self HLA on incoming index cells, a process that would directly depend on the level of allogeneic KIR/HLA incompatibility between sexual partners. In line with this hypothesis, we found that HIV-1–discordant couples were characterized by a mismatched recipient KIR2DL1/HLA-C2 with index HLA-C1/C1 combination allowing licensed missing self NK cell killing of index cells through KIR2DL1. HIV-1–concordant couples on the other hand were characterized by a matched allogeneic KIR/HLA combination consisting of recipient homozygous KIR2DL3 with index HLA-C1/C2 preventing missing self NK cell killing of index cells through KIR2DL3 (and KIR2DL1 which is present in the majority of subjects). This latter matched combination is likely independent of the HLA genotype of the recipient, because recipient NK cells will be inhibited by index HLA regardless of whether they have been educated or not. In a multivariate regression model, these 2 allogeneic KIR/HLA combinations strongly and independently predicted couple HIV-1 serostatus with no or little additional effect for the homozygous KIR2DL2 or KIR2DL3 genotypes of the recipient partners. This suggests that within the context of a stable heterosexual relationship, recipient KIR genotype depends largely on index HLA genotype to influence HIV-1 transmission.
These genotype findings were confirmed in functional experiments using healthy blood donor–derived NK cells and HIV-1 patient-derived CD4+ T cells showing that NK cell–mediated CD4+ T-cell killing correlated with the presence of matched or mismatched allogeneic KIR/HLA combinations. These combinations included the matched and mismatched KIR/HLA combinations characteristic of HIV-1–discordant and concordant couples in addition to other KIR2DL/HLA-C combinations with similar expected effects. Interestingly, the allogeneic KIR3DS1/HLA-Bw4 combination also correlated with increased levels of in vitro NK cell alloreactivity, extending the known role of autologous KIR3DS1/HLA-Bw4 genotypes in protection against HIV-1 disease progression and in vitro inhibition of viral replication.20,27,40 Carriage of a centromeric KIR BB genotype (homozygous KIR2DL2) on its own was not sufficient to induce higher levels of CD4+ T-cell killing, confirming that target cell HLA is an important factor determining NK cell alloreactivity.8-11 The fact that other KIR/HLA genotypes influenced in vitro NK cell alloreactivity in addition to those identified in our Senegalese cohort possibly reflects the fact that samples from European origin were used for the functional experiments. Therefore, future studies should analyze in vitro NK cell alloreactivity in samples from African origin, as well as include larger numbers of NK/CD4 cocultures to allow for a more detailed analysis of the independent contributions of KIR/HLA mismatches, KIR3DS1/HLA-Bw4 genotype, and recipient KIR genotype. Functional experiments should also include samples from HESN subjects and their partners to confirm the presence of alloreactive NK cells in these individuals. Indeed, several functional studies showed increased levels of NK cell activation in HESN subjects,29,41-44 but NK cell alloreactivity has never been shown. In that regard, although we limited IL-2 preactivation of NK cells to only 2 days to mimic ex vivo conditions, it would also be relevant to investigate whether our findings can be reproduced using freshly isolated resting NK cells.
Our data confirm and extend previous studies of HIV-1–discordant couples and mother-infant pairs showing HLA class I allele discordance between transmission pairs to be associated with a decreased risk of sexual or perinatal HIV-1 transmission.15-19 HLA class I discordance was proposed to result in more efficient HLA-restricted CTL targeting of incoming viruses preadapted to the index partners' HLA. In addition, alloreactivity was considered as a plausible explanation as well. Indeed, HLA class I discordant transmission pairs are more likely to be also KIR ligand mismatched, implying a role for NK cell alloreactivity in previous transmission studies.
Our findings are in accordance with our current understanding of NK cell alloreactivity, which occurs when NK cells educated by inhibitory KIR recognizing a self HLA respond to allogeneic target cells that are lacking this HLA (missing self). This model emerged from studies of haploidentical hematopoietic stem cell transplantation as a treatment for acute myeloid leukemia.8-11 In these studies, allogeneic KIR/HLA mismatches resulted in the expansion of a donor-derived alloreactive NK cell subset in the recipient with strong alloreactivity toward residual immune and leukemia cells leading to leukemia eradication and protection from graft-versus-host disease. However, other studies of hematopoietic stem cell transplantation observed better prognosis when donors had activating KIR2DS1 and KIR2DS2 or when they carried a centromeric KIR BB genotype.37,45 These data are also in agreement with our findings, although in our study the effect of recipient KIR genotype appeared largely secondary to that of recipient/index allogeneic KIR/HLA combinations. Recent data showed the importance of KIR2DS1-positive HLA-C2–negative stem cell donors in leukemia outcome,46 reflecting the negative effect of self HLA-C2 on the responsiveness of KIR2DS1-positive NK cells.47 Although this latter interaction could not be tested as result of the low KIR2DS1 frequency in our West African population, it suggests that additional KIR/HLA mechanisms, possibly including activating KIR, could be involved in HIV-1 transmission.
HIV-1–discordant couples in our study population reported a relatively high frequency of condom use and a high proportion of their HIV-1–infected partners were under antiretroviral therapy, suggesting that the actual HIV-1 transmission risk may be relatively low. Although we do not know the total duration of HIV-1 exposure, it is probable that in several HIV-1–discordant couples HIV exposure started several years before HIV diagnosis of the HIV-infected partner, in the absence of antiretroviral therapy or condom use. HIV transmission during the acute and/or undiagnosed phase of the infection is highly efficient.48 HIV-1–discordant couples can therefore be considered as selected survivors at lower risk of transmission, regardless of the actual levels of HIV exposure. Nevertheless, our findings should be confirmed in other cohorts of HESN individuals with available index partners, including those with higher frequencies of documented HIV exposures. In addition, large-scale longitudinal cohorts of transmitting and nontransmitting HIV-1–discordant couples49,50 will be required to confirm the effect of KIR/HLA incompatibility in the context of other known behavioral, host, viral, and environmental risk factors.
In summary, we found that HIV-1 transmission and lack thereof in a population of Senegalese HIV-1–discordant and concordant couples is associated with mismatched allogeneic KIR/HLA combinations predictive of alloreactive NK cells targeting incoming HIV-1–infected index cells. These data suggest that alloreactive NK cells operating at the boundary of transmitting hosts could play an important role in HIV-protective immunity. Therapeutic interventions aiming at harnessing the NK cell response should be explored as a strategy for the prevention of HIV-1 transmission.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the couples in Dakar for their cooperation; Dr Ndeye Fatou Ngom Gueye, Dr Ibrahima Ndiaye, Khady Ba Fall, and Marianne Ndiaye for the recruitment, counseling, and follow-up of the couples; Abdoul Aziz Diallo, Marema Fall, and Aïssatou Gueye Ndiaye for technical assistance; Odin Goovaerts for critically reading the paper.
This work was supported by the Belgian Fund for Scientific Research (FWO-Vlaanderen), grant G.0660.06, and the Belgian Directorate-General for Development Cooperation.
Authorship
Contribution: W.J., S.V., C.D., and L.K. designed the research; W.J., S.V., and J.W.M. performed the research; W.J. analyzed and interpreted the data; C.M., M.S., T.N.D., and S.M. enrolled the study cohort and collected samples and data: W.J. wrote the paper; and all authors revised the paper and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wim Jennes, Laboratory of Immunology, Department of Biomedical Sciences, Institute of Tropical Medicine, Nationalestraat 155, 2000 Antwerp, Belgium; e-mail: wjennes@itg.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal