To the editor:
Horse antithymocyte globulin (hATG) and cyclosporine have been used as standard therapy for children with acquired aplastic anemia (AA) for whom an HLA-matched family donor is unavailable. However, in 2009, hATG (lymphoglobulin; Genzyme) was withdrawn and replaced by rabbit ATG (rATG; thymoglobulin; Genzyme) in Japan. Many other countries in Europe and Asia are facing the same situation.1 Marsh et al recently reported outcomes for 35 adult patients with AA who were treated with rATG and cyclosporine as a first-line therapy.2 Although the hematologic response rate was 40% at 6 months, several patients subsequently achieved late responses. The best response rate was 60% compared with 67% in a matched-pair control group of 105 patients treated with hATG. The overall and transplantation-free survival rates appeared to be significantly inferior with rATG compared with hATG at 68% versus 86% (P = .009) and 52% versus 76% (P = .002), respectively. These results are comparable to those from a prospective randomized study reported by Scheinberg et al comparing hATG and rATG.3 Both studies showed the superiority of hATG over rATG.2,3
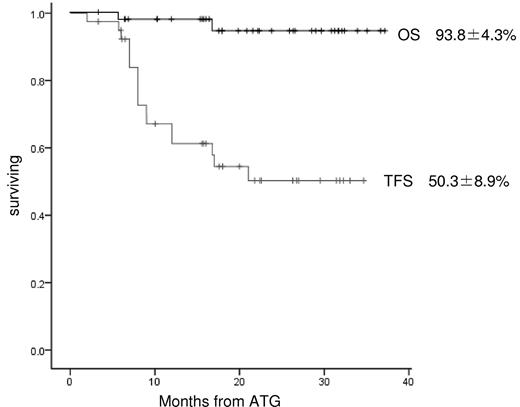
We recently analyzed outcomes for 40 Japanese children (median age, 9 years; range, 1-15) with AA treated using rATG and cyclosporine. The median interval from diagnosis to treatment was 22 days (range, 1-203). The numbers of patients with very severe, severe, and nonsevere disease were 14, 10, and 16, respectively. The ATG dose was 3.5 mg/kg/day for 5 days. The median follow-up time for all patients was 22 months (range, 6-38). At 3 months, no patients had achieved a complete response (CR) and partial response (PR) was seen in only 8 patients (20.0%). At 6 months, the numbers of patients with CR and PR were 2 (5.0%) and 17 (42.5%), respectively. After 6 months, 5 patients with PR at 6 months had achieved CR and 4 patients with no response at 6 months had achieved PR, offering a total best response rate of 57.5%. Two patients relapsed at 16 and 19 months without receiving any second-line treatments. Two patients with no response received a second course of rATG at 13 and 17 months, but neither responded. Sixteen patients underwent hematopoietic stem cell transplantation (HSCT) from alternative donors (HLA-matched unrelated donors, n = 13; HLA-mismatched family donors, n = 3). Two deaths occurred after rATG therapy, but no patients died after HSCT. Causes of death were intracranial hemorrhage at 6 months and acute respiratory distress syndrome at 17 months. The overall 2-year survival rate was 93.8% and the 2-year transplantation-free survival rate was 50.3% (Figure 1).
Kaplan-Meier estimates of overall survival (OS) and transplantation-free survival (TFS) in 40 Japanese children with AA. Survival was investigated using Kaplan-Meier methods. OS for all patients with AA after rATG and cyclosporine as first-line therapy included patients who later received HSCT for nonresponse to rATG. In the analysis of TFS for all patients treated with rATG and CSA, transplantation was considered an event.
Kaplan-Meier estimates of overall survival (OS) and transplantation-free survival (TFS) in 40 Japanese children with AA. Survival was investigated using Kaplan-Meier methods. OS for all patients with AA after rATG and cyclosporine as first-line therapy included patients who later received HSCT for nonresponse to rATG. In the analysis of TFS for all patients treated with rATG and CSA, transplantation was considered an event.
In our previous prospective studies with hATG, the response rates after 6 months were 68% and 70%, respectively, with no increases in response rates observed after 6 months.4,5 Our results support the notion that rATG is inferior to hATG for the treatment of AA in children. First-line HSCT from an alternative donor may be justified, considering the excellent outcomes in children who received salvage therapies using alternative donor HSCT.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Seiji Kojima, Nagoya Graduate School of Medicine, Tsurumai-cho 65, Showa-ku, Nagoya, Ai, Japan 466-8550; e-mail: kojimas@med.nagoya-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal