Key Points
Antigen cross-presentation is regulated by the activity of deubiquitylase YOD1 that influences the control of viral infections.
The enhanced cross-presentation depends on the proteasomal activity and the acidification status of APCs but is independent of functional TAP1.
Abstract
Antigen presenting cells (APCs) that express a catalytically inactive version of the deubiquitylase YOD1 (YOD1-C160S) present exogenous antigens more efficiently to CD8+ T cells, both in vitro and in vivo. Compared with controls, immunization of YOD1-C160S mice led to greater expansion of specific CD8+ T cells and showed improved control of infection with a recombinant γ-herpes virus, MHV-68, engineered to express SIINFEKL peptide, the ligand for the ovalbumin-specific TCR transgenic OT-I cells. Enhanced expansion of specific CD8+ T cells was likewise observed on infection of YOD1-C160S mice with a recombinant influenza A virus expressing SIINFEKL. YOD1-C160S APCs retained antigen longer than did control APCs. Enhanced cross-presentation by YOD1-C160S APCs was transporter associated with antigen processing (TAP1)–independent but sensitive to inclusion of inhibitors of acidification and of the proteasome. The activity of deubiquitylating enzymes may thus help control antigen-specific CD8+ T-cell responses during immunization.
Introduction
CD8+ T-cell responses constitute a critical component of host defense against intracellular pathogens. Antigen-specific CD8+ T cells are primed by antigen presenting cells (APCs), such as dendritic cells (DCs), which process and present antigens derived from intracellular pathogens either by becoming infected themselves (direct presentation) or by phagocytosis of infected cells (cross-priming).1,2 However, infected DCs may be compromised in their function and be less efficient at inducing an immune response,3-5 making the cross-presentation pathway all the more important.6 Pathogen-derived antigens are then presented to naive CD8+ T cells by DCs via class I MHC molecules. The ensuing activation and expansion of CD8+ T cells of the appropriate specificity allows them to kill the infected target cells in the acute phase of response. After the pathogen is cleared, the pool of effector cells contracts. A small pool of self-renewing memory cells survives to respond to an eventual infection with the same pathogen.7 Although the intricacies of direct presentation are fairly well understood, the mechanisms involved in cross-presentation are not fully resolved and are probably diverse.1,2,8
DCs are particularly good at presenting exogenous antigens to CD8+ T cells, attributes that derive from their morphology, strategic location in vivo, efficient antigen uptake, low lysosomal proteolysis, and from the presence of specific protease inhibitors.9 These traits allow them to retain antigen and carry it to the draining lymph node (LN) where immune responses are induced.10,11 DCs take up not only soluble, but also particulate antigens released by apoptotic or pathogen-damaged cells and process them to cross-prime CD8+ T cells.12,13 The latter event is particularly relevant for the generation of immune responses against pathogens that do not directly infect APCs.8
The endoplasmic reticulum-associated degradation machinery is responsible for degradation and disposal of misfolded proteins from the ER, and its possible relevance for the processing of exogenous antigens is now apparent.14,15 Thus, p97, a crucial player in this pathway, has been implicated in cross-presentation.14 A dominant-negative form of p97 impairs processing of exogenously provided chicken ovalbumin (Ova) and its subsequent presentation to Ova-specific CD8+ T cells. The deubiquitylating enzyme YOD1 interacts with p97 and is involved in the dislocation reaction.16 Cells that express a mutant form of YOD1 devoid of deubiquitylating activity (YOD1-C160S) accumulate various polyubiquitylated dislocation intermediates that would otherwise have been cleared from the ER. If the ER-to-cytosol pathway were indeed an important component of cross-presentation, then expression of the dominant negative version of YOD1 might well prove informative.
Here we investigated the role of YOD1 in antigen processing and presentation. We generated a transgenic mouse in which the expression of a dominant-negative YOD1 transgene (YOD1-C160S) was dependent on the activity of a doxycycline-inducible transcriptional activator. Exposure of cells obtained from such mice to doxycycline or directly supplementing the mice with doxycycline in their drinking water activated the transcriptional transactivator that reversibly controlled the expression of catalytically inactive YOD1. We demonstrate that YOD1-C160S APCs presented exogenous antigens efficiently in vitro and in vivo, and show significantly improved cross-presentation. This enhanced cross-presentation was largely independent of the transporter associated with antigen processing-1 (TAP1) and transport of peptide/MHC complexes from the ER to the Golgi, but was inhibited by interference with acidification and with proteasomal activity.
Methods
Mice, virus, and cell lines
Generation of YOD1-C160S (YOD1-C160S) mice is described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CD45.2+ C57BL/6 and CD45.1+ C57BL/6 and TAP−/− mice were purchased from The Jackson Laboratory. Kb-MHV-68-(ORF8) TCR TN mice were generated as described.17 OT I RAG−/− and OT II RAG−/− mice were purchased from Taconic Farms. All animals were bred and housed in the AALAC-accredited animal facility of the Whitehead Institute for Biomedical Research, Cambridge, MA. Age and gender-matched mice were used for experiments. MHV-68 was grown and titrated in 3T12-3 cell lines obtained from ATCC and stored in aliquots at − 80°C until further use. MHV-68-M2-SIINFEKL was provided by Pedro Simas of the University of Lisbon, Portugal. The recombinant influenza A virus (WSN-SIINFEKL) was described elsewhere.18 All experiments were approved by the IACUC.
Treatment of mice and cells with doxycycline
Mice were given doxycycline (2 mg/mL) in drinking water as indicated. Mice were killed and internal organs were extracted and processed as described in supplemental Methods. Bone marrow–derived dendritic cells (BMDCs) were obtained by culturing bone marrow cells for 7 days in the presence of IL-4 (1 ng/mL) and GM-CSF (1 ng/mL). To induce expression of YOD1-C160S, cultures were supplemented with filter-sterilized doxycycline (1 μg/mL). We routinely obtained > 70% of CD11b+CD11c+ cells as measured by flow cytometry (data not shown). Induction of YOD1-C160S was ascertained by immunoblotting and flow cytometry using anti-HA antibodies.
Antigen presentation assays and the effect of inhibitors
The procedure for measuring direct presentation is described in supplemental Methods. To assess the influence of YOD1-C160S expression on antigen cross-presentation ex vivo, 2 types of experiments were performed. First, BMDCs generated in the presence or absence of doxycycline were pulsed for 6 hours with the indicated doses of ovalbumin and washed 3 times with complete RPMI medium. Ova-pulsed BMDCs were cocultured with CFSE-labeled OT-I cells for various time periods. Expression of CD69 and CFSE dilution in OT-I cells were measured by flow cytometry.
In the second set of experiments, mouse embryonic fibroblasts (MEFs) were infected overnight with MHV-68 at different MOIs. Infected cells were irradiated at 500 rads to induce apoptosis. Cidofovir (1 μg/mL) was added to block any remaining viral replication.19 Cells were detached by trypsin treatment, washed multiple times and added to BMDCs cultures for 12 to 16 hours. CFSE-labeled ORF8 TN cells were added to these BMDCs for 16 or 72 hours. Surface expression of CD69 and proliferation were measured to assess activation. A schematic for measuring the influence of various inhibitors on cross-presentation is shown in supplemental Figure 5.
Statistical analysis
Statistical analysis to compare responses between groups was done by student t test and ANOVA test as indicated in the figure legends. The results were presented as mean ± SEM or SD as indicated in the figure legends. The P values are shown in the figures or figure legends and were represented as *P ≤ .05 or **P ≤ .01.
Results
Expression of YOD1-C160S in tissues and cells upon supplementation with doxycycline
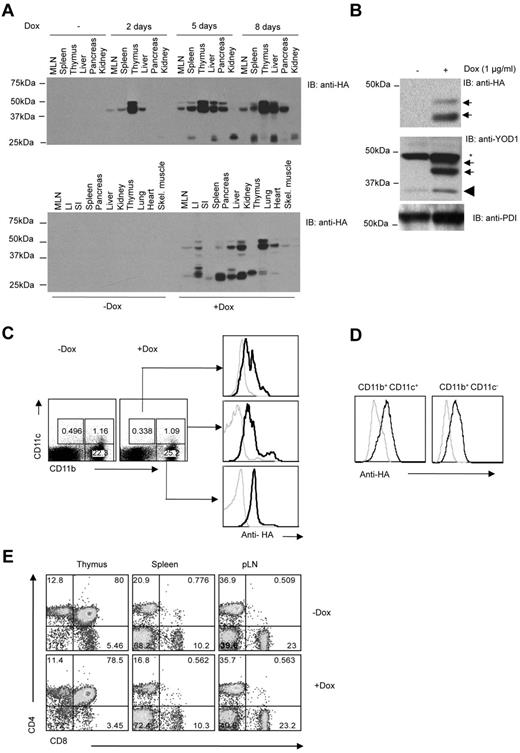
Different organs were isolated from mice whose drinking water had been supplemented with doxycycline. At different times after exposure to doxycycline, we assessed the expression of YOD1-C160S by immunoblotting using anti-HA antibody. Whereas tissues isolated from mice given normal water did not exhibit any HA immunoreactivity (Figure 1A), expression of YOD1-C160S (apparent molecular mass 38.5kDa), was evident as early as day 2 in lymph node, spleen, thymus, and pancreas, but not in kidney. Levels of YOD1-C160S increased further at later time points, with a plateau between days 5 and 8 (Figure 1A top panel).
Characterization of YOD1-C160S transgenic mice. (A) YOD1-C160S mice were fed with normal or doxycycline (2 mg/mL) supplemented water. Lysates of indicated organs from euthanized mice were resolved by electrophoresis and immunoblotted using an anti-HA antibody. The top panel shows the kinetic of YOD1-C160S expression in mice on doxycycline for 2, 5, or 8 days. The bottom panel shows expression of YOD1-C160S in different organs as indicated at 8 days of doxycycline supplementation. (B) HA immunoblots show expression of YOD1-C160S (top panel) and total YOD1 (middle panel, arrows: YOD1-C160S, wedge: endogenous YOD1, asterisk: background band) in BMDCs on day 7. Anti-PDI blot served as loading control. (C) YOD1-C160S mice were given doxycycline-water for 7 days and splenocytes were isolated thereafter. Intracellular staining in surface stained CD11c+ and CD11b+ cells was performed using Alexa Fluor 488–conjugated anti-HA antibody. (D) Intracellular staining for YOD1-C160S expression in control and doxycycline supplemented BMDCs using anti-HA Alexa Fluor 488 conjugated antibody is shown. (E) Mice were given doxycycline water for ∼ 20 days and cellular distribution in the lymphoid organs of control and doxycycline supplemented mice is shown by representative FACS plots.
Characterization of YOD1-C160S transgenic mice. (A) YOD1-C160S mice were fed with normal or doxycycline (2 mg/mL) supplemented water. Lysates of indicated organs from euthanized mice were resolved by electrophoresis and immunoblotted using an anti-HA antibody. The top panel shows the kinetic of YOD1-C160S expression in mice on doxycycline for 2, 5, or 8 days. The bottom panel shows expression of YOD1-C160S in different organs as indicated at 8 days of doxycycline supplementation. (B) HA immunoblots show expression of YOD1-C160S (top panel) and total YOD1 (middle panel, arrows: YOD1-C160S, wedge: endogenous YOD1, asterisk: background band) in BMDCs on day 7. Anti-PDI blot served as loading control. (C) YOD1-C160S mice were given doxycycline-water for 7 days and splenocytes were isolated thereafter. Intracellular staining in surface stained CD11c+ and CD11b+ cells was performed using Alexa Fluor 488–conjugated anti-HA antibody. (D) Intracellular staining for YOD1-C160S expression in control and doxycycline supplemented BMDCs using anti-HA Alexa Fluor 488 conjugated antibody is shown. (E) Mice were given doxycycline water for ∼ 20 days and cellular distribution in the lymphoid organs of control and doxycycline supplemented mice is shown by representative FACS plots.
Bone marrow cells cultured in the presence of IL-4 and GM-CSF for 7 days yielded BMDCs. Addition of doxycycline (1 μg/mL) induced expression of YOD1-C160S, as shown by the presence of an HA-reactive polypeptide at ∼ 38.5kD (Figure 1B). We confirmed expression by flow cytometry (Figure 1C-D). The cell types responsible for antigen presentation, that is, CD11b+ and CD11c+ cells, showed expression of YOD1-C160S (Figure 1C). Similarly, BMDCs generated in the presence of doxycycline showed expression of YOD1-C160S (Figure 1D). BMDCs generated in the presence or absence of doxycycline from control mice showed no expression of YOD1-C160S (supplemental Figure 1A). Hereafter, we shall refer to YOD1-C160S mice or YOD1-C160S BMDCs exposed to doxycycline as YOD1-C160S mice or YOD1-C160S BMDCs, respectively, and their transgenic counterparts not exposed to doxycycline will be referred to as controls.
T-cell distribution in lymphoid organs
Absence of another deubiquitylase, such as CYLD, can cause defective T-cell development.20 We therefore investigated whether induction of YOD1-C160S affects the CD4+ and CD8+ T-cell compartments. Both YOD1-C160S mice and their littermate controls had similar relative and absolute numbers of CD4+ and CD8+ T cells (Figure 1E and data not shown). YOD1-C160S mice displayed no obvious signs of pathology when fed doxycycline-containing water for up to 2 months.
Expression of YOD1-C160S in APCs has no obvious effect on direct antigen presentation
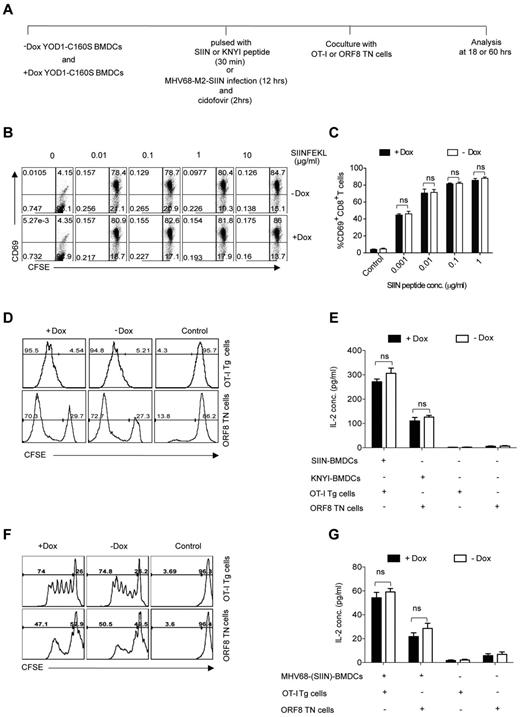
To investigate the influence of YOD1-C160S on direct antigen presentation, we performed 2 types of experiment. First, we pulsed control and YOD1-C160S BMDCs with different concentrations of SIINFEKL, the class I MHC-restricted peptide derived from chicken ovalbumin, or with the MHV-68 ORF8-derived epitope KNYIFEEKL. These BMDCs were then cocultured with CFSE labeled OT-I Tg21 or ORF8 TN cells.17 After 18 and 60 hours of coculture we measured activation, proliferation, and cytokine production (Figure 2A). As expected, very few OT-I cells cocultured with control BMDCs showed signs of activation, as measured by increased expression of CD69. On coculturing with SIINFEKL-pulsed BMDCs, most OT-I cells acquired the CD69 marker (Figure 2B-C). We observed similar levels of CD69 expression in OT-I cells stimulated with peptide-pulsed control or YOD1-C160S BMDCs (Figure 2B-C). Control and YOD1-C160S BMDCs were analyzed by flow cytometry for the expression of costimulatory molecules, such as CD80, CD86, and CD40. No differences were found in the surface expression of any of the markers (supplemental Figure 1B). Proliferation (Figure 2D) and IL-2 production (Figure 2E) by either OT-I Tg cells or ORF8 TN cells cocultured with the relevant peptide-pulsed BMDCs were also comparable. Although levels of H-2Kb were marginally higher in YOD1-C160S BMDCs (supplemental Figure 1C) this did not affect stimulation of T cells.
YOD1-C160S expressed in BMDCs has no effect on direct antigen presentation. (A) Schematic of direct antigen presentation experiments. Expression of YOD1-C160S in BMDCs was induced by adding 1 μg/mL of doxycycline to the culture. BMDCs were either pulsed with the class I restricted peptides SIINFEKL or KNYIFEEKL, or infected with recombinant MHV-68 virus expressing SIINFEKL. BMDCs were then cocultured with CFSE labeled OT-I or ORF8 TN cells. Activation, proliferation, and cytokine production by T cells was analyzed. (B-C) FACS plots and bar diagram show the activation of OT-I cells at different SIINFEKL concentration as indicated by CD69 surface expression. (D) FACS plots show the proliferation of OT-I Tg (top panel) or ORF8 TN cells (bottom panel) when cocultured with the respective peptide-pulsed BMDCs. (E) IL-2 levels in the culture supernatants as measured by ELISA are shown. (F-G) Proliferation (F) and IL-2 production (G) by ORF8 TN and OT-I cells are shown. All experiments were repeated 3 times and data are represented as mean ± SEM.
YOD1-C160S expressed in BMDCs has no effect on direct antigen presentation. (A) Schematic of direct antigen presentation experiments. Expression of YOD1-C160S in BMDCs was induced by adding 1 μg/mL of doxycycline to the culture. BMDCs were either pulsed with the class I restricted peptides SIINFEKL or KNYIFEEKL, or infected with recombinant MHV-68 virus expressing SIINFEKL. BMDCs were then cocultured with CFSE labeled OT-I or ORF8 TN cells. Activation, proliferation, and cytokine production by T cells was analyzed. (B-C) FACS plots and bar diagram show the activation of OT-I cells at different SIINFEKL concentration as indicated by CD69 surface expression. (D) FACS plots show the proliferation of OT-I Tg (top panel) or ORF8 TN cells (bottom panel) when cocultured with the respective peptide-pulsed BMDCs. (E) IL-2 levels in the culture supernatants as measured by ELISA are shown. (F-G) Proliferation (F) and IL-2 production (G) by ORF8 TN and OT-I cells are shown. All experiments were repeated 3 times and data are represented as mean ± SEM.
In a second set of experiments, we tested whether YOD1-C160S influences the direct antigen presentation pathway for class I MHC-restricted peptides by provision of intact protein antigens instead of short synthetic peptides. Control and YOD1-C160S BMDCs were infected overnight with a recombinant strain of MHV-68 that expresses an M2-SIINFEKL fusion protein. Recognition of the SIINFEKL epitope by OT-I Tg T cells would therefore require proteolytic excision of SIINFEKL from the fusion protein and its loading onto the H-2Kb class I MHC molecule. Infected BMDCs were treated with cidofovir to kill any remaining replicating virus. We cocultured infected BMDCs for 3 days either with OT-I Tg or with ORF8 TN cells to measure antigen presentation. Antigen-specific CD8+ T cells proliferated to a similar extent and produced similar amounts of IL-2 under both conditions (Figure 2F-G). We conclude that expression of YOD1-C160S does not interfere with direct antigen presentation through class I MHC.
Next, we established that YOD1 is not involved in class II MHC-restricted antigen presentation. Control and YOD1-C160S BMDCs were equally capable of stimulating ovalbumin-specific class II MHC restricted CD4+ T cells (OT-II) when exposed to ovalbumin or the corresponding peptide epitope, as measured by surface levels of CD69 on CD4+ OT-II cells (supplemental Figure 2). Processing and presentation of ovalbumin in the context of class II MHC is unaffected by the expression of YOD1-C160S in APCs.
Expression of YOD1-C160S in APCs enhances antigen cross-presentation
Having established that expression of YOD1-C160S does not affect direct antigen presentation, we tested whether its expression affects antigen cross-presentation.
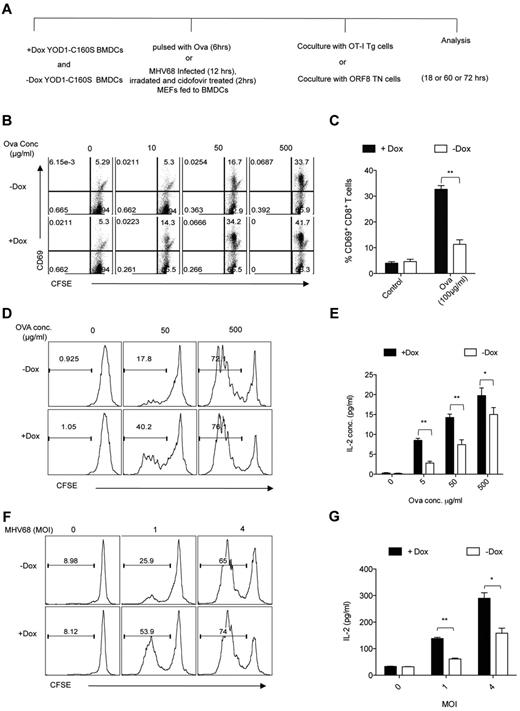
First, BMDCs were fed with various doses of soluble ovalbumin and were then cocultured with OT-I Tg cells (Figure 3A). At all concentrations of ovalbumin tested, YOD1-C160S BMDCs induced more OT-I Tg cells to up-regulate CD69 than did control BMDCs (Figure 3B-C). Likewise, YOD1-C160S BMDCs induced enhanced proliferation and IL-2 production, especially at lower concentrations of ovalbumin (Figure 3D-E). The percentages of IL-2 and IFN-γ producing OT-I Tg cells stimulated by ovalbumin-pulsed YOD1-C160S BMDCs and their controls (supplemental Figure 3) showed that more OT-I Tg cells produced both cytokines (double producers) when stimulated with YOD1-C160S BMDCs (control: 30%-40% double producers; YOD1-C160S BMDCs: 50%-60% double producers; supplemental Figure 3). Finally, overexpression of YOD1-C160S enhanced cross-presentation in a dose-dependent manner, directly correlated with the levels of YOD1-C160S (supplemental Figure 4). Neither BMDCs generated in the presence of doxycycline from genetic control mice that express, either a transgenes or a transactivator alone, nor BMDCs generated in the presence of doxycycline from WT C57BL/6 mice showed enhanced cross-presentation.
YOD1-C160S expressing BMDCs enhance antigen cross-presentation. (A) A schematic of antigen cross-presentation experiments is shown. Control and YOD1-C160S expressing BMDCs were either pulsed with Ova or were fed apoptotic MHV-68–infected MEFs and cocultured with OT-I cells or ORF8 TN cells, respectively. (B-C) FACS plots (B) and bar diagram (C) show expression of CD69 by OT-I cells measured after 18 hours of cocultures with BMDCs that were pulsed with indicated concentrations of ovalbumin. (D-E) Proliferation (D) and IL-2 production (E) by OT-I cells was measured after 60 hours of coculture with Ova-pulsed control and YOD1-C160S BMDCs at indicated concentrations of Ova. (F-G) Proliferation (F) and IL-2 production (G) by ORF8 TN cells was measured after 72 hours of coculture with control and YOD1-C160S BMDCs, which were fed with MHV-68–infected apoptotic MEFs. All experiments were repeated at least 3 times and data are represented as mean ± SEM.
YOD1-C160S expressing BMDCs enhance antigen cross-presentation. (A) A schematic of antigen cross-presentation experiments is shown. Control and YOD1-C160S expressing BMDCs were either pulsed with Ova or were fed apoptotic MHV-68–infected MEFs and cocultured with OT-I cells or ORF8 TN cells, respectively. (B-C) FACS plots (B) and bar diagram (C) show expression of CD69 by OT-I cells measured after 18 hours of cocultures with BMDCs that were pulsed with indicated concentrations of ovalbumin. (D-E) Proliferation (D) and IL-2 production (E) by OT-I cells was measured after 60 hours of coculture with Ova-pulsed control and YOD1-C160S BMDCs at indicated concentrations of Ova. (F-G) Proliferation (F) and IL-2 production (G) by ORF8 TN cells was measured after 72 hours of coculture with control and YOD1-C160S BMDCs, which were fed with MHV-68–infected apoptotic MEFs. All experiments were repeated at least 3 times and data are represented as mean ± SEM.
We next explored whether presentation of viral antigens derived from phagocytosed MHV-68–infected apoptotic cells to MHV-68–specific CD8+ T cells is also modulated by YOD1-C160S. MEFs were infected with MHV-68 and then γ-irradiated to induce apoptosis. When presented by BMDCs, such virus-infected apoptotic remnants are an efficient source of antigen for CD8+ T cells.13,22 ORF8 TN CD8+ T cells proliferated to a greater extent and produced more IL-2 when stimulated by YOD1-C160S BMDCs compared with control BMDCs (Figure 3F-G). YOD1-C160S BMDCs thus prime a more effective CD8+ T-cell response by enhanced cross-presentation of both soluble and particulate antigen.
Prolonged antigen retention in YOD1-C160S APCs
YOD1-C160S lacks deubiquitylating activity, causes accumulation of various polyubiquitylated substrates, and leads to retention of proteins in the ER that should otherwise have been targeted for dislocation and proteasomal destruction in the cytoplasm.16 We reasoned that exogenous antigen, taken up by YOD1-C160S APCs, might likewise be retained longer in membrane-delimited compartments, which, could then act as a depot of antigen and as a source for T-cell stimulation. We performed antigen retention assays using fluorochrome-labeled ovalbumin. Control and YOD1-C160S BMDCs were pulsed with Ova-FITC for 30 minutes and the fluorescence retained by CD11c+ cells was tracked over time. Antigen uptake was similar for both cell types (Figure 4A) but YOD1-C160S BMDCs retained antigen longer than did controls. We measured the level of SIINFEKL-H2Kb complexes on the surface of control and YOD1-C160S-BMDCs pulsed with ovalbumin. Compared with controls, greater numbers of YOD1-C160S BMDCs displayed SIINFEKL-H2Kb complexes on the surface at 12 hours (Figure 4B) and at later time points (data not shown). For in vivo experiments, YOD1-C160S mice exposed to doxycycline and their littermate controls were immunized with Ova-FITC in the footpads. Similar levels of Ova-FITC acquisition were recorded for CD8+CD11c+ cells in the popliteal LNs after 6 hours. However, the Ova-FITC signal again persisted longer in YOD1-C160S DCs than in control DCs (Figure 4C). These data establish that, although levels of initial antigen acquisition are similar, retention of antigen is extended in YOD1-C160S APCs compared with controls, a parameter that correlated with presentation of H-2Kb-SIINFEKL complexes.
Exploring potential mechanism of enhanced cross-presentation by YOD1-C160S APCs. (A) Control and YOD1-C160S BMDCs were pulsed with 50 μg/mL of Ova-FITC for 30 minutes and the intensities of Ova-FITC were measured over time. FACS plots overlay show the percentage of FITC+ cells (text box) and the mean fluorescence intensities of FITC below the respective histograms in control (regular letters) and YOD1-C160S expressing cells (bold letters). (B) Fifty μg of Ova-FITC was injected into footpads of control and YOD1-C160S mice and the percentages of Ova-FITC+ CD8+CD11c+ cells were measured at 6 and 24 hours in the draining popliteal LNs by flow cytometry. Overlay FACS plots are shown. Thin and thick lines represent the Ova-FITC staining in cells isolated from control and YOD1-C160S mice, respectively. C. BMDCs were pulsed with 200 μg/mL of Ova for 30 minutes and surface displayed SIINFEKL-peptide/H-2Kb complexes on control and YOD1-C160S BMDCs are shown after 12 hours (IC stands for isotype control). (D-G) Influence of chemical/pharmacologic inhibitors or TAP1-deficiency on antigen cross-presentation. Control and YOD1-C160S BMDCs were pulsed with 50, 100, or 500 μg/mL of ovalbumin for 5 hours. Different chemical inhibitors were added during the pulse period as described in supplemental Figure 5A. Treated BMDCs were then cocultured with CFSE labeled OT-I cells and their proliferation and cytokine production were measured. Proliferation (D) and IL-2 production (E) by OT-I cells under indicated conditions is shown. Experiments were repeated 4 times with similar results and data are shown as mean values ± SEM. (F-G) Control and YOD1-C160S BMDCs pulsed with 500 μg/mL of ovalbumin were treated with indicated doses of proteasome inhibitor (zL3VS) and cocultured with CFSE labeled OT-I cells. Proliferation (F) and IL-2 levels (G) under indicated conditions are shown. Experiments were repeated 3 times and mean values ± SEM are shown. (H-J) BMDCs from TAP1−/−YOD1-C160S and YOD1-C160S mice were generated in the presence or absence of doxycycline. These cells were pulsed with different doses of Ova and cocultured with CFSE labeled OT-I cells. After 72 hours of coculture, proliferation of OT-I cells (H) IL-2 production (I) in culture supernatants were measured. (J) Bar diagrams show the fold changes in the levels of IL-2 from OT-I cells cocultured with control and YOD1-C160S BMDCs pulsed with 50 μg/mL of Ova. (K-L) TAP1−/−YOD1-C160S mice fed with regular or doxycycline supplemented water for 7 days were transferred with 5 × 105 OT-I cells. These mice were then immunized subcutaneously in the base of tail with 200 μg of Ova emulsified in incomplete Freund adjuvant (IFA) and the frequencies of Kb-SIINFEKL-Tet+ cells were measured at 7 days after immunization in the draining iliac LNs. K. Representative FACS plots show the frequencies of Kb-SIINFEKL-Tet+ cells. (L) Bar diagram show the cumulative frequencies of SIINFEKL-Tet+ cells in 2 groups of mice. Three animals were included in each group and experiments were repeated twice.
Exploring potential mechanism of enhanced cross-presentation by YOD1-C160S APCs. (A) Control and YOD1-C160S BMDCs were pulsed with 50 μg/mL of Ova-FITC for 30 minutes and the intensities of Ova-FITC were measured over time. FACS plots overlay show the percentage of FITC+ cells (text box) and the mean fluorescence intensities of FITC below the respective histograms in control (regular letters) and YOD1-C160S expressing cells (bold letters). (B) Fifty μg of Ova-FITC was injected into footpads of control and YOD1-C160S mice and the percentages of Ova-FITC+ CD8+CD11c+ cells were measured at 6 and 24 hours in the draining popliteal LNs by flow cytometry. Overlay FACS plots are shown. Thin and thick lines represent the Ova-FITC staining in cells isolated from control and YOD1-C160S mice, respectively. C. BMDCs were pulsed with 200 μg/mL of Ova for 30 minutes and surface displayed SIINFEKL-peptide/H-2Kb complexes on control and YOD1-C160S BMDCs are shown after 12 hours (IC stands for isotype control). (D-G) Influence of chemical/pharmacologic inhibitors or TAP1-deficiency on antigen cross-presentation. Control and YOD1-C160S BMDCs were pulsed with 50, 100, or 500 μg/mL of ovalbumin for 5 hours. Different chemical inhibitors were added during the pulse period as described in supplemental Figure 5A. Treated BMDCs were then cocultured with CFSE labeled OT-I cells and their proliferation and cytokine production were measured. Proliferation (D) and IL-2 production (E) by OT-I cells under indicated conditions is shown. Experiments were repeated 4 times with similar results and data are shown as mean values ± SEM. (F-G) Control and YOD1-C160S BMDCs pulsed with 500 μg/mL of ovalbumin were treated with indicated doses of proteasome inhibitor (zL3VS) and cocultured with CFSE labeled OT-I cells. Proliferation (F) and IL-2 levels (G) under indicated conditions are shown. Experiments were repeated 3 times and mean values ± SEM are shown. (H-J) BMDCs from TAP1−/−YOD1-C160S and YOD1-C160S mice were generated in the presence or absence of doxycycline. These cells were pulsed with different doses of Ova and cocultured with CFSE labeled OT-I cells. After 72 hours of coculture, proliferation of OT-I cells (H) IL-2 production (I) in culture supernatants were measured. (J) Bar diagrams show the fold changes in the levels of IL-2 from OT-I cells cocultured with control and YOD1-C160S BMDCs pulsed with 50 μg/mL of Ova. (K-L) TAP1−/−YOD1-C160S mice fed with regular or doxycycline supplemented water for 7 days were transferred with 5 × 105 OT-I cells. These mice were then immunized subcutaneously in the base of tail with 200 μg of Ova emulsified in incomplete Freund adjuvant (IFA) and the frequencies of Kb-SIINFEKL-Tet+ cells were measured at 7 days after immunization in the draining iliac LNs. K. Representative FACS plots show the frequencies of Kb-SIINFEKL-Tet+ cells. (L) Bar diagram show the cumulative frequencies of SIINFEKL-Tet+ cells in 2 groups of mice. Three animals were included in each group and experiments were repeated twice.
Antigen cross-presentation by YOD1-C160S APCs requires acidification, is insensitive to brefeldin A, and does not require a functional TAP1 complex
Several entry routes for antigen cross-presentation have been proposed.8,23 We used TAP-deficient mice and chemical inhibitors to probe which aspects might be affected by the presence of YOD1-C160S in APCs (supplemental Figure 5).
First, YOD1-C160S BMDCs were pulsed with soluble ovalbumin for 5 hours and incubated with NH4Cl or chloroquine, compounds that interfere with acidification of vacuolar compartments. BMDCs treated with the different inhibitors at indicated concentrations were not compromised in their ability to stimulate OT-I Tg cells when loaded with SIINFEKL (supplemental Figure 5B-C). Both NH4Cl and chloroquine-treated BMDCs pulsed with ovalbumin inhibited proliferation and cytokine production of OT-I Tg cells (Figure 4D-E). Second, treatment of APCs with the proteasome inhibitors ZL3VS and MG132 abrogated the presentation of exogenous antigen in a dose-dependent manner. Although inhibition of proteasomal activity affected antigen cross-presentation by both types of BMDCs, we saw a consistently stronger inhibitory effect in YOD1-C160S BMDCs (Figure 4F-G). In contrast, treatment of ovalbumin-pulsed BMDCs with brefeldin A, which blocks anterograde transport from the ER to the Golgi, inhibited neither proliferation nor cytokine production by OT-I Tg cells (Figure 4D-E).
We next bred YOD1-C160S mice onto a TAP-deficient background. In these mice, as expected, few CD8+ T cells remained, whereas CD4+ T cells were abundant (supplemental Figure 5B-C). Control and YOD1-C160S BMDCs generated from these mice were pulsed with ovalbumin and cocultured with OT-I Tg cells as described. Neither proliferation nor cytokine production by OT-I Tg cells cocultured with ovalbumin-pulsed YOD1-C160S BMDCs were affected by TAP deficiency (Figure 4H-J). The effect of antigen concentration on cross-presentation by TAP-deficient YOD1-C160S BMDCs suggests that the contribution of TAP-dependent pathway(s) is more pronounced at lower antigen concentrations, presumably through the overall reduction in class I MHC levels, although at higher antigen concentrations the TAP-dependent pathway(s) may be obscured by TAP-independent pathways.
Similar results were obtained when we immunized TAP-deficient YOD1-C160S mice with ovalbumin and measured proliferation of CFSE-labeled transferred OT-I cells. Both the fraction and absolute numbers of SIINFEKL-H2Kb Tet+ cells that divided were greater in TAP-proficient than in TAP-deficient mice, irrespective of the presence of YOD1-C160S, confirming a role of TAP in cross-presentation in vivo.24,25 Nevertheless, more SIINFEKL-H2Kb-Tet+ cells were found in the draining iliac LN of YOD1-C160S mice than in controls (Figure 4K-L). Overall enhancement of cross-presentation by YOD1-C160S was evident especially at higher doses of antigen and despite TAP-deficiency.
YOD1-C160S mice mount a better CD8+ T-cell response on immunization with protein
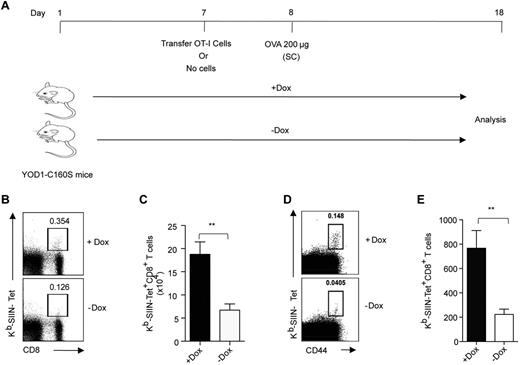
Are CD8+ T-cell responses enhanced upon protein immunization in YOD1-C160S mice? YOD1-C160S mice and their controls received 5 × 105 CFSE labeled OT-I Tg cells 1 day before immunization with ovalbumin. Ten days after immunization we measured the numbers of H2Kb−Tet+ cells in the draining iliac LN (Figure 5A). Frequencies and numbers of H2Kb-SIINFEKL-Tet+ cells were greater in the LNs of YOD1-C160S mice than in controls (Figure 5B-C). Upon immunization, endogenously arising SIINFEKL-H2Kb-SIINFEKL-Tet+ cells were present in ∼ 3-fold greater numbers than in controls (Figure 5D-F). Thus, induction of YOD1-C160S in mice promotes specific CD8+ T-cell responses by cross-presentation.
YOD1-C160S mice mount enhanced antigen-specific CD8+ T-cell response on immunization. (A) Schematic of in vivo experiments is shown. Control and YOD1-C160S mice, which either received OT-I cells or no cells, were immunized subcutaneously with 200 μg of Ova. Five × 105 OT-I cells were transferred into control and YOD1-C160S mice, which were then immunized subcutaneously with 200 μg of Ova in IFA. Frequencies and numbers of Kb-SIINFEKL-Tet+ CD8+ T cells in draining iliac LNs after 7 days were measured by flow cytometry. (B) Representative FACS plots show the frequencies of Kb-SIINFEKL-Tet+ cells. (C) Absolute numbers of Kb-SIINFEKL-Tet+ cells in draining iliac LNs of 2 groups of mice are shown. (D-E) Control and YOD1-C160S mice were immunized subcutaneously with 200 μg of Ova in incomplete Freund adjuvant (IFA) and the frequencies and numbers of endogenous Kb-SIINFEKL-Tet+ cells were analyzed in iliac LN 7 days after transfer. (D) Representative FACS plots show frequencies of endogenous Kb-SIINFEKL-Tet+ cells. (E) Total numbers of Kb-SIINFEKL-Tet+ cells in the iliac LNs of control and YOD1-C160S mice are shown. In each experiment, 4 mice were included and experiments were repeated at least 3 times. Mean values ± SEM are shown.
YOD1-C160S mice mount enhanced antigen-specific CD8+ T-cell response on immunization. (A) Schematic of in vivo experiments is shown. Control and YOD1-C160S mice, which either received OT-I cells or no cells, were immunized subcutaneously with 200 μg of Ova. Five × 105 OT-I cells were transferred into control and YOD1-C160S mice, which were then immunized subcutaneously with 200 μg of Ova in IFA. Frequencies and numbers of Kb-SIINFEKL-Tet+ CD8+ T cells in draining iliac LNs after 7 days were measured by flow cytometry. (B) Representative FACS plots show the frequencies of Kb-SIINFEKL-Tet+ cells. (C) Absolute numbers of Kb-SIINFEKL-Tet+ cells in draining iliac LNs of 2 groups of mice are shown. (D-E) Control and YOD1-C160S mice were immunized subcutaneously with 200 μg of Ova in incomplete Freund adjuvant (IFA) and the frequencies and numbers of endogenous Kb-SIINFEKL-Tet+ cells were analyzed in iliac LN 7 days after transfer. (D) Representative FACS plots show frequencies of endogenous Kb-SIINFEKL-Tet+ cells. (E) Total numbers of Kb-SIINFEKL-Tet+ cells in the iliac LNs of control and YOD1-C160S mice are shown. In each experiment, 4 mice were included and experiments were repeated at least 3 times. Mean values ± SEM are shown.
Enhanced CD8+ T-cell responses on immunization help control viral infection
Do the improved CD8+ T-cell responses induced by cross-presentation in YOD1-C160S mice confer a protective advantage to mice upon a subsequent viral infection? We transferred 5 × 105 OT-I Tg cells 1 day before immunization with ovalbumin (Figure 6A). At day 7 after immunization, we examined the frequencies of H2Kb-SIINFEKL-Tet+ cells in the peripheral blood of immunized mice to monitor the response in 2 groups of mice. YOD1-C160S mice had approximately 2-fold greater numbers of SIINFEKL-H2Kb Tet+ cells than controls (Figure 6B-C). We then challenged mice intranasally with a recombinant MHV-68 virus that expresses the SIINFEKL epitope fused to the M2 protein (MHV-68-M2 SIIN). One week after challenge, specific CD8+ T-cell responses were analyzed in the draining mediastinal LNs and viral burden was determined in the lung tissues of infected mice by plaque assay. Frequencies of SIINFEKL-H2Kb Tet+ cells were more than 2-fold higher in YOD1-C160S mice than in controls (Figure 6D-E). MHV-68 infection did not affect the frequencies of CD8+ T cells specific for other virus-derived epitopes tested here, such as ORF8 (Figure 6D-E) or ORF61 and 75c (data not shown). The expanded SIINFEKL-H2-Kb−Tet+ cells reduced viral titers by up to 1000-fold in the YOD1-C160S mice (Figure 6F).
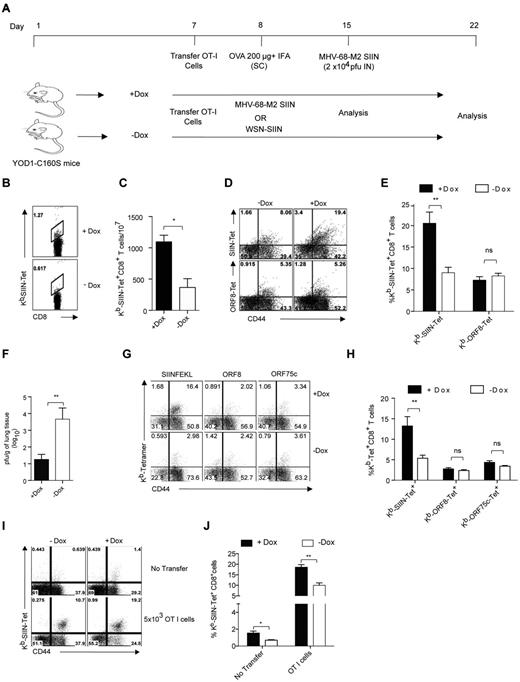
Differential expansion of antigen-specific CD8+ T cells in immunized YOD1-C160S mice on viral infections. (A) A schematic of in vivo experiments is shown. Control and YOD1-C160S mice, which received OT-I cells, were immunized subcutaneously with 200 μg of Ova in IFA. The mice were challenged with a recombinant γ-herpes virus (MHV-68-M2-SIINFEKL) and analyses were performed 7 days after infection, respectively. In some experiments (G-J) OT-I cells recipient mice were infected with MHV-68-M2-SIINFEKL or influenza A virus (WSN-SIINFEKL) and analyses were performed 7 or 10 days after infection, respectively. (B-F) Control and YOD1-C160S mice were transferred with 5 × 105 of OT-I cells and immunized with 200μg of Ova in IFA. Seven days after immunization frequencies of Kb−SIINFEKL-Tet+ cells were measured in peripheral blood and mice were then infected intranasally with 2 × 104 PFU of recombinant MHV-68-M2-SIINFEKL virus. At 7 days after immunization, the activation status, frequencies, and numbers of antigen specific cells were measured in the draining mediastinal LN and viral burden was measured in the extracted lung tissues. Representative FACS plots (B) and bar diagrams (C) show the frequencies of Kb-SIINFEKL-Tet+ cells in the peripheral blood of immunized mice before viral infection. Representative FACS plots (D) and bar diagram (E) show the frequencies of Kb-SIINFEKL-Tet+ and ORF8-Tet+ CD8+ T cells in the mediastinal LN of mice 7 days after infection. (F) Viral titers in lung homogenates of 2 groups of mice are shown. (G-H) Five × 105 naive OT-I cells were transferred intraperitoneally into control and YOD1-C160S mice 1 day before infection with 5 × 105 PFU of MHV-68-M2-SIINFEKL virus. Representative FACS plots (G) and bar diagram (H) show the frequencies of antigen-specific cells in spleens of infected mice 7 days after infection. (I-J) Untransferred or OT I cells (5 × 103) recipient mice control and YOD1-C160S mice were infected with 100 PFU of WSN-SIINFEKL intranasally and the expansion of Kb-SIINFEKL-Tet+ cells were measured 10 days after infection in mediastinal LNs. Representative FACS plots (I) and bar diagrams (J) show the frequencies of Tet+ cells. Experiments were repeated twice with similar results.
Differential expansion of antigen-specific CD8+ T cells in immunized YOD1-C160S mice on viral infections. (A) A schematic of in vivo experiments is shown. Control and YOD1-C160S mice, which received OT-I cells, were immunized subcutaneously with 200 μg of Ova in IFA. The mice were challenged with a recombinant γ-herpes virus (MHV-68-M2-SIINFEKL) and analyses were performed 7 days after infection, respectively. In some experiments (G-J) OT-I cells recipient mice were infected with MHV-68-M2-SIINFEKL or influenza A virus (WSN-SIINFEKL) and analyses were performed 7 or 10 days after infection, respectively. (B-F) Control and YOD1-C160S mice were transferred with 5 × 105 of OT-I cells and immunized with 200μg of Ova in IFA. Seven days after immunization frequencies of Kb−SIINFEKL-Tet+ cells were measured in peripheral blood and mice were then infected intranasally with 2 × 104 PFU of recombinant MHV-68-M2-SIINFEKL virus. At 7 days after immunization, the activation status, frequencies, and numbers of antigen specific cells were measured in the draining mediastinal LN and viral burden was measured in the extracted lung tissues. Representative FACS plots (B) and bar diagrams (C) show the frequencies of Kb-SIINFEKL-Tet+ cells in the peripheral blood of immunized mice before viral infection. Representative FACS plots (D) and bar diagram (E) show the frequencies of Kb-SIINFEKL-Tet+ and ORF8-Tet+ CD8+ T cells in the mediastinal LN of mice 7 days after infection. (F) Viral titers in lung homogenates of 2 groups of mice are shown. (G-H) Five × 105 naive OT-I cells were transferred intraperitoneally into control and YOD1-C160S mice 1 day before infection with 5 × 105 PFU of MHV-68-M2-SIINFEKL virus. Representative FACS plots (G) and bar diagram (H) show the frequencies of antigen-specific cells in spleens of infected mice 7 days after infection. (I-J) Untransferred or OT I cells (5 × 103) recipient mice control and YOD1-C160S mice were infected with 100 PFU of WSN-SIINFEKL intranasally and the expansion of Kb-SIINFEKL-Tet+ cells were measured 10 days after infection in mediastinal LNs. Representative FACS plots (I) and bar diagrams (J) show the frequencies of Tet+ cells. Experiments were repeated twice with similar results.
We also investigated the responsiveness of transferred naive OT-I Tg cells to MHV-68 infection. On transfer of OT-I Tg cells followed by infection with MHV-68-M2 SIIN virus, the frequencies of H2Kb-SIINFEKL-Tet+ cells were up to 3-fold higher in YOD1-C160S mice compared with controls (Figure 6G-H). Frequencies of CD8+ T cells specific for other epitopes of the virus remained unaltered (Figure 6G-H). Next we tested whether infection of YOD1-C160S mice result in an enhanced CD8+ T-cell response when challenged with a different virus that can also provide the SIINFEKL epitope. To this end, control and YOD1-C160S mice having received OT-I cells were infected with a recombinant influenza A virus that encodes a version of HA that includes the SIINFEKL sequence. Expansion of SIINFEKL-Tet+ cells was measured 10 days later. We found greater numbers of SIINFEKL-Tet+ cells in the mediastinal LNs of YOD1-C160S mice compared with controls (Figure 6I-J). We conclude that the response to CD8 T-cell epitopes may be enhanced on viral infection of YOD1-C160S mice, with functional consequences for the control of viral growth.
Antigen-pulsed YOD1-C160S BMDCs promote specific CD8+ T-cell responses on adoptive transfer in mice
Last, we assessed whether or not YOD1-C160S DCs pulsed with antigen ex vivo enhance specific CD8+ T-cell responses on transfer into WT mice. To this end, YOD1-C160S BMDCs and their controls were pulsed with 200 μg/mL ovalbumin for 5 hours. Pulsed BMDCs were transferred together with 2 × 106 OT-I splenocytes into WT mice (supplemental Figure 6A). Eight days after transfer, we saw that frequencies and numbers of H2Kb-SIINFEKL- Tet+CD8+ T cells were increased up to 3-fold in the spleens of recipients of antigen-loaded YOD1-C160S BMDCs (supplemental Figure 6B-D). Furthermore, frequencies and numbers of IFN-γ producing cells were also increased up to 3-fold by antigen-loaded YOD1-C160S BMDCs compared with controls (supplemental Figure 6E-G).
Discussion
We show that the expression of a dominant-negative YOD1 transgene (YOD1-C160S) results in a gain-of-function to favor cross-presentation of exogenous antigens by APCs, enhancing CD8+ T-cell responses both in vitro and in vivo. Enhanced cross-presentation is observed for both soluble antigen (ovalbumin) and virus-infected phagocytosed apoptotic cells. The enhancement in cross-presentation afforded by YOD1-C160S is sufficient to improve the control of an MHV-68 infection. Direct presentation of exogenously added peptides by class I MHC and presentation of class II MHC-restricted peptides are not affected by YOD1-C160S.
Our previous work established a role for YOD1 in the dislocation of terminally misfolded proteins from the ER,16 but did not address the possible involvement of YOD1 in translocation events at other intracellular compartments. If proteins destined for cross-presentation must enter the cytoplasm to undergo proteasomal proteolysis, how could expression of YOD1-C160S, which inhibits dislocation, enhance rather than inhibit cross-presentation? We show that the presence of YOD1-C160S prolongs antigen retention within APCs as could also be achieved by the prior treatment of BMDCs with WP1130, a broadly specific deubiquitylase inhibitor (data not shown).26 Prolonged antigen retention by APCs improves cross-presentation.9,27 Several explanations may account for prolonged antigen retention in YOD1-C160S APCs. First, slower endo/phago-lysosomal acidification is a decisive factor that favors antigen cross-presentation. For example, DCs are less efficient at acidification of phagosomes than macrophages, leading to reduced antigen proteolysis in DCs, which in turn favors presentation.27 Furthermore, human monocytes, when differentiated into DCs in the presence of IFN-α, displayed enhanced cross-presentation compared with DCs generated with IL-4.28 This effect was mediated by prolonged antigen retention in IFN-generated DCs. Lysosomal proteolysis in DCs is also regulated by the levels of active lysosomal proteases,9,29 the incomplete recruitment of V-ATPase30 and efficient Rab27a-dependent recruitment of NADPH oxidase NOX-2 to endosomes/phagosomes, which delays the fusion and maturation of endo/phagolysosomal compartments.31-33 Thus, the presence of YOD1-C160S could affect not only antigen retention and delivery to the compartment(s) involved in cross-presentation, but might also have less direct effects, for example through altering the expression of genes involved in the regulation of endo-/phagosomal maturation (S.S., P.-A.K., and H.L.P., unpublished data).
We should also consider the possibility of (a) dislocation pathway(s) operative in endosomes, comparable in concept (if not in composition of its component parts) to those implicated in ER dislocation. ER-resident proteins are recruited to phagosomes and the parasitophorous vacuole of T gondii–infected cells. Inhibition of this recruitment compromises antigen cross-presentation.34 Given YOD1's described role in ER dislocation16 and its interaction with the AAA-ATPase p97, mechanistically involved in a diverse array of membrane-associated phenomena, YOD1 might likewise function as part of an endosome/phagosome-associated dislocation machinery. In this view, YOD1-C160S would favor cross-presentation by blocking dislocation from endosomes, thereby retaining antigen at a site within the DC that favors the production of epitopes suitable for loading onto class I MHC products. Moreover, in a manner potentially independent of its catalytic activity, YOD1 could serve as a factor for recruitment of other molecules necessary to create organelles competent for cross-presentation, or it might regulate trafficking to such organelles. Indeed, recruitment of ER-ERGIC components to phagosomes inhibits phagolysosomal maturation by preventing lysosomal fusion.34 Accordingly, YOD1-C160S expression might result in enhanced recruitment of factors that convert endosomes/phagosomes into a compartment with less proteolytic activity than that found in lysosomes, which would favor cross-presentation.9 Is there a preferred compartment that is responsible for increased retention of ovalbumin by YOD1-C160S DCs? Fluorescence microscopy studies were done to localize internalized ovalbumin. Colocalization of ovalbumin with EEA1, an early endosomal marker, was slightly higher in YOD1-C160S BMDCs than in control cells (supplemental Figure 5E-F). This might account for the increased retention of exogenous antigen and possibly favor its delivery to a subcellular compartment(s) capable of cross-presentation. The overall colocalization of exogenously added antigen with class I MHC did not significantly differ between control and YOD1-C160S BMDCs (supplemental Figure 5G-H). Our results show that enhanced cross-presentation seen in YOD1-C160S BMDCs is reduced by inclusion of proteasome inhibitors (Figure 4D-G). Although there are no known examples of proteasomes gaining access to the lumen of the endo/lysosomal compartment, as none of the proteasome subunits contain the requisite targeting signals, it is conceivable that active proteasomes could be sequestered by autophagosomes, and on fusion with lysosomes, the digestion products might become available for cross-presentation. Similarly, antigen might be degraded in a proteasome-dependent pathway but delivered to endo/lysosomes independent of TAP by autophagy.35
The notion that endolysosomal compartments may possess dislocation machinery derives support from the mechanism of action of a wide variety of bacterial toxins. Cytolethal distending toxins escape into the cytoplasm from an unknown location, presumed to be endolysosomal. The composition of channel(s) used by cholera or pertussis toxin to escape into the cytoplasm has not been unambiguously established.36 Because none of these toxins are known to be capable of pore formation, they must recruit host factors to accomplish discharge of the catalytic subunit and thereby intoxicate cells to which they bind. Genetic analysis of the pathways exploited by such bacterial toxins demonstrates unanticipated complexity and involvement of membrane proteins of unknown function and location.36,37 Although it is possible to block intoxication by these proteins through ablation of specific genes, or by impeding specific trafficking steps pharmacologically, there is no single pathway that can account for all of the observed effects. Thus, when one escape route is blocked, others may take their place.36 Cross-presentation may well follow similar rules. Indeed, we would argue that no single pathway is likely to account for all the observed instances of cross-presentation, nor is it clear that only a single cell type contributes to this phenomenon in vivo. For example, although much emphasis has been placed on the role of the CD8+ subset of DCs,22 numerous reports have documented cross-presentation by APCs outside of the CD8+ DC subset.38-42
Regardless of the underlying mechanism(s), our study shows that interference with a deubiquitylating activity known to be involved in dislocation of misfolded proteins from the ER affects cross-presentation in unexpected ways. The YOD1-C160S mutation exerts a dominant negative effect on dislocation from the ER, yet causes a gain of function when assayed in the setting of cross-presentation. We show that enhanced cross-presentation caused by YOD1-C160S measurably affects the control of a virus infection in vivo. YOD1-C160S reduced production of infectious MHV-68 by ∼ 1000-fold. This finding may have implications for vaccine design, especially for situations where a strong CD8+ T-cell response is desired. For some antigens, such as antigens delivered by recombinant vaccinia virus-based vaccines, cross-presentation is the major pathway for the priming of CD8+ T cells.43,44 Interference with individual DUBs45 such as YOD1, for example through the design of specific inhibitors, might be an interesting approach to tune vaccine efficacy when a strong cross-presentation component is called for.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the National Institutes of Health. Microscopy was performed at Kech Facility of Whitehead Institute for Biomedical Research.
National Institutes of Health
Authorship
Contribution: S.S., P.A.K., M.F., O.K., C.S., and H.L.P. designed research; S.S., P.A.K., M.F., O.K., and C.S. performed research; S.S., P.A.K., M.F., and H.L.P. analyzed and interpreted data; S.S. and P.A.K. performed statistical analysis; and S.S., P.A.K., and H.L.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hidde L. Ploegh, Whitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, MA 02142; e-mail: ploegh@wi.mit.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal