To the editor:
The relative benefits and risks of peripheral blood stem cells (PBSCs) versus bone marrow (BM) for allogeneic hematopoietic stem cell transplantation (SCT) are still a matter of highly controversial debates.1-3 The first randomized study comparing the 2 stem cell sources in unrelated donor SCT recently documented comparable overall and event-free survival, but indicated a higher risk for chronic graft-versus-host disease (GVHD) with PBSCs.4 Only a few pediatric patients were included in this study even though the long-term sequelae of chronic GVHD are of particular concern in this patient group.
We retrospectively compared the long-term outcome of contemporaneous unrelated donor SCT in 220 children transplanted with BM (n = 102) or PBSCs (n = 118) for hematologic malignancies and reported to the German/Austrian pediatric registry for SCT. All patients had received myeloablative conditioning followed by unmanipulated SCT from HLA-matched unrelated donors. The PBSC and BM groups were comparable with regard to patient and donor age, sex, cytomegalovirus (CMV) serostatus, disease status at transplantation, GVHD prophylaxis, growth factor use, and degree of HLA matching. The groups differed with regard to disease category with slightly more myelodysplastic syndrome patients (P = .02) and a higher CD34-cell dose (P = .001) in the PBSC group.
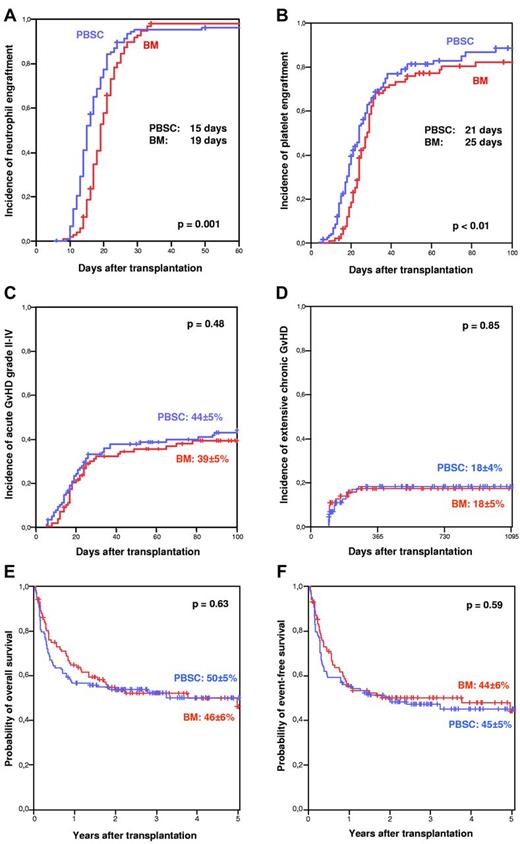
Neutrophil and platelet engraftment were achieved significantly faster after PBSC than BM transplantation (Figure 1A-B). In this entirely pediatric cohort, the incidence of clinically relevant grade II-IV acute GVHD (Figure 1C) did not differ. Most importantly, the incidence of chronic GVHD (PBSCs vs BM: 35% vs 33%, respectively; P = .9) and extensive chronic GVHD (Figure 1D) proved low and was virtually identical in the 2 groups. With a median follow-up time of 3 years, overall survival (PBSCs vs BM: 50% ± 5% vs 46% ± 6%, respectively; P = .63) and event-free survival (PBSCs vs BM: 45% ± 5% vs 44% ± 6%, respectively; P = .59) were comparable (Figure 1E-F). In multivariable analysis, taking into account all parameters with P < .2 in univariate analysis, the only significant independent risk factor for treatment failure was advanced disease status at the time of transplantation (relative risk = 2.4, 95% confidence interval, 1.5-3.8; P = .001). In contrast, stem cell source (PBSCs vs BM) had no effect (relative risk = 1.1, 95% confidence interval, 0.7-1.6; P = .8).
Comparative outcome after PBSC versus BM transplantation in children. Panels A through D depict cumulative incidences of neutrophil engraftment (neutrophils > 500/μL), platelet engraftment (platelets > 20.000/μL), acute GVHD grade II-IV, and extensive chronic GVHD after PBSC versus BM transplantation. Panels E and F show overall survival and event-free survival after PBSC versus BM transplantation in a Kaplan-Meier plot.
Comparative outcome after PBSC versus BM transplantation in children. Panels A through D depict cumulative incidences of neutrophil engraftment (neutrophils > 500/μL), platelet engraftment (platelets > 20.000/μL), acute GVHD grade II-IV, and extensive chronic GVHD after PBSC versus BM transplantation. Panels E and F show overall survival and event-free survival after PBSC versus BM transplantation in a Kaplan-Meier plot.
Our registry-based analysis provides evidence that in pediatric recipients of HLA-matched unrelated-donor transplantation with consistent antithymocyte globulin (ATG) use during conditioning, transplantation with PBSCs and BM results in comparable clinical outcomes without detectable differences in the risk of acute or, more importantly, chronic GVHD. Consistent with a recent study underscoring the role of ATG for the prevention of acute and chronic GVHD,5 the use of ATG in 96% of our transplantation procedures compared with only 27% in the above-mentioned randomized study by Anasetti et al4 might be one of the key factors responsible for the overall low and comparable incidence of chronic GVHD in pediatric PBSC and BM recipients. Therefore, we consider the results of our registry study to be highly relevant for pediatric transplantation physicians. The ongoing controversy on the preferential stem cell source needs to recognize differences in patient characteristics, targeted disease, and GVHD prophylaxis as well as the volunteer donor's preference with regard to stem cell source, which may be disregarded when one stem cell source is considered to be superior.
Authorship
The online version of this letter contains a data supplement.
Acknowledgments: Because this study is a retrospective analysis of anonymized registry data that were collected with informed consent from thepatients and/or their legal guardians, there was no requirement for formal institutional review board approval according to institutional guidelines.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a list of contributing transplantation centers and physicians, see the supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online letter.
Correspondence: Roland Meisel, MD, Clinic for Pediatric Oncology, Hematology and Clinical Immunology, Center for Child & Adolescent Health, Heinrich-Heine-University, Moorenstrasse 5, 40225 Düsseldorf, Germany; e-mail: meisel@med.uni-duesseldorf.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal