Key Points
Neutrophils mobilized by Am80 display greater bactericidal activity than those by G-CSF.
These findings suggest a molecular rationale for developing new therapy for neutropenia by using Am80 as a cost-effective treatment option.
Abstract
Despite advances in the therapeutic use of recombinant granulocyte colony-stimulating factor (G-CSF) to promote granulopoiesis of human hematopoietic stem cells (HSCs), neutropenia remains one of the most serious complications of cancer chemotherapy. We discovered that retinoid agonist Am80 (tamibarotene) is more potent than G-CSF in coordinating neutrophil differentiation and immunity development. Am80-induced neutrophils (AINs) either in vitro or in neutropenic mouse model displayed strong bactericidal activities, similar to those of human peripheral blood neutrophils (PBNs) or mouse peripheral blood neutrophils (MPBNs) but markedly greater than did G-CSF–induced neutrophils (GINs). In contrast to GINs but similar to PBNs, the enhanced bacterial killing by AINs accompanied both better granule maturation and greater coexpression of CD66 antigen with the integrin β2 subunit CD18. Consistently, anti-CD18 antibody neutralized Am80-induced bactericidal activities of AINs. These studies demonstrate that Am80 is more effective than G-CSF in promoting neutrophil differentiation and bactericidal activities, probably through coordinating the functional interaction of CD66 with CD18 to enhance the development of neutrophil immunity during granulopoiesis. Our findings herein suggest a molecular rationale for developing new therapy against neutropenia using Am80 as a cost-effective treatment option.
Introduction
Neutrophils, the most common granulocytes, constitute up to 70% of circulating leukocytes that primarily defend pathogen infections. Cancer chemotherapy-induced neutropenia is a hematologic disorder marked by large decrease in the number of neutrophils in the bloodstream. It has been more than 2 decades since G-CSF was first used to treat acquired and congenital neutropenia1 by promoting granulopoiesis of hematopoietic stem cells (HSCs). Despite the considerable clinic benefits of this agent when used as primary prophylaxis,2 neutropenia induced by chemotherapy in cancer patients still remains a devastating issue with substantial morbidity, mortality, and cost, which places a significant burden on the individual patient and the healthcare system.1,3-6 An earlier pioneering study with G-CSF administered to normal individuals reveals that this agent adversely affects neutrophil chemotaxis and bactericidal activity against Staphylococcus aureus (S aureus), due in part to the reduced assembly of neutrophil F-actin and altered cytosolic calcium mobilization.7 Recent studies also show that in contrast to peripheral blood neutrophils (PBNs), the impaired bacterial killing in neutrophils induced by G-CSF from CD34+ cells is associated with lack of mature granules, because of abnormal granulopoiesis early in the differentiation process.8 Thus, success in devising more cost-effective therapy for neutropenia may depend on determining how granulopoiesis is coordinated with the development of neutrophil-based immunity.
The retinoid agonist Am809,10 is designed to ameliorate the side effects of all-trans retinoic acid (RA) through its selective binding to retinoic acid receptor α (RARα),9,11 a transcription factor activated by RA12 to regulate granulocytic differentiation of both leukemic myeloblasts and HSCs.13-17 RA, a naturally occurring form of vitamin A, plays key roles in the development of the body plan and induces the differentiation of many types of normal and malignant cells.18-20 To date, RA treatment of acute promyelocytic leukemia (APL) represents the best example of successful differentiation-induction therapy in clinical oncology21 ; however, the side effects associated with RA therapy are generally serious and RA resistance is a common event.22-24 Several studies have demonstrated that RARα regulates Am80-induced granulocytic differentiation.25-27 Moreover, Am80 is approximately 10-fold more efficient, with lower toxicity, than either RA or other retinoids used as differentiation therapy in APL patients.10,28 Currently, Am80 has been approved for the treatment of APL in Japan10 and tested clinically for several other cancers/diseases in the United States and Europe (http://www.cytrx.com/tamibarotene; http://clinicaltrials.gov). The advances in the use of Am80 to induce granulocytic differentiation led us to test this agent as a means to enhance neutrophil bactericidal activity arising from granulopoiesis during immune development. We report here that Am80 possesses significantly greater activity than G-CSF as an inducer of neutrophil differentiation and immune development, probably through its promotion of HSC-derived granulopoiesis by mediating the differential effects of CD66 on CD18 activation.
Methods
Human PBNs, cells, and cell culture
Human peripheral blood (PB) was taken from healthy volunteers in accord with a protocol approved by the Children's Hospital Los Angeles/University of Southern California Keck School of Medicine (CHLA/USC) Committee on Clinical Investigations. Informed consent was obtained in accordance with the Declaration of Helsinki. Details for cells, cell culture, and purification of PBNs are available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
For transmission electron microscopy and magnetic sorting of CD15+ neutrophils, see supplemental Methods.
Phagocytotic and bacterial killing assays
Each 1 × 105 to 2 × 106 of freshly purified PBNs, mouse peripheral blood neutrophils (MPBNs), cyclophosphamide (CPA)–neutropenic mice PB neutrophils (C-MPBNs), G-CSF–induced neutrophils (GINs), and Am80-induced neutrophils (AINs) were suspended with 500 μL of DMEM with 10% FBS and incubated with log-phase Escherichia coli DH5α (ATCC), or Staphylococcus aureus (ATCC) at an MOI of 5 or 10 at 37°C for up to 60 minutes, whereas bacteria in the absence of neutrophils were used to determine growth. See details in supplemental Methods.
Degranulation and neutrophil elastase release analyses
See details in supplemental Methods.
Cell proliferation, cell death, and morphologic analysis of granulocytic differentiation
Western blotting
Western blotting (WB) was performed as described.15 Antibodies for lactoferrin (Abcam), MMP-9 (Merck Chemicals), MPO (Cell Signaling Technology), and LL-37 (Biolegend) were used in analyses.
In situ bactericidal killing and anti-CD18 antibody neutralization of bactericidal activity
In situ neutrophil bactericidal activities were determined using LIVE/DEAD BacLight Viability Kit (Life Technologies), as detailed in supplemental Methods. Anti-CD18 antibody neutralization is described in supplemental Methods.
Immunofluorescence detection of bacterial infection
Flow cytometric analysis
Neutropenic mouse model
Mouse work was performed according to guidelines under protocols approved by the Children's Hospital Los Angeles Institutional Animal Care and Use Committee. Twenty female C57BL6/J mice (The Jackson Laboratory), aged 6 to 8 weeks, were randomly divided into 1 control and 3 experimental groups. To create neutropenic mouse model, as described,31,32 experimental mice received a single 200 mg/kg intraperitoneal dose of cyclophosphamide (CPA; Baxter Healthcare) at day 0, whereas control mice with PBS. To induce neutrophil recovery, experimental mice at day 3 were treated with either 250 μg/kg G-CSF by subcutaneous injection32 or 5 mg/kg Am80 or PBS intraperitoneally for consecutive 2 or 6 days. Each 50 μL of PB was collected from the tail vein every other day for monitoring the baseline of leukocytes (WBCs) and neutrophils with Vetscan HM5 (Abaxis). Mice were killed by CO2 asphyxiation at days 5 or 9, and PB samples were collected by cardiac puncture immediately after sacrificing of mice.
Statistical analysis
See supplemental Methods for statistical analysis.
Results
Am80 promotes neutrophil differentiation more effectively than does G-CSF
Am80, CH55, and ITYA are a group of retinoid agonists that were synthesized by introducing heteroatoms into RA-like structures (supplemental Figure 1). We first compared the efficiencies of these compounds with G-CSF and RA in mediating granulopoiesis from CD34+ cells, using our established methodology.17,29 We demonstrated that G-CSF was less effective than RA at inducing morphologic differentiation of CD34+ cells to granulocytes, accompanied by a higher induction of monocytes (supplemental Figure 2A-B). However, G-CSF induced greater cell proliferation and a lower rate of cell death compared with RA (supplemental Figure 2C). Of the other retinoid agonists, Am80 (10nM) and CH55 (5nM) promoted > 75% granulocytic differentiation by day 13, in contrast to ITYA (5nM) that produced > 60% monocytes (supplemental Figure 2D-E). Although both Am80 and CH55 inhibited cell proliferation (supplemental Figure 2F), the cell death rate associated with Am80 treatment was lower than seen with either CH55 or RA (supplemental Figure 2C,F). Together, these data show that G-CSF is significantly less efficacious as an inducer of granulocytic differentiation than are RA, CH55, and Am80, whereas ITYA mainly induces monocytic differentiation. Importantly, although Am80, RA, and CH55 promote granulocytic differentiation with similar effectiveness, Am80 induces a lower rate of cell death.
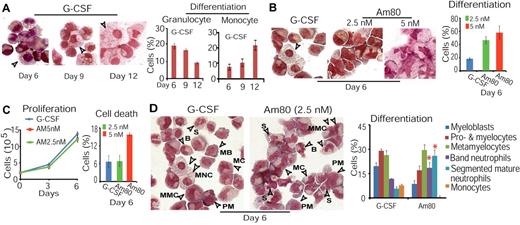
Because the lower level of CD34+ cell differentiation to granulocytes induced by G-CSF on day 12 was associated with a higher level of monocytic induction (supplemental Figure 2A-B), we considered that G-CSF might induce granulocytic differentiation more effectively in a short period of time. Thus, we treated CD34+ cells with G-CSF for 6, 9, and 12 days. By analysis of morphologic differentiation of those cells, we found more effective granulocytic differentiation with the lowest rate of monocytic induction on day 6 compared with either day 9 or day 12 (Figure 1A). Using this optimal 6-day induction condition, we again compared G-CSF and Am80 for their ability to induce granulocytic differentiation of CD34+ cells. Because 10nM of Am80 induced more cell death (supplemental Figure 2F), a reduced Am80 concentration (2.5nM or 5nM) was substituted in the tests. We found that, similar to the effect of 5nM of Am80, 2.5nM of this agent markedly induced granulocytic differentiation with negligible monocytic induction compared with G-CSF (Figure 1B). In contrast to 5nM of Am80 but similar to G-CSF, however, 2.5nM of Am80 stimulated proliferation while preventing cell death (Figure 1C). Hence, these results indicate that the 6-day optimal induction period for G-CSF is also suitable for Am80 (2.5nM), promoting us to apply such drug exposure time and drug dose for the remainders of the study.
Am80 promotes neutrophil differentiation more effectively than G-CSF while stimulating proliferation and preventing cell death similar to G-CSF. (A) Better granulocytic induction associated with lower monocytic induction in CD34+ cells treated with G-CSF for 6 versus 9 or 12 days. (B-C) Reduced concentration of Am80 (2.5nM) leads to more effective induction of granulocytic differentiation with negligible monocytic induction compared with G-CSF (B). Similar to G-CSF, 2.5nM of Am80 displayed capacity of stimulating proliferation while preventing cell death (C). (D) Overall comparison of Am80 and G-CSF under conditions found to be optimal for granulocytic differentiation. Granulopoiesis from CD34+ cells was assessed by light microscopy; arrows indicate examples of cells at their differentiation states. MB indicates myeloblast; PM, promyelocyte; MC, myelocyte; MMC, metamyelocyte; B, band neutrophils; S, segmented neutrophils; and MNC, monocyte (*band neutrophils, P < .03; segmented neutrophils, P < .0025).
Am80 promotes neutrophil differentiation more effectively than G-CSF while stimulating proliferation and preventing cell death similar to G-CSF. (A) Better granulocytic induction associated with lower monocytic induction in CD34+ cells treated with G-CSF for 6 versus 9 or 12 days. (B-C) Reduced concentration of Am80 (2.5nM) leads to more effective induction of granulocytic differentiation with negligible monocytic induction compared with G-CSF (B). Similar to G-CSF, 2.5nM of Am80 displayed capacity of stimulating proliferation while preventing cell death (C). (D) Overall comparison of Am80 and G-CSF under conditions found to be optimal for granulocytic differentiation. Granulopoiesis from CD34+ cells was assessed by light microscopy; arrows indicate examples of cells at their differentiation states. MB indicates myeloblast; PM, promyelocyte; MC, myelocyte; MMC, metamyelocyte; B, band neutrophils; S, segmented neutrophils; and MNC, monocyte (*band neutrophils, P < .03; segmented neutrophils, P < .0025).
We next analyzed neutrophil differentiation of CD34+ cells on the promyelocyte, myelocyte, metamyelocyte, and band neutrophil stages to segmented mature neutrophil stage. CD34+ cells were treated with G-CSF or Am80 for 6 days. Granulocytic morphologic analysis showed that sequential development of neutrophils was sufficiently induced by Am80. By contrast, G-CSF induced more myeloblasts as well as promyelocytes and myelocytes than banded and segmented neutrophils (Figure 1D). In addition, G-CSF consistently induced monocytes (approximately 10%; Figure 1A,D). These results demonstrate that Am80 is more effective than G-CSF in inducing neutrophil morphologic differentiation, with a similar rate of cellular toxicity.
AINs are more effective than GINs in producing and secreting granules
During neutrophil differentiation, heterogeneous populations of granule proteins are produced sequentially and stored in cytoplasm for first-line defense against different pathogens.33,34 We thus investigated whether Am80-enhanced neutrophil maturation is associated with increased granule production. Neutrophils induced for 6 days from CD34+ cells by G-CSF or Am80 were analyzed by transmission electron microscopy. The ultrastructural images showed that, at the segmented neutrophil level, GINs possessed variable number of vesicles often containing less dense and amorphous material, together with few primary and secondary-like granules (Figure 2A), similar to the observations reported before.8 By contrast, vesicles found in AINs were frequently filled with dense material or with both amorphous and dense material (Figure 2B). Compared with GINs, AINs contained increased numbers of primary and secondary-like granules, though their numbers were considerably less than those in PBNs (Figure 2A-C). Thus, our data indicate marked differences in vesicle formation and granule production between GINs and AINs.
AINs produce and secrete granules more effectively than do GINs. (A-C) Ultrastructural images of GINs (A), AINs (B), or freshly isolated PBNs (C) with electron microscope. The vesicles in GINs appeared to contain less dense and amorphous material, whereas those in AINs showed dense material or both dense and amorphous material. AINs appeared to have more primary and secondary-like granules than do GINs. Arrows indicate examples of vesicles and granules. Ve indicates vesicle; Pg, primary granule; and Sg, secondary granule. (D) NE activity challenged by fMLP stimuli. CB indicates cytochalasin B. (E) AINs had a greater production and secretion of the cleaved 38-kDa product of MPO upon E coli stimuli. Images at the top of the horizontal line were derived from 8% gel, whereas images under the horizontal line from 12% gel. The band intensities of Actin detected in 12% gel are similar to those in 8% gel (data not shown). (F) Effective secretion of lactoferrin by AINs versus GINs upon E coli stimuli. To make the sample orders in the medium section match those in the lysate section, the positions between nonstimulated and stimulated samples in the medium section have been switched, as indicated by a vertical line. (G) Greater production and degranulation of LL37 by AINs versus GINs. (H) Increased abundance of intracellular MMP-9 upon E coli stimuli but insufficient degranulation in both GINs and AINs. To make the sample orders in the medium section match those in the lysate section, the positions between nonstimulated and stimulated samples in the medium section have been switched, as indicated by a vertical line. In addition, the samples loaded in lysate and medium sections in panels E through H were originally separated by a molecular weight (MW) marker. This MW marker was deleted so that a blank space is shown between lysate and medium samples.
AINs produce and secrete granules more effectively than do GINs. (A-C) Ultrastructural images of GINs (A), AINs (B), or freshly isolated PBNs (C) with electron microscope. The vesicles in GINs appeared to contain less dense and amorphous material, whereas those in AINs showed dense material or both dense and amorphous material. AINs appeared to have more primary and secondary-like granules than do GINs. Arrows indicate examples of vesicles and granules. Ve indicates vesicle; Pg, primary granule; and Sg, secondary granule. (D) NE activity challenged by fMLP stimuli. CB indicates cytochalasin B. (E) AINs had a greater production and secretion of the cleaved 38-kDa product of MPO upon E coli stimuli. Images at the top of the horizontal line were derived from 8% gel, whereas images under the horizontal line from 12% gel. The band intensities of Actin detected in 12% gel are similar to those in 8% gel (data not shown). (F) Effective secretion of lactoferrin by AINs versus GINs upon E coli stimuli. To make the sample orders in the medium section match those in the lysate section, the positions between nonstimulated and stimulated samples in the medium section have been switched, as indicated by a vertical line. (G) Greater production and degranulation of LL37 by AINs versus GINs. (H) Increased abundance of intracellular MMP-9 upon E coli stimuli but insufficient degranulation in both GINs and AINs. To make the sample orders in the medium section match those in the lysate section, the positions between nonstimulated and stimulated samples in the medium section have been switched, as indicated by a vertical line. In addition, the samples loaded in lysate and medium sections in panels E through H were originally separated by a molecular weight (MW) marker. This MW marker was deleted so that a blank space is shown between lysate and medium samples.
We next verified whether Am80-induced granulocytic differentiation was indeed associated with sufficient granule production, and tested the degranulation ability of AINs upon bacterial stimuli. CD34+ cells were treated with G-CSF or Am80 for 6 days. The resultant GINs and AINs were then incubated with or without E coli or formyl-met-leu-phe (fMLP), followed by analyzing granule production and secretion. By assessing the activity of primary granule neutrophil elastase (NE) release, we found that both AINs and GINs showed much lower activity of NE release than did PBNs, upon challenge with fMLP (Figure 2D). On the other hand, WB analysis using both cell lysates and supernatants showed that compared with GINs (Figure 2E), AINs displayed a greater production and secretion of the cleaved 38-kDa product of primary myeloperoxidase (MPO) granule.35 Moreover, we found that lactoferrin, a secondary granule that has potent broad-spectrum anti-microbial activity,36,37 was stored in AINs and secreted into the medium in sufficient quantities upon bacterial stimuli. By contrast, although the level of lactoferrin was increased with bacterial stimuli in GINs, the efficiency of lactoferrin secretion by GINs was much lower than seen with AINs (Figure 2F). Similarly, a high level of secondary granule LL-37 was observed in AINs, and upon bacterial stimuli, LL-37 granules were effectively released into the medium. GINs, on the other hand, showed both lack of LL-37 production and degranulation (Figure 2G). Thus, MPO, lactoferrin, and LL-37 are effectively produced and/or secreted by AINs but not GINs.
Interestingly, even in the absence of E coli, we found that GINs secreted MMP-9 (tertiary granules) into the medium, and that this secretion was inhibited by bacterial stimuli (Figure 2H). On the other hand, although E coli increased the level of MMP-9 in AINs, bacterial stimuli failed to induce AINs to secrete MMP-9 (Figure 2H). These observations indicate possible defects in MMP-9 induction or degranulation (or both) in ex vivo–differentiated GINs and AINs.
AINs possess significantly higher phagocytotic and bactericidal activities than do GINs
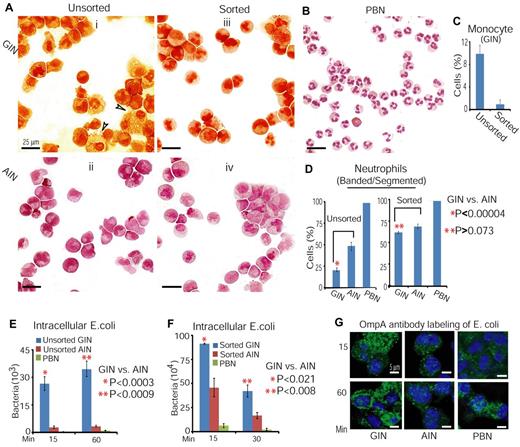
The above studies show that Am80 is more effective than G-CSF in promoting both granulocytic morphologic differentiation and granule production/secretion (Figures 1–2). We therefore asked whether this higher degree of neutrophil differentiation induced by Am80 translates into greater neutrophil immunity against bacterial infection. Because Am80 induces more band-form and segmented neutrophils than does G-CSF (Figure 1), we first tested whether sorted band and segmented neutrophils from GINs and AINs possessed similar bactericidal activities. Because neutrophils are CD15+ cells,38 we purified GINs and AINs using anti-CD15 antibody conjugated to magnetic MicroBeads. After purification, the proportion of band/segmented neutrophils in unsorted GINs increased from 21% to 63% in sorted GINs, similar to findings in sorted AINs (Figure 3Ai-Aii versus Aiii,Aiv, D). Moreover, the fraction of residual monocytes in sorted GINs was only approximately 1% (Figure 3C). In addition, the size of these ex vivo–differentiated neutrophils is larger than that of freshly isolated PBNs from healthy donor (Figure 3A versus B), as previously observed.8 These unsorted and sorted GINs and AINs, together with PBNs consisting of > 95% segmented neutrophils (Figure 3B,D), were then tested for their capacity to phagocytize E coli. Of note, the CFU counts indicated that only a few viable bacteria were recovered from intracellular compartments of PBNs and unsorted AINs compared with unsorted GINs (Figure 3E). Moreover, the sorted GINs were also significantly less able than sorted AINs to clear the ingested bacteria (Figure 3F). Such enhanced bacterial clearance in AINs was further confirmed by in situ labeling of E coli, using anti-OmpA antibody that specifically recognizes the outer membrane of E coli (Figure 3G). These results indicate that the levels of neutrophil differentiation arising from granulopoiesis induced by Am80 are essential to effective neutrophil immunity against bacterial infection. This observation is supported by the data that GINs with segmented neutrophil morphology still display a lower level of granule-like molecules and contain a greater number of less dense, amorphous vesicles (Figure 2A-C).
Both unsorted and sorted AINs display a higher capacity for clearance of bacteria than do unsorted and sorted GINs. (A) Morphology of GINs and AINs before and after sorting with anti-CD15 antibody. Arrows indicate examples of monocytes. (B) Morphology of freshly isolated PBNs. (C-D) Quantification of monocytes (C) and band/segmented neutrophils (D) in panels A and B. (E-F) Comparison of the effect of unsorted and sorted GINs and AINs on the clearance of intracellular bacteria. Clearance efficiency was determined from the numbers of viable bacteria recovered from the intracellular compartment after infection. E coli DH5α at an MOI of 100 were used to infect sorted GINs, sorted AINs, and PBNs (F). (G) In situ labeling of bacteria with anti-OmpA antibodies in unsorted GINs and AINs.
Both unsorted and sorted AINs display a higher capacity for clearance of bacteria than do unsorted and sorted GINs. (A) Morphology of GINs and AINs before and after sorting with anti-CD15 antibody. Arrows indicate examples of monocytes. (B) Morphology of freshly isolated PBNs. (C-D) Quantification of monocytes (C) and band/segmented neutrophils (D) in panels A and B. (E-F) Comparison of the effect of unsorted and sorted GINs and AINs on the clearance of intracellular bacteria. Clearance efficiency was determined from the numbers of viable bacteria recovered from the intracellular compartment after infection. E coli DH5α at an MOI of 100 were used to infect sorted GINs, sorted AINs, and PBNs (F). (G) In situ labeling of bacteria with anti-OmpA antibodies in unsorted GINs and AINs.
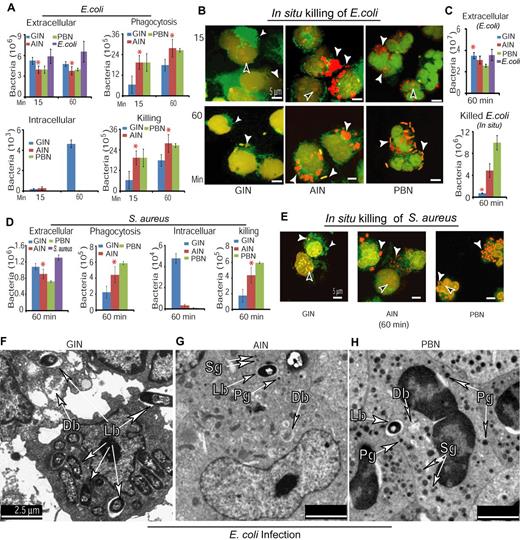
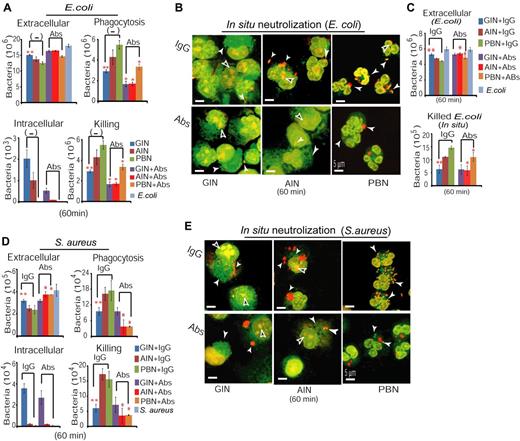
To thoroughly compare bactericidal activities between AINs and GINs, we treated CD34+ cells with G-CSF or Am80 for 6 days, followed by analyses of phagocytosis and bacterial killing. Using the methodology previously described,30,39 we incubated GINs, AINs, or PBNs with E coli at an MOI of 5 for 15 or 60 minutes, or use of E coli in the absence of neutrophils to monitor bacterial growth. The results showed (Figure 4A) that the extracellular bacteria were significantly decreased in both PBN and AIN samples. PBNs rapidly killed bacteria within 15 minutes, while by 60 minutes there were only a few viable intracellular bacteria in either PBN or AIN samples. By contrast, GINs showed substantially impaired clearance of intracellular bacteria by 60 minutes of infection and was deficient in both phagocytosis and bacterial killing. To confirm that AINs possess greater bactericidal activity than do GINs, we examined bacterial killing in situ, using confocal microscopy of both viable bacteria labeled with SYTO9 fluorescent dye (green) and killed bacteria labeled with propidium iodide (PI) fluorescent dye (red). The results demonstrated that significantly more surviving E coli (green) were retained in GIN samples, where we found much less dead bacteria (red) in contrast to observations in AIN or PBN samples (Figure 4B). Quantifying both extracellular bacteria after infection as well as in situ dead bacteria confirmed that both AINs and PBNs possessed greater bactericidal activities than did GINs (Figure 4C). Because S aureus remains a significant cause of morbidity and mortality in neutropenic patients40 whereas superoxide-producing activity of mature neutrophils is involved in oxygen-dependent killing of this bacterium,41,42 we examined the oxidative activity of GINs, AINs, and PBNs, followed by testing their bactericidal activities against S aureus. The results showed that significantly higher oxidative activities were observed in both AINs and PBNs compared with GINs (supplemental Figure 3). Moreover, similar to the effectiveness of AINs on inhibiting E coli infection (Figure 4A-C), AINs displayed significantly higher bactericidal activities against S aureus infection compared with GINs (Figure 4D-E). Furthermore, ultrastructural images of E coli infection by electron microscope showed that numerous intact/surviving bacteria were retained in GINs (Figure 4F) containing less dense vesicles (Figure 2A); whereas similar to PBNs (Figure 4H), only a few intact/surviving bacteria were identified in AINs (Figure 4G) whose cytoplasm contained dense vesicles together with increased numbers of primary and secondary-like granules (Figure 2B). In GINs, AINs, and PBNs, there were also a few vesicles with degraded materials suggestive of dead bacteria (Figure 4F-H). Considered together, these data suggest that Am80-induced granulocytic differentiation is associated with enhanced neutrophil innate immunity against bacterial infection.
AINs possess significantly higher phagocytotic and bactericidal activities than do GINs. (A) Phagocytotic and bactericidal activities against E coli infection were determined by the number of extracellular bacteria, phagocytized bacteria, recovered intracellular bacteria, and killed bacteria. There was a 1.26- ± 0.01-fold increase in bacterial numbers over the 45 minutes of the experiment (*GINs versus AINs, P < .03 at least). (B) Confocal fluorescence microscopy of surviving and dead bacteria in GINs, AINs and PBNs. White arrow indicates surviving (green) or dead bacteria (red), whereas black arrow indicates example of neutrophil nucleus stained in red simultaneously by PI fluorescent dye. Representative confocal fluorescence images of bacteria were reproduced at 60× magnification. (C) Quantification of both extracellular bacteria after infection and in situ killed bacteria in panel B (*GINs versus AINs or PBNs, P < .04 at least). (D-E) Similar to panels A and B, phagocytic and bactericidal activities against S aureus infection were determined in GINs, AINs, and PBNs (*GINs versus AINs, P < .04 at least). (F-H) Ultrastructural images of bacterial infection of GINs (F), AINs (G), and PBNs (H) with electron microscope. Lb indicates living bacteria; Db, suggestive of dead bacteria; Pg, primary granule; and Sg, secondary granule.
AINs possess significantly higher phagocytotic and bactericidal activities than do GINs. (A) Phagocytotic and bactericidal activities against E coli infection were determined by the number of extracellular bacteria, phagocytized bacteria, recovered intracellular bacteria, and killed bacteria. There was a 1.26- ± 0.01-fold increase in bacterial numbers over the 45 minutes of the experiment (*GINs versus AINs, P < .03 at least). (B) Confocal fluorescence microscopy of surviving and dead bacteria in GINs, AINs and PBNs. White arrow indicates surviving (green) or dead bacteria (red), whereas black arrow indicates example of neutrophil nucleus stained in red simultaneously by PI fluorescent dye. Representative confocal fluorescence images of bacteria were reproduced at 60× magnification. (C) Quantification of both extracellular bacteria after infection and in situ killed bacteria in panel B (*GINs versus AINs or PBNs, P < .04 at least). (D-E) Similar to panels A and B, phagocytic and bactericidal activities against S aureus infection were determined in GINs, AINs, and PBNs (*GINs versus AINs, P < .04 at least). (F-H) Ultrastructural images of bacterial infection of GINs (F), AINs (G), and PBNs (H) with electron microscope. Lb indicates living bacteria; Db, suggestive of dead bacteria; Pg, primary granule; and Sg, secondary granule.
Granulocytes induced by Am80 show higher coexpression of CD66 and CD18 surface markers than do those induced by G-CSF
Our observation, AINs possessed profound bactericidal activity similar to that PBNs but greater than GINs (Figure 4), raises an intriguing question: What signaling regulation against bacterial infection has developed during neutrophil differentiation? Previous studies suggest that both CD66 antigens and complement receptor-3 (CR3), a heterodimeric integrin αMβ2 that consists of CD11b and CD18, regulate neutrophil innate immunity against diverse pathogens.43,44 Hence, we investigated the expression of CD66, CD11b, and CD18 surface markers in these cells. Flow cytometric analysis showed that granulocytes induced by G-CSF or Am80 expressed similar levels of CD11b, CD18, or CD66 surface markers with CD11b surface marker expression being significantly lower in GINs and AINs than in PBNs (supplemental Figure 4A-D). Moreover, GINs and AINs coexpressed CD11b-CD18 surface markers at similar levels that were lower than found in PBNs (supplemental Figure 4E). These data indicate that both GINs and AINs are characterized by defective expression of CD11b or coexpression of CD11b-CD18 surface markers.
We next compared the coexpression of CD66 with CD11b or CD18 surface markers in GINs versus AINs versus PBNs using flow cytometry analysis. The results revealed an additional subpopulation coexpressing either CD66-CD18 (supplemental Figure 5A top panel) or CD66-CD11b (supplemental Figure 5B top panel) surface markers in G-CSF–induced granulocytes. Such subpopulations were not observed in either Am80-induced granulocytes or PBNs. Moreover, there was higher coexpression of CD66-CD18 or CD66-CD11b surface markers in Am80-induced granulocytes than in those induced by G-CSF (supplemental Figure 5A-B). These data indicate that during differentiation, AINs gain some properties similar to those of PBNs, as shown by a single population expressing CD66-CD18 or CD66-CD11b surface markers.
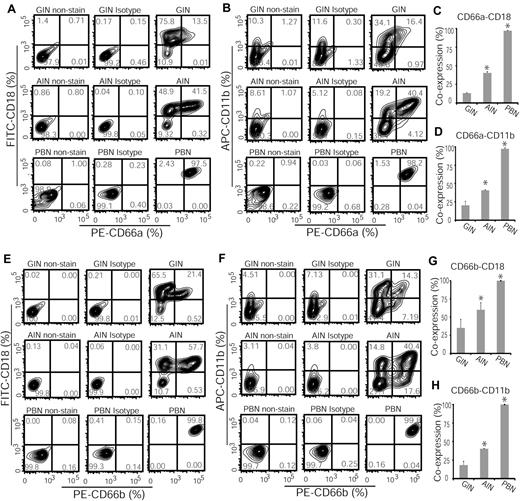
Because CD66a and CD66b are involved in mediating neutrophil aggregation45 and CR3 activation,46,47 we next investigated coexpression of CD66a-CD18, CD66a-CD11b, CD66b-CD18, and CD66b-CD11b surface markers in granulocytes induced from CD34+ cells by G-CSF or Am80. Flow cytometric analyses showed that coexpression of CD66a-CD18 (Figure 5A,C) and CD66a-CD11b (Figure 5B,D) surface markers in GINs was significantly lower than in AINs or PBNs samples (Figure 5A-D). Furthermore, we found that similar to PBNs, significantly higher coexpression of CD66b-CD18 (Figure 5E,G) and CD66b-CD11b (Figure 5F,H) surface markers were induced in Am80-induced granulocytes compared with those induced by G-CSF (Figure 5E-H). These findings suggest that Am80 is more effective than G-CSF to enhance the development of a CD66-CR3 signaling network during differentiation.
Granulocytes induced by Am80 coexpress CD66 and CD18 surface markers at a higher level than do those induced by G-CSF. (A-D) Flow cytometric analyses of G-CSF– versus Am80-induced granulocytes in coexpression of CD66a-CD18 and CD66a-CD11b surface markers (*GINs versus AINs. CD66a-CD18, P < .003; CD66a-CD11b, P < .028). (E-H) Increased induction of both CD66b-CD18 and CD66b-CD11b surface markers in Am80 versus G-CSF–induced granulocytes (*GINs versus AINs; CD66b-CD18, P < .047; CD66b-CD11b, P < .033). Corresponding isotypes and nonstaining (blank cells) served as controls.
Granulocytes induced by Am80 coexpress CD66 and CD18 surface markers at a higher level than do those induced by G-CSF. (A-D) Flow cytometric analyses of G-CSF– versus Am80-induced granulocytes in coexpression of CD66a-CD18 and CD66a-CD11b surface markers (*GINs versus AINs. CD66a-CD18, P < .003; CD66a-CD11b, P < .028). (E-H) Increased induction of both CD66b-CD18 and CD66b-CD11b surface markers in Am80 versus G-CSF–induced granulocytes (*GINs versus AINs; CD66b-CD18, P < .047; CD66b-CD11b, P < .033). Corresponding isotypes and nonstaining (blank cells) served as controls.
Anti-CD18 antibody neutralizes Am80-enhanced neutrophil bactericidal activity
Because Am80-enhanced neutrophil bactericidal activity (Figure 4) was associated with higher coexpression of CD66-CD18 surface markers (supplemental Figure 5; Figure 5), this suggests that during granulopoiesis, Am80 simultaneously promotes neutrophil differentiation and development of the CD66-CD18 signaling network against bacterial infection. To test this idea using bacterial killing assay, we disrupted CD66-CD18 signaling by neutralization of CD18 function with anti-CD18 antibody. Freshly isolated PBNs, AINs, GINs were first incubated with or without anti-CD18 antibody for 30 minutes. The cells were then exposed to log-phase E coli for 60 minutes. E coli in the neutrophil-free condition were used to monitor bacterial growth. The changes in phagocytotic and bactericidal activities in the presence and absence of anti-CD18 antibody were determined by calculating extracellular, phagocytized, intracellular, and killed bacteria. The results (Figure 6A) showed that similar to PBNs, AINs displayed significantly higher phagocytotic and bactericidal activities than did GINs, and that addition of anti-CD18 antibody significantly neutralized such activities, as reflected by recovered numbers of extracellular bacteria, suppressed phagocytotic capacity, and reduced bacterial killing. Further to assess the effect of CD66-CD18 signaling on regulating neutrophil immunity we performed in situ bacterial infection and killing in the presence of normal IgG (control) or anti-CD18 antibody. Confocal microscope imaging showed that both AINs and PBNs indeed had higher in situ bactericidal activities in killing of E coli than did GINs, whereas addition of anti-CD18 antibody significantly reversed such bacterial killing in either AIN or PBN samples (Figure 6B-C). Furthermore, such Am80-promoted neutrophil differentiation and development of the CD66-CD18 signaling network were evaluated by testing the effect of AINs against S aureus infection. We found that similar to the results in Figure 6A-C, the markedly higher bactericidal activity of AINs against S aureus infection was significantly neutralized in the presence of anti-CD18 antibodies (Figure 6D-E). Hence, higher coexpression of CD66-CD18 surface markers induced by Am80 (supplemental Figure 5; Figure 5) correlates with Am80-enhanced neutrophil immunity arising from granulopoiesis, supporting the notion that in AINs, CD18 cross-links the differential effect of CD66 on neutrophil activation with CR3-dependent neutrophil innate immunity against bacterial infection.
Anti-CD18 antibody neutralizes Am80-enhanced neutrophil bactericidal activities. (A) Effects of anti-CD18 antibody on neutralization of AINs, GINs, and PBNs phagocytic and bactericidal activities against E coli (**GINs versus AINs without Abs, P < .037 at least; *AINs versus AINs+Abs, P < .036 at least; GINs versus GINs+Abs, P < .006 at least; PBNs versus PBNs+Abs, P < .007 at least). Abs indicates antibodies. (B) In situ bacterial killing imaged by confocal microscopy. White arrow indicates surviving (green) or dead bacteria (red), whereas black arrow indicates example of neutrophil nucleus stained in red simultaneously by PI fluorescent dye. (C) Quantification of both extracellular bacteria after infection and in situ killed bacteria in panel B (**GINs versus AINs or PBNs, P < .006 at least; *AINs+IgG versus AINs+Abs, P < .008; PBNs+IgG versus PBNs+Abs, P < .03). (D-E) Effects of anti-CD18 antibody on neutralization of AINs, GINs, and PBNs phagocytic and bactericidal activities against S aureus (**GINs versus AINs or PBNs, P < .03 at least; *AINs+IgG versus AINs+Abs, P < .007 at least; PBNs+IgG versus PBNs+Abs, P < .02 at least).
Anti-CD18 antibody neutralizes Am80-enhanced neutrophil bactericidal activities. (A) Effects of anti-CD18 antibody on neutralization of AINs, GINs, and PBNs phagocytic and bactericidal activities against E coli (**GINs versus AINs without Abs, P < .037 at least; *AINs versus AINs+Abs, P < .036 at least; GINs versus GINs+Abs, P < .006 at least; PBNs versus PBNs+Abs, P < .007 at least). Abs indicates antibodies. (B) In situ bacterial killing imaged by confocal microscopy. White arrow indicates surviving (green) or dead bacteria (red), whereas black arrow indicates example of neutrophil nucleus stained in red simultaneously by PI fluorescent dye. (C) Quantification of both extracellular bacteria after infection and in situ killed bacteria in panel B (**GINs versus AINs or PBNs, P < .006 at least; *AINs+IgG versus AINs+Abs, P < .008; PBNs+IgG versus PBNs+Abs, P < .03). (D-E) Effects of anti-CD18 antibody on neutralization of AINs, GINs, and PBNs phagocytic and bactericidal activities against S aureus (**GINs versus AINs or PBNs, P < .03 at least; *AINs+IgG versus AINs+Abs, P < .007 at least; PBNs+IgG versus PBNs+Abs, P < .02 at least).
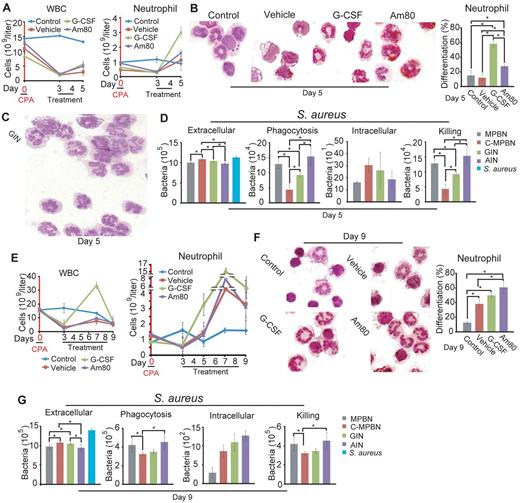
Neutrophils mobilized by Am80 in neutropenic mice display greater bactericidal activity than those by G-CSF
Using a neutropenic mouse model induced by a single dose of CPA, as described,31,32 we tested whether in vivo mobilized neutrophils by Am80 indeed possess the same greater neutrophil immunity against bacterial infection than those by G-CSF, as observed in the ex vivo model (Figures 4, 6). We found that a severe reduction of both WBCs and neutrophils occurred in all experimental mice 3 days after injection of CPA, compared with control mice (Figure 7A). Rapidly thereafter at day 5 with injection of G-CSF or Am80 or vehicle for 2 consecutive days, a remarkably accelerated neutrophil recovery was induced by G-CSF compared with Am80, whereas neutrophil counts in vehicle group also returned nearly to control value (Figure 7A-B). These neutrophils were purified from PB of different mice, as shown by GIN sample (Figure 7C), and used for analyzing of bactericidal activities against S aureus infection. We found (Figure 7D) that extracellular bacteria were eliminated significantly by either MPBNs, AINs, or GINs than neutrophils isolated from C-MPBNs treated with vehicle, whereas AINs were markedly more effective on eliminating bacteria than both GINs and C-MPBNs. Similar to MPBNs, AINs phagocytized and killed significantly more bacteria than either GINs or C-MPBNs. Because the accelerated neutrophil recovery ceased at day 7 (Figure 7E), we purified neutrophils from PB of different mice at day 9 (Figure 7F) to compare their bactericidal activities after cessation of accelerated neutrophil recovery. The results showed that both MPBNs and AINs still displayed significantly higher bactericidal activity than did C-MPBNs, whereas there was no difference in either phagocytosis or bacterial killing between GINs and C-MPBNs (Figure 7G). These findings demonstrate that similar to MPBNs, neutrophils mobilized by Am80 in neutropenic mice are significantly more efficacious against S aureus infection than those by G-CSF, even though G-CSF can induce remarkably more neutrophils than do Am80 at earlier stage of neutrophil recovery. Moreover, although C-MPBN counts reach significantly higher level than control values at later stage of neutrophil recovery, the bactericidal activities of C-MPBNs are still significantly lower than MPBNs or AINs.
Neutrophils mobilized by Am80 in neutropenic mice display greater bactericidal activity than those by G-CSF. Twenty C57BL6/J mice were randomly divided into 4 groups for the experiments. (A) G-CSF induced a remarkably accelerated neutrophil recovery compared with mice treated with Am80 or vehicle at day 5. (B) Morphologic analysis of PB neutrophils at day 5 (*G-CSF versus Am80, P < 1.2 × 10−6; G-CSF versus control, P < 4.0 × 10−5; G-CSF versus vehicle, P < 8.2 × 10−8; Am80 versus vehicle, P < 1.3 × 10−6; Am80 versus control, P < .016. (C) Representative purity of PB neutrophils, as shown by freshly isolated GINs from mice. (D) Phagocytotic and bactericidal activities of neutrophils, isolated from PB of different mice, were reflected by the number of extracellular bacteria, phagocytized bacteria, and killed bacteria. *Extracellular: AINs versus GINs, P < .043; AINs versus C-MPBNs, P < 1.1 × 10−4; MPBNs versus C-MPBNs, P < 3.7 × 10−4; GINs versus C-MPBNs, P < .049. *Phagocytosis: AINs versus GINs, P < .026; AINs versus C-MPBNs, P < .02; MPBNs versus GINs, P < .042; MPBNs versus C-MPBNs, P < .003; GINs versus C-MPBNs, P < .009. *Killing: AINs versus GINs, P < .026; AINs versus C-MPBNs, P < .005; MPBNs versus GINs, P < .015; MPBNs versus C-MPBNs, P < .003; GINs versus C-MPBNs, P < .009. (E) Accelerated recoveries of WBC and neutrophil were ceased at day 7 after 96 hours of stimuli with G-CSF or Am80 or vehicle. (F) Morphologic analysis of PB neutrophils at day 9 (*G-CSF versus control, P < .005; Am80 versus control, P < 3.9 × 10−4; Am80 versus vehicle, P < .03; Vehicle versus control, P < .03). (G) AINs possessed significantly higher phagocytotic and bactericidal activities than do GINs 48 hours after cessation of accelerated neutrophil recovery (E). *Extracellular: AINs versus GINs, P < .02; AINs versus C-MPBNs, P < .007; MPBNs versus GINs, P < .04; MPBNs versus C-MPBNs, P < .02. *Phagocytosis: AINs versus C-MPBNs, P < .034; MPBNs versus C-MPBNs, P < .043. *Killing: AINs versus C-MPBNs, P < .034; MPBNs versus C-MPBNs, P < .043.
Neutrophils mobilized by Am80 in neutropenic mice display greater bactericidal activity than those by G-CSF. Twenty C57BL6/J mice were randomly divided into 4 groups for the experiments. (A) G-CSF induced a remarkably accelerated neutrophil recovery compared with mice treated with Am80 or vehicle at day 5. (B) Morphologic analysis of PB neutrophils at day 5 (*G-CSF versus Am80, P < 1.2 × 10−6; G-CSF versus control, P < 4.0 × 10−5; G-CSF versus vehicle, P < 8.2 × 10−8; Am80 versus vehicle, P < 1.3 × 10−6; Am80 versus control, P < .016. (C) Representative purity of PB neutrophils, as shown by freshly isolated GINs from mice. (D) Phagocytotic and bactericidal activities of neutrophils, isolated from PB of different mice, were reflected by the number of extracellular bacteria, phagocytized bacteria, and killed bacteria. *Extracellular: AINs versus GINs, P < .043; AINs versus C-MPBNs, P < 1.1 × 10−4; MPBNs versus C-MPBNs, P < 3.7 × 10−4; GINs versus C-MPBNs, P < .049. *Phagocytosis: AINs versus GINs, P < .026; AINs versus C-MPBNs, P < .02; MPBNs versus GINs, P < .042; MPBNs versus C-MPBNs, P < .003; GINs versus C-MPBNs, P < .009. *Killing: AINs versus GINs, P < .026; AINs versus C-MPBNs, P < .005; MPBNs versus GINs, P < .015; MPBNs versus C-MPBNs, P < .003; GINs versus C-MPBNs, P < .009. (E) Accelerated recoveries of WBC and neutrophil were ceased at day 7 after 96 hours of stimuli with G-CSF or Am80 or vehicle. (F) Morphologic analysis of PB neutrophils at day 9 (*G-CSF versus control, P < .005; Am80 versus control, P < 3.9 × 10−4; Am80 versus vehicle, P < .03; Vehicle versus control, P < .03). (G) AINs possessed significantly higher phagocytotic and bactericidal activities than do GINs 48 hours after cessation of accelerated neutrophil recovery (E). *Extracellular: AINs versus GINs, P < .02; AINs versus C-MPBNs, P < .007; MPBNs versus GINs, P < .04; MPBNs versus C-MPBNs, P < .02. *Phagocytosis: AINs versus C-MPBNs, P < .034; MPBNs versus C-MPBNs, P < .043. *Killing: AINs versus C-MPBNs, P < .034; MPBNs versus C-MPBNs, P < .043.
Discussion
Am80-enhanced bactericidal activity arises from granulopoiesis during neutrophil differentiation
Granulopoiesis refers to the stage-specific differentiation of HSCs to common myeloid progenitors to granulocyte progenitors and finally to mature granulocytes. Neutrophil innate immunity develops over the entirety of granulocytic differentiation. Recent studies demonstrate that impaired bacterial killing by neutrophils induced from CD34+ cells with G-CSF is associated with the lack of mature granules.8 We now show that Am80 is more effective than G-CSF in inducing neutrophil differentiation of CD34+ cells (Figure 1). This property can be attributed to the sequential manner in which Am80 induces this process, beginning with the promyelocyte stage and expanding finally to segmented neutrophils (Figure 1D), all associated with the production of granule proteins and their secretions upon bacterial stimulation (Figure 2). By contrast, GINs with segmented neutrophil morphology exhibit much less dense/amorphous vesicles and a lower level of granules than do AINs and PBNs (Figure 2A-C). Such deficiency in GINs is accompanied by defects in degranulation compared with AINs (Figure 2E-G). Moreover, coexpression of CD66-CD18 surface markers in AINs and PBNs are significantly higher than in GINs (supplemental Figure 5; Figure 5). Such higher levels of CD66-CD18 correlate with the significantly greater bactericidal activity of AINs, PBNs, and MPBNs versus GINs (Figures 4, 6, 7), explaining why Am80-enhanced phagocytosis and bacterial killing can be neutralized with anti-CD18 antibody (Figure 6). These findings support the notion that CD66-CD18 signaling is essential in the regulation of neutrophil innate immunity against bacterial infection. Further evidence favoring the cost-effective use of Am80 against neutropenia needs to be generated from determining that in a neutropenic mouse model, Am80 is more effective than G-CSF against neutropenia-related different microbial infections in both prophylaxis and treatment settings.
Retinoid-mediated development of neutrophil innate immunity and CD66-CD18 signaling during granulopoiesis
During the past 2 decades, the results of clinical therapy for neutropenia have shown that despite an increased number of neutrophils and reduced neutropenia duration with administration of G-CSF, there are no benefits consistently on reducing infection and infection-related mortality or all-cause mortality.1,3,5,6 This raises a crucial question: How could G-CSF increase the number of neutrophils and shorten the neutropenia duration but only have a marginal effect on reducing of infection, morbidity, and mortality? In 1998, Ambruso's group initiated a critical in vivo study to address this issue by administering G-CSF to normal individuals.7 They found that neutrophils mobilized by G-CSF displayed significantly lower chemotaxis and bacterial killing, probably because of the reduced assembly of neutrophil F-actin and altered calcium signaling. This study revealed, for the first time, that the real benefit of G-CSF therapy might lie in enhanced number and survival of neutrophils, although these cells harbor defects in bacterial killing. In later studies using an ex vivo granulopoiesis system, Dick et al show that neutrophils induced by G-CSF are defective in normal granule development, leading to marked impairment in bacterial killing.8 The studies we report here demonstrate that neutrophils mobilized by Am80 in vitro (Figures 1–2) or in vivo (Figure 7) possess greater innate immunity against bacterial infection than do those induced with G-CSF (Figures 3, 4, 6, 7). Such enhanced innate immunity arises during granulopoiesis in the differentiation process (Figures 1, 2, 5; supplemental Figure 5), probably via coordination of the granule production, neutrophil maturation, and immunity development through the CD66-CD18 signaling pathway (Figures 5–6, supplemental Figure 5). Hence, our data based on comparing the neutrophil maturation and bactericidal activity mobilized by Am80 and G-CSF support the notion: the defect of neutrophil innate immunity mobilized by G-CSF during granulopoiesis primarily results in the lower efficacy against bacterial infection. However, how retinoid signaling coordinates granulopoiesis with development of neutrophil immunity is currently unknown. Am80 is designed to eliminate the side effects of RA by binding to RARα in a more selective manner9,11 to induce granulocytic differentiation.25,27 Using Am80 to induce granulopoiesis of CD34+ cells, we found that AINs and PBNs coexpress CD66-CD18 surface markers at significantly greater levels than do GINs (Figure 5; supplemental Figure 5). Such up-regulated surface marker expression in AINs is associated with increased granule production, sufficient degranulation of MPO, lactoferrin, and LL-37 on bacterial stimuli, and enhanced bactericidal activities (Figures 2,Figure 3–4). Moreover, the enhanced phagocytotic and bactericidal activities of AINs can be neutralized with anti-CD18 antibody (Figure 6). Thus, the enhanced bactericidal activity of AINs appears to operate through Am80-induced RARα signaling to coordinate CD66-dependent neutrophil maturation/activation with CD18-mediated host defense (supplemental Figure 6). Further evaluation of this regulatory signaling using loss-of-function approaches, including shRNA blockade of CD66a, CD66b, and CD18 expression during granulopoiesis, should systemically determine the mechanisms by which Am80-mediated development of neutrophil immunity against bacterial infection proceeds through the CD66-CD18 signaling pathway. This information, in turn, should provide new insights into how CD66 leads to CD18 activation and how CD66-CD18 signaling coordinates granulocytic maturation-activation with the host defense.
Defects arising from ex vivo granulopoiesis and their implications
CD66 and CR3 signaling receptors play essential roles in cross-linking granulocyte activation with neutrophil immunity against bacterial infection,43,44 whereas granule proteins produced and stored sequentially during granulopoiesis provide an innate defense armory against microbial infections.33,48 Thus, adequate coordination of both innate immune receptor expression and granule production during neutrophil differentiation is critical to securing neutrophil bactericidal activity. Indeed, the relatively lower effectiveness with which AINs coexpress CD11b-CD18 (supplemental Figure 4) and secrete NE or MMP-9 (Figure 2D,H) may reduce the anti-microbial activity of these neutrophils against other microorganisms, a possibility that remains to be tested. Moreover, Sabroe's group shows that compared with PBNs, G-CSF–induced neutrophils have markedly higher surface expression of Toll-like receptors (TLR),8 similar to our observation that the level of TLR4 surface marker is significantly high in both GINs and AINs (data not shown). Interestingly, although neutrophils induced by G-CSF show a greater increase in IL-8 production than do PBNs in response to the TLR agonists, they fail to migrate toward low concentrations of IL-8, whereas PBNs show chemotaxis in response to this chemokine.8 However, we observed that GINs showed the highest chemotaxis compared with AINs and PBNs in response to fMLP stimuli (data not shown). Despite this difference, a higher surface expression of TLR detected by Sabroe's group and by us indicates an abnormal development of TLR signaling regulation in those ex vivo–differentiated neutrophils.8 Moreover, we have found that in contrast to PBNs, ex vivo–differentiated neutrophils induced by either G-CSF or Am80 display an altered expression pattern of fragment crystallizable γ receptors FcγR, including FcγRI (CD64) and FcγRIII (CD16; supplemental Figures 7-8), which are involved in maintenance of immune homeostasis and regulation of proinflammatory responses.49 Further studies to elucidate the mechanisms of Am80-enhanced neutrophil immunity as well as identify the defects in those Am80-mobilized neutrophils should establish a framework of molecular insights into Am80 signaling, which may catalyze the design of improved retinoid agonists to more effectively mimic human granulopoiesis and development of neutrophil immunity in vivo simultaneously.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Xiaopeng Zhang for providing assistance in phagocytotic experiment and Ms Minerva Mongeotti in electron microscopy analysis.
This work was supported by grants from the National Institutes of Health (R01 CA120512 and ARRA-R01CA120512) and Winzer Fund from Department of Pathology (CHLA/USC) to L.W.
National Institutes of Health
Authorship
Contribution: W.D. designed the research, performed data collection, analysis, and interpretation, and wrote the paper; H.S. performed data collection, analysis, and interpretation, and prepared the manuscript; L.L. designed the research and performed data collection, analysis, and interpretation, and prepared the manuscript; R.M. performed data collection and analysis; X.Z. designed the research and prepared the manuscript; K.S. provided retinoids and related test conditions; Q.H. designed the research; N.V.P. designed the research, performed data analysis and interpretation, provided study material, and prepared the manuscript; and L.W. conceived and designed the research, performed data analysis and interpretation, wrote the paper, provided financial support, and approved the final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lingtao Wu, Dept of Pathology, MS #103, Children's Hospital Los Angeles, USC Keck School of Medicine, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail: lwu@chla.usc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal