Abstract
Patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) have a poor prognosis with conventional chemotherapy. In the present study, we retrospectively analyzed the outcome of patients with BPDCN who underwent allogeneic stem cell transplantation (allo-SCT) or autologous stem cell transplantation (auto-SCT). A total of 39 patients (allo-SCT, n = 34; auto-SCT, n = 5) were identified in the European Group for Blood and Marrow Transplantation registry. The 34 allo-SCT patients had a median age of 41 years (range, 10-70) and received transplantations from sibling (n = 11) or unrelated donors (n = 23) between 2003 and 2009. MAC was used in 74% of patients. Nineteen allo-SCT patients (56%) received transplantations in first complete remission. The 3-year cumulative incidence of relapse, disease-free survival, and overall survival was 32%, 33%, and 41%, respectively. By univariate comparison, being in first remission at allo-SCT favorably influenced survival, whereas age, donor source, and chronic GVHD had no significant impact. We conclude that high-dose therapy followed by allo-SCT from related or unrelated donors can provide durable remission even in elderly patients with BPDCN. However, it remains to be shown if graft-versus-malignancy effects can contribute significantly to BPDCN control after allo-SCT.
Key Points
BPDCN is a rare hematopoietic malignancy characterized by a poor prognosis and unusual resistance to conventional chemotherapy.
This study indicates that high-dose therapy followed by allo-SCT can provide durable disease control in this otherwise inevitably fatal condition.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN), formerly known as blastic natural killer cell lymphoma or CD4+/CD56+ hematodermic neoplasm, is a rare hematopoietic malignancy derived from the precursors of plasmacytoid dendritic cells.1-6 The term BPDCN has been adopted by the 2008 World Health Organization classification after the description of its immunophenotypic, molecular, and functional characteristics such as CD123 and TCL1 expression and production of type 1 IFN, which suggested its pathogenetic relation to the plasmacytoid dendritic cell lineage.
This rare entity is a clinically aggressive tumor preferentially involving the skin, BM, and lymph nodes1,5 that mainly affects elderly patients with the maximum incidence peaking at approximately 65 years of age. The overall prognosis for BPDCN is remarkably poor. Most patients show an initial response to acute leukemia–like chemotherapy, but relapses with subsequent drug resistance occur in virtually all patients, resulting in a median overall survival (OS) of only 12-14 months.7-9 Preliminary results from molecular studies suggest that abnormalities of genes known to confer a poor prognosis in other hematopoietic malignancies, such as CDKN2A/CDKN2B, TET2, and TP53, are frequently seen in BPDCN.10,11
Because of its rarity, no standardized therapeutic approach has yet been established for BPDCN, but some case reviews have nevertheless reported anecdotal long-term remissions in young patients who underwent early allogeneic stem cell transplantation (allo-SCT) or autologous SCT (auto-SCT).7,9 Conclusions that guide clinical decisions are difficult to draw from these preliminary reports because patient numbers were small and important transplantation details such as conditioning regimens and donor sources were not provided. Recently, Dietrich et al reported a small series of 4 patients all older than 55 years who had undergone moderately reduced allo-SCT from unrelated mismatched donors.12 Two of these patients were in long-term remission 16 and 57 months after transplantation. This study provided the first evidence that allo-SCT might also induce long-term disease control in elderly patients.
Information on the feasibility and outcome of SCT in this otherwise incurable disease is very limited. We therefore conducted a retrospective analysis of BPDCN patients identified in the European Group for Blood and Marrow Transplantation (EBMT) registry. Using comprehensive diagnostic criteria, we evaluated the outcome of allo-SCT and auto-SCT to determine whether long-term disease-free survival (DFS) can be achieved in this otherwise rapidly fatal disease.
Methods
Data source
The EBMT is a voluntary organization comprising more than 600 transplantation centers mainly in Europe. Accreditation as a member center requires the submission of minimal essential data (ie, the MED-A form) from all consecutive patients to a central registry in which patients may be identified by the diagnosis of underlying disease and type of transplantation. MED-A data are collected by paper forms or an electronic data management system and are updated annually. Since 1996, accredited EBMT centers are subject to onsite audits to assess data accuracy and consecutive reporting.
Informed consent for transplantation and data collection was obtained locally according to regulations applicable at the time of transplantation. Since January 1, 2003, all transplantation centers have been required to obtain written informed consent before data registration with the EBMT following the Helsinki Declaration of 1975.
Patient eligibility
All patients with a diagnosis of “blastic plasmacytoid dendritic cell neoplasm,” “blastic natural killer cell lymphoma,” “CD4+/CD56+ hematodermic neoplasm,” or “dendritic cell leukemia” who received auto-SCT or allo-SCT between January 2000 and March 2009 were identified in the EBMT database. Baseline information and transplantation characteristics of the selected patients were downloaded. Centers were then contacted to provide written histopathology and flow cytometry reports to confirm the diagnosis of BPDCN. In addition, a questionnaire was sent out to collect additional data on clinical presentation, prior therapies, and update of follow-up. Data from 4 patients have already been published.12
The diagnosis of BPDCN was then confirmed by central review of pathology and flow cytometry reports using the diagnostic criteria proposed by Garnache-Ottou et al,13 which are summarized in Table 1. Only patients with a diagnostic score ≥ 2 were included. The immunophenotypic profiles and calculated scores of all individual patients are summarized in Table 2.
Diagnostic criteria for BPDCN as proposed by Garnache-Ottou et al13
| . | Present . | Absent . |
|---|---|---|
| Profile: CD4+, CD56+/−, CD11−, MPO−, cCD79a−, cCD3− | 1 point | BPDCN excluded |
| CD123 | 1 point | 0 point |
| BDCA-2 | 2 points | 0 point |
| BDCA-4 | 1 point | 0 point |
| . | Present . | Absent . |
|---|---|---|
| Profile: CD4+, CD56+/−, CD11−, MPO−, cCD79a−, cCD3− | 1 point | BPDCN excluded |
| CD123 | 1 point | 0 point |
| BDCA-2 | 2 points | 0 point |
| BDCA-4 | 1 point | 0 point |
Immunophenotypic profiles of patients included or not in the analysis and calculated scores as proposed by Garnache-Ottou et al13
| Patient no. . | BPDCN-associated markers . | Lineage markers . | Others . | Garnache-Ottou score13 . | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 . | CD56 . | CD123 . | TCL1 . | BDCA2 . | BDCA4 . | MPO . | CD14 . | CD11c . | CD3 . | CD79a . | CD19 . | CD20 . | CD15 . | CD10 . | CD16 . | CD57 . | TIA1 . | Tdt . | CD34 . | CD117 . | CD33 . | CD2 . | CD7 . | HLADR . | CD68 . | ||
| 1 | + | + | + | NA | NA | NA | − | − | NA | − | − | − | − | NA | NA | NA | NA | NA | − | − | − | − | − | + | + | NA | 2 |
| 2 | + | + | + | NA | NA | NA | − | NA | − | − | − | NA | − | NA | − | NA | − | − | − | − | NA | NA | − | − | + | + | 2 |
| 3 | + | + | + | NA | NA | NA | − | NA | NA | − | − | NA | − | − | − | NA | NA | NA | NA | − | NA | NA | − | − | + | − | 2 |
| 4 | + | + | + | + | NA | NA | − | − | − | − | − | − | NA | − | NA | − | − | NA | − | − | NA | + | NA | NA | + | NA | 2 |
| 5 | + | + | + | NA | NA | NA | − | − | NA | − | NA | − | − | NA | NA | NA | NA | NA | − | NA | + | + | − | + | + | NA | 2 |
| 6 | + | + | + | + | NA | NA | − | − | − | − | − | − | NA | − | NA | − | − | NA | − | − | NA | + | NA | NA | + | NA | 2 |
| 7 | + | + | + | NA | NA | + | − | − | NA | − | NA | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | NA | + | + | NA | 3 |
| 8 | + | + | NA | + | + | NA | − | − | NA | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | + | − | 3 |
| 9 | + | + | + | NA | NA | NA | − | − | NA | − | − | − | − | − | − | NA | NA | NA | + | − | − | − | − | − | + | NA | 2 |
| 10 | + | + | + | NA | NA | NA | − | NA | − | − | − | NA | − | NA | − | NA | NA | NA | − | NA | NA | − | + | NA | + | NA | 2 |
| 11 | + | + | + | NA | NA | NA | − | NA | − | − | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | − | NA | + | NA | 2 |
| 12 | + | + | + | NA | NA | NA | − | − | − | − | − | − | − | − | − | NA | − | NA | NA | − | − | + | − | + | + | NA | 2 |
| 13 | + | + | + | + | NA | NA | − | − | NA | − | − | − | − | NA | NA | NA | NA | NA | NA | − | NA | + | + | NA | NA | NA | 2 |
| 14 | + | + | + | + | + | NA | − | − | NA | − | − | NA | − | NA | NA | − | − | NA | − | − | NA | − | NA | NA | + | NA | 4 |
| 15 | + | + | + | + | + | NA | − | − | NA | − | − | NA | − | NA | NA | − | − | NA | − | − | NA | − | NA | NA | + | NA | 4 |
| 16 | + | + | + | NA | + | + | − | − | NA | − | − | − | NA | − | − | NA | NA | NA | − | − | + | + | NA | − | NA | NA | 5 |
| 17 | + | + | + | + | + | NA | − | − | NA | − | − | − | − | NA | − | NA | NA | NA | + | − | − | − | + | + | + | + | 4 |
| 18 | + | + | + | NA | NA | NA | − | NA | − | − | NA | − | − | − | NA | NA | NA | NA | − | NA | − | NA | + | − | + | NA | 2 |
| 19 | + | + | + | NA | NA | NA | − | NA | − | − | − | NA | − | NA | − | NA | NA | + | NA | − | NA | NA | NA | NA | NA | + | 2 |
| 20 | + | + | + | NA | NA | NA | − | − | NA | − | NA | NA | − | − | − | NA | NA | NA | − | − | − | − | NA | + | NA | NA | 2 |
| 21 | + | + | + | + | NA | NA | − | − | NA | − | − | NA | NA | − | NA | NA | NA | NA | − | − | − | − | − | NA | + | NA | 2 |
| 22 | + | + | + | NA | NA | NA | − | NA | − | − | − | − | − | NA | − | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | + | 2 |
| 23 | + | + | + | + | NA | NA | − | NA | NA | − | NA | − | NA | NA | − | NA | + | NA | − | NA | NA | NA | NA | NA | NA | NA | 2 |
| 24 | + | + | + | NA | + | + | − | − | NA | − | − | − | − | − | − | − | NA | NA | − | − | − | − | + | − | + | − | 5 |
| 25 | + | + | + | + | NA | NA | − | NA | − | − | NA | − | − | NA | NA | NA | NA | NA | − | − | − | − | − | − | + | + | 2 |
| 26 | + | + | NA | NA | + | NA | − | − | NA | − | − | − | NA | − | − | NA | NA | − | + | − | NA | − | + | − | + | NA | 3 |
| 27 | + | + | + | NA | + | + | − | − | NA | − | − | − | − | − | − | − | NA | NA | + | − | + | + | − | − | + | + | 5 |
| 28 | + | + | + | NA | NA | NA | − | NA | NA | − | − | NA | NA | NA | − | − | − | NA | NA | NA | NA | NA | + | + | + | NA | 2 |
| 29 | + | + | NA | NA | NA | + | − | NA | NA | − | − | NA | − | NA | NA | NA | NA | NA | + | − | NA | − | − | + | + | NA | 2 |
| 30 | + | + | + | NA | NA | NA | − | − | NA | − | NA | − | NA | − | − | NA | NA | NA | NA | NA | NA | − | NA | NA | + | NA | 2 |
| 31 | + | + | + | NA | NA | NA | − | − | NA | − | − | − | − | − | NA | NA | NA | NA | − | − | − | + | + | + | + | + | 2 |
| 32 | + | + | + | NA | NA | NA | − | NA | NA | − | − | NA | − | NA | − | NA | NA | − | − | − | NA | NA | NA | NA | NA | − | 2 |
| 33 | + | + | + | + | NA | NA | − | NA | − | − | − | NA | NA | NA | − | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | 2 |
| 34 | + | + | NA | NA | + | NA | − | NA | − | − | − | NA | − | NA | NA | NA | NA | NA | NA | − | NA | NA | − | NA | NA | NA | 3 |
| 35 | + | − | NA | NA | NA | NA | NA | NA | NA | + | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Excluded |
| 36 | − | + | NA | NA | NA | NA | − | − | − | − | NA | − | NA | − | − | NA | NA | NA | NA | − | − | − | + | − | + | NA | Excluded |
| 37 | + | + | NA | NA | NA | NA | − | − | NA | + | − | − | + | NA | NA | NA | NA | NA | − | NA | − | NA | + | + | + | NA | Excluded |
| 38 | NA | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | − | + | − | NA | + | − | NA | Excluded |
| 39 | NA | − | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | NA | NA | NA | + | NA | Excluded |
| 40 | + | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | − | − | NA | NA | NA | NA | NA | + | Excluded |
| 41 | + | + | NA | NA | NA | NA | NA | NA | NA | − | NA | − | NA | NA | − | − | NA | NA | − | + | − | NA | + | − | + | NA | Excluded |
| 42 | + | + | NA | NA | NA | NA | NA | − | NA | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | − | NA | + | NA | Excluded |
| 43 | NA | NA | NA | NA | NA | NA | − | − | NA | − | − | − | NA | − | − | NA | NA | NA | − | + | − | + | − | + | + | NA | Excluded |
| 44 | + | + | NA | NA | NA | NA | NA | NA | NA | − | NA | − | − | NA | − | NA | NA | NA | NA | NA | NA | NA | − | NA | NA | NA | Excluded |
| 45 | + | + | NA | NA | NA | NA | NA | NA | NA | − | − | NA | NA | NA | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | + | NA | Excluded |
| 46 | + | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | − | NA | NA | − | NA | NA | − | Excluded |
| 47 | − | + | NA | NA | NA | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Excluded |
| 48 | + | + | NA | NA | NA | NA | − | − | NA | − | NA | − | − | − | − | − | − | NA | NA | − | + | NA | − | − | NA | NA | Excluded |
| 49 | + | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | Excluded |
| 50 | + | + | NA | NA | NA | NA | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | + | − | + | + | Excluded |
| 51 | + | + | NA | NA | NA | NA | − | NA | NA | − | NA | NA | NA | NA | NA | − | NA | NA | NA | − | + | NA | NA | + | + | NA | Excluded |
| 52 | NA | NA | NA | NA | NA | NA | − | − | NA | − | − | − | − | − | − | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | Excluded |
| 53 | + | + | NA | NA | NA | NA | NA | NA | NA | − | − | NA | − | NA | NA | NA | − | − | NA | − | NA | NA | + | − | + | NA | Excluded |
| Patient no. . | BPDCN-associated markers . | Lineage markers . | Others . | Garnache-Ottou score13 . | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 . | CD56 . | CD123 . | TCL1 . | BDCA2 . | BDCA4 . | MPO . | CD14 . | CD11c . | CD3 . | CD79a . | CD19 . | CD20 . | CD15 . | CD10 . | CD16 . | CD57 . | TIA1 . | Tdt . | CD34 . | CD117 . | CD33 . | CD2 . | CD7 . | HLADR . | CD68 . | ||
| 1 | + | + | + | NA | NA | NA | − | − | NA | − | − | − | − | NA | NA | NA | NA | NA | − | − | − | − | − | + | + | NA | 2 |
| 2 | + | + | + | NA | NA | NA | − | NA | − | − | − | NA | − | NA | − | NA | − | − | − | − | NA | NA | − | − | + | + | 2 |
| 3 | + | + | + | NA | NA | NA | − | NA | NA | − | − | NA | − | − | − | NA | NA | NA | NA | − | NA | NA | − | − | + | − | 2 |
| 4 | + | + | + | + | NA | NA | − | − | − | − | − | − | NA | − | NA | − | − | NA | − | − | NA | + | NA | NA | + | NA | 2 |
| 5 | + | + | + | NA | NA | NA | − | − | NA | − | NA | − | − | NA | NA | NA | NA | NA | − | NA | + | + | − | + | + | NA | 2 |
| 6 | + | + | + | + | NA | NA | − | − | − | − | − | − | NA | − | NA | − | − | NA | − | − | NA | + | NA | NA | + | NA | 2 |
| 7 | + | + | + | NA | NA | + | − | − | NA | − | NA | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | NA | + | + | NA | 3 |
| 8 | + | + | NA | + | + | NA | − | − | NA | − | NA | − | − | − | − | − | − | NA | − | − | − | − | − | − | + | − | 3 |
| 9 | + | + | + | NA | NA | NA | − | − | NA | − | − | − | − | − | − | NA | NA | NA | + | − | − | − | − | − | + | NA | 2 |
| 10 | + | + | + | NA | NA | NA | − | NA | − | − | − | NA | − | NA | − | NA | NA | NA | − | NA | NA | − | + | NA | + | NA | 2 |
| 11 | + | + | + | NA | NA | NA | − | NA | − | − | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | − | NA | + | NA | 2 |
| 12 | + | + | + | NA | NA | NA | − | − | − | − | − | − | − | − | − | NA | − | NA | NA | − | − | + | − | + | + | NA | 2 |
| 13 | + | + | + | + | NA | NA | − | − | NA | − | − | − | − | NA | NA | NA | NA | NA | NA | − | NA | + | + | NA | NA | NA | 2 |
| 14 | + | + | + | + | + | NA | − | − | NA | − | − | NA | − | NA | NA | − | − | NA | − | − | NA | − | NA | NA | + | NA | 4 |
| 15 | + | + | + | + | + | NA | − | − | NA | − | − | NA | − | NA | NA | − | − | NA | − | − | NA | − | NA | NA | + | NA | 4 |
| 16 | + | + | + | NA | + | + | − | − | NA | − | − | − | NA | − | − | NA | NA | NA | − | − | + | + | NA | − | NA | NA | 5 |
| 17 | + | + | + | + | + | NA | − | − | NA | − | − | − | − | NA | − | NA | NA | NA | + | − | − | − | + | + | + | + | 4 |
| 18 | + | + | + | NA | NA | NA | − | NA | − | − | NA | − | − | − | NA | NA | NA | NA | − | NA | − | NA | + | − | + | NA | 2 |
| 19 | + | + | + | NA | NA | NA | − | NA | − | − | − | NA | − | NA | − | NA | NA | + | NA | − | NA | NA | NA | NA | NA | + | 2 |
| 20 | + | + | + | NA | NA | NA | − | − | NA | − | NA | NA | − | − | − | NA | NA | NA | − | − | − | − | NA | + | NA | NA | 2 |
| 21 | + | + | + | + | NA | NA | − | − | NA | − | − | NA | NA | − | NA | NA | NA | NA | − | − | − | − | − | NA | + | NA | 2 |
| 22 | + | + | + | NA | NA | NA | − | NA | − | − | − | − | − | NA | − | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | + | 2 |
| 23 | + | + | + | + | NA | NA | − | NA | NA | − | NA | − | NA | NA | − | NA | + | NA | − | NA | NA | NA | NA | NA | NA | NA | 2 |
| 24 | + | + | + | NA | + | + | − | − | NA | − | − | − | − | − | − | − | NA | NA | − | − | − | − | + | − | + | − | 5 |
| 25 | + | + | + | + | NA | NA | − | NA | − | − | NA | − | − | NA | NA | NA | NA | NA | − | − | − | − | − | − | + | + | 2 |
| 26 | + | + | NA | NA | + | NA | − | − | NA | − | − | − | NA | − | − | NA | NA | − | + | − | NA | − | + | − | + | NA | 3 |
| 27 | + | + | + | NA | + | + | − | − | NA | − | − | − | − | − | − | − | NA | NA | + | − | + | + | − | − | + | + | 5 |
| 28 | + | + | + | NA | NA | NA | − | NA | NA | − | − | NA | NA | NA | − | − | − | NA | NA | NA | NA | NA | + | + | + | NA | 2 |
| 29 | + | + | NA | NA | NA | + | − | NA | NA | − | − | NA | − | NA | NA | NA | NA | NA | + | − | NA | − | − | + | + | NA | 2 |
| 30 | + | + | + | NA | NA | NA | − | − | NA | − | NA | − | NA | − | − | NA | NA | NA | NA | NA | NA | − | NA | NA | + | NA | 2 |
| 31 | + | + | + | NA | NA | NA | − | − | NA | − | − | − | − | − | NA | NA | NA | NA | − | − | − | + | + | + | + | + | 2 |
| 32 | + | + | + | NA | NA | NA | − | NA | NA | − | − | NA | − | NA | − | NA | NA | − | − | − | NA | NA | NA | NA | NA | − | 2 |
| 33 | + | + | + | + | NA | NA | − | NA | − | − | − | NA | NA | NA | − | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | 2 |
| 34 | + | + | NA | NA | + | NA | − | NA | − | − | − | NA | − | NA | NA | NA | NA | NA | NA | − | NA | NA | − | NA | NA | NA | 3 |
| 35 | + | − | NA | NA | NA | NA | NA | NA | NA | + | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Excluded |
| 36 | − | + | NA | NA | NA | NA | − | − | − | − | NA | − | NA | − | − | NA | NA | NA | NA | − | − | − | + | − | + | NA | Excluded |
| 37 | + | + | NA | NA | NA | NA | − | − | NA | + | − | − | + | NA | NA | NA | NA | NA | − | NA | − | NA | + | + | + | NA | Excluded |
| 38 | NA | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | − | + | − | NA | + | − | NA | Excluded |
| 39 | NA | − | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | NA | NA | NA | + | NA | Excluded |
| 40 | + | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | − | − | NA | NA | NA | NA | NA | + | Excluded |
| 41 | + | + | NA | NA | NA | NA | NA | NA | NA | − | NA | − | NA | NA | − | − | NA | NA | − | + | − | NA | + | − | + | NA | Excluded |
| 42 | + | + | NA | NA | NA | NA | NA | − | NA | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | − | NA | + | NA | Excluded |
| 43 | NA | NA | NA | NA | NA | NA | − | − | NA | − | − | − | NA | − | − | NA | NA | NA | − | + | − | + | − | + | + | NA | Excluded |
| 44 | + | + | NA | NA | NA | NA | NA | NA | NA | − | NA | − | − | NA | − | NA | NA | NA | NA | NA | NA | NA | − | NA | NA | NA | Excluded |
| 45 | + | + | NA | NA | NA | NA | NA | NA | NA | − | − | NA | NA | NA | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | + | NA | Excluded |
| 46 | + | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | − | NA | NA | − | NA | NA | − | Excluded |
| 47 | − | + | NA | NA | NA | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Excluded |
| 48 | + | + | NA | NA | NA | NA | − | − | NA | − | NA | − | − | − | − | − | − | NA | NA | − | + | NA | − | − | NA | NA | Excluded |
| 49 | + | + | NA | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | Excluded |
| 50 | + | + | NA | NA | NA | NA | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | + | − | + | + | Excluded |
| 51 | + | + | NA | NA | NA | NA | − | NA | NA | − | NA | NA | NA | NA | NA | − | NA | NA | NA | − | + | NA | NA | + | + | NA | Excluded |
| 52 | NA | NA | NA | NA | NA | NA | − | − | NA | − | − | − | − | − | − | NA | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | Excluded |
| 53 | + | + | NA | NA | NA | NA | NA | NA | NA | − | − | NA | − | NA | NA | NA | − | − | NA | − | NA | NA | + | − | + | NA | Excluded |
NA indicates not available.
Definitions
Regimens intended to be myeloablative conditioning (MAC) were defined as those that included ≥ 8 Gy of total-body irradiation (TBI), ≥ 10 mg/kg of busulfan, or ≥ 150 mg/m2 of melphalan, as described previously.14,15 All other regimens were considered to be reduced-intensity conditioning (RIC). GVHD was graded according to consensus criteria. Nonrelapse mortality (NRM) was defined as death not related to progression of BPDCN.
Statistical analyses
Probabilities of DFS and OS were calculated using the Kaplan-Meier estimate. Survival curves were compared using the log-rank test. Cumulative incidence curves were used for incidence of relapse and NRM and compared using the Gray test as competing events.16,17 Univariate comparison and estimation of hazard ratios used Cox regression model for OS and DFS and Fine and Gray competing risk regression model for incidence of relapse and NRM.16,17 To study its impact on outcome, chronic GVHD was analyzed as a time-dependent covariate. All P values were 2-sided and significance was set at the level of 5%. Approximate 95% confidence intervals (CIs) are provided for survival probabilities or cumulative incidences.
Results
Patients and transplantation characteristics
A total of 139 patients fulfilling the inclusion criteria were identified in the EBMT registry (allo-SCT, n = 100; auto-SCT, n = 39). Of these, the requested additional data could be retrieved for 74 patients (allo-SCT, n = 53; auto-SCT, n = 19). After central review of immunophenotype and written histology reports, the diagnosis of BPDCN was finally confirmed in 39 patients (allo-SCT, n = 34; auto-SCT, n = 5; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). There were no significant differences between the 19 excluded and the 34 included patients in terms of age, sex, remission status at SCT, time from diagnosis to SCT, and use of RIC, but the excluded patients had been allografted in a significantly earlier time period and had skin infiltration at diagnosis less frequently than the included patients (supplemental Table 1 and supplemental Figure 1).
Because of the low number of evaluable patients remaining in the auto-SCT group, the present analysis was restricted to the allo-SCT cohort.
Patient and transplantation characteristics for the 34 allografted patients are summarized in Table 3. The median age was 41 years (range, 10-70). The majority of patients had received an acute-leukemia-type first-line treatment. Because more than half of the patients received transplantations in first complete remission (CR1), the median time from diagnosis to allo-SCT was short at 6 months (range, 3-63). Two-thirds of the patients were grafted from alternative donors. A MAC regimen was used in the majority of patients (n = 25, 74%). MAC regimens consisted of combinations of TBI and cyclophosphamide (n = 17), or busulfan and cyclophosphamide (n = 6). The most frequently used RIC regimens (26%, n = 9) were busulfan/fludarabine–based regimens (n = 5). All MAC and RIC regimens used are listed in Table 4.
Main characteristics of allo-SCT patients
| Characteristic . | Whole population (N = 34) . | MAC (n =25) . | RIC (n = 9) . | P* . |
|---|---|---|---|---|
| Age at allo-SCT, y | ||||
| Median | 41 | 36 | 62 | .005 |
| Range | 10-70 | 10-64 | 15-70 | |
| Sex, n (%) | ||||
| Female | 13 (38) | 12 (48) | 1 (11) | .10 |
| Male | 21 (62) | 13 (52) | 8 (89) | |
| Clinical presentation at diagnosis, n (%) | ||||
| Skin involvement | 26 (79) | 21 (84) | 5 (56) | .81 |
| Lymph nodes | 13 (38) | 9 (36) | 4 (44) | |
| Blood involvement | 18 (53) | 15 (60) | 3 (33) | |
| BM infiltration | 29 (85) | 23 (92) | 6 (67) | |
| No. of prior therapies, range | 1-4 | 1-4 | 1-4 | .50 |
| Type of first-line treatment, n (%) | ||||
| AML or ALL-type | 27 (82) | 21 (84) | 6 (67) | .35 |
| NHL-type | 7 (8) | 4 (16) | 3 (33) | |
| Time from diagnosis to allo-SCT, mo | ||||
| Median | 6 | 6 | 8 | .58 |
| Range | 3-63 | 3-63 | 5-16 | |
| Status at allo-SCT, n (%) | ||||
| CR1 | 19 (56) | 15 (60) | 4 (44) | .46 |
| > CR1 or refractory | 15 (44) | 10 (40) | 5 (66) | |
| Donor type, n (%) | ||||
| Related | 11 (32) | 7 (28) | 4 (44) | .43 |
| Unrelated | 23 (68) | 18 (72) | 5 (66) | |
| Stem cell source, n (%) | ||||
| BM | 19 (56) | 16 (64) | 3 (33) | .18 |
| PBSCs | 9 (26) | 6 (24) | 3 (33) | |
| Cord blood | 6 (18) | 3 (12) | 3 (33) | |
| T-cell depletion, n (%) | ||||
| No T-cell depletion | 23 (68) | 19 (76) | 4 (44) | .11 |
| Antithymocyte globulin | 9 (26) | 4 (16) | 5 (66) | |
| Alemtuzumab | 2 (6) | 2 (8) | 0 (0) | |
| Characteristic . | Whole population (N = 34) . | MAC (n =25) . | RIC (n = 9) . | P* . |
|---|---|---|---|---|
| Age at allo-SCT, y | ||||
| Median | 41 | 36 | 62 | .005 |
| Range | 10-70 | 10-64 | 15-70 | |
| Sex, n (%) | ||||
| Female | 13 (38) | 12 (48) | 1 (11) | .10 |
| Male | 21 (62) | 13 (52) | 8 (89) | |
| Clinical presentation at diagnosis, n (%) | ||||
| Skin involvement | 26 (79) | 21 (84) | 5 (56) | .81 |
| Lymph nodes | 13 (38) | 9 (36) | 4 (44) | |
| Blood involvement | 18 (53) | 15 (60) | 3 (33) | |
| BM infiltration | 29 (85) | 23 (92) | 6 (67) | |
| No. of prior therapies, range | 1-4 | 1-4 | 1-4 | .50 |
| Type of first-line treatment, n (%) | ||||
| AML or ALL-type | 27 (82) | 21 (84) | 6 (67) | .35 |
| NHL-type | 7 (8) | 4 (16) | 3 (33) | |
| Time from diagnosis to allo-SCT, mo | ||||
| Median | 6 | 6 | 8 | .58 |
| Range | 3-63 | 3-63 | 5-16 | |
| Status at allo-SCT, n (%) | ||||
| CR1 | 19 (56) | 15 (60) | 4 (44) | .46 |
| > CR1 or refractory | 15 (44) | 10 (40) | 5 (66) | |
| Donor type, n (%) | ||||
| Related | 11 (32) | 7 (28) | 4 (44) | .43 |
| Unrelated | 23 (68) | 18 (72) | 5 (66) | |
| Stem cell source, n (%) | ||||
| BM | 19 (56) | 16 (64) | 3 (33) | .18 |
| PBSCs | 9 (26) | 6 (24) | 3 (33) | |
| Cord blood | 6 (18) | 3 (12) | 3 (33) | |
| T-cell depletion, n (%) | ||||
| No T-cell depletion | 23 (68) | 19 (76) | 4 (44) | .11 |
| Antithymocyte globulin | 9 (26) | 4 (16) | 5 (66) | |
| Alemtuzumab | 2 (6) | 2 (8) | 0 (0) | |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; NHL, non-Hodgkin lymphoma; and PBSCs, peripheral blood stem cells.
The t test was used for continuous variables; the Fisher test for categorical variables.
Allo-SCT regimens
| . | No. (no. of TCDs) . | % . |
|---|---|---|
| MAC regimens* | 25 (6) | 74 |
| TBI ≥ 10 Gy + Cy | 17 (3) | 50 |
| Bu ≥ 10 mg/kg + Cy ± Mel ± Flu | 5 (3) | 15 |
| Bu ≥ 10 mg/kg + Flu ± thiotepa | 3 (0) | 9 |
| RIC regimens | 9 (5) | 26 |
| Bu ≤ 6 mg/kg + Flu ± thiotepa ± Cy | 5 (4) | 15 |
| TBI 2 Gy + Flu + Cy | 2 (0) | 6 |
| TBI 2 Gy + Flu + AraC | 1 (0) | 2,5 |
| Flu + treosulfan | 1 (1) | 2,5 |
| . | No. (no. of TCDs) . | % . |
|---|---|---|
| MAC regimens* | 25 (6) | 74 |
| TBI ≥ 10 Gy + Cy | 17 (3) | 50 |
| Bu ≥ 10 mg/kg + Cy ± Mel ± Flu | 5 (3) | 15 |
| Bu ≥ 10 mg/kg + Flu ± thiotepa | 3 (0) | 9 |
| RIC regimens | 9 (5) | 26 |
| Bu ≤ 6 mg/kg + Flu ± thiotepa ± Cy | 5 (4) | 15 |
| TBI 2 Gy + Flu + Cy | 2 (0) | 6 |
| TBI 2 Gy + Flu + AraC | 1 (0) | 2,5 |
| Flu + treosulfan | 1 (1) | 2,5 |
AraC indicates cytosine arabinoside; Bu, busulfan; Cy, cyclophosphamide; Flu, fludarabine; Mel, melphalan; TBI, total body irradiation; and TCDs, T-cell depletions.
Doses for the MAC regimens are provided in “Methods.”
Outcome
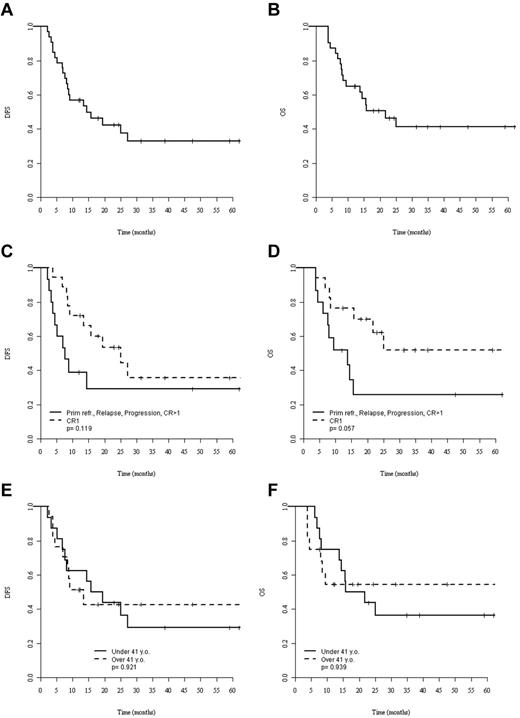
At the last follow-up, 16 patients (47%) were alive after allo-SCT with a median observation time of 28 months (range, 4-77). Eleven patients (32%) experienced relapse at a median of 8 months (range, 2-27) after allo-SCT, whereas no further relapse was observed after 27 months, translating into a cumulative incidence of relapse of 32% at 3 years. Eight of these 11 patients had died of disease progression at the most recent follow-up. Nine patients died without signs of disease recurrence at a median of 9 months (range, 4-25) after allo-SCT, corresponding to a 3-year NRM of 30% (95% CI, 13%-48%). For the whole cohort of allo-SCT patients, 3-year DFS and OS were 33% (95% CI, 15%-51%) and 41% (95% CI, 22%-60%), respectively (Figure 1A-B).
Survival after allo-SCT. (A-B) DFS (A) and OS (B) of the whole population. (C-D) DFS (C) and OS (D) for patients allografted in CR1 (dashed lines) or more advanced status (solid lines). (E-F) DFS (E) and OS (F) for patients younger (solid lines) and older (dashed lines) than 41 years of age. The median age of the younger group was 24 years (range, 9-40) and the median age of the older group was 57 years (range, 41-70). P values were calculated by log-rank test.
Survival after allo-SCT. (A-B) DFS (A) and OS (B) of the whole population. (C-D) DFS (C) and OS (D) for patients allografted in CR1 (dashed lines) or more advanced status (solid lines). (E-F) DFS (E) and OS (F) for patients younger (solid lines) and older (dashed lines) than 41 years of age. The median age of the younger group was 24 years (range, 9-40) and the median age of the older group was 57 years (range, 41-70). P values were calculated by log-rank test.
GVHD
After allo-SCT, grade 2-4 acute GVHD was observed in 17 patients (50%), but was life-threatening (grade 3-4) in only 4 of them (12%). Of 32 evaluable patients, chronic GVHD was observed in 13 patients (41%), of which 5 (16%) were graded as extensive.
Prognostic factor analyses
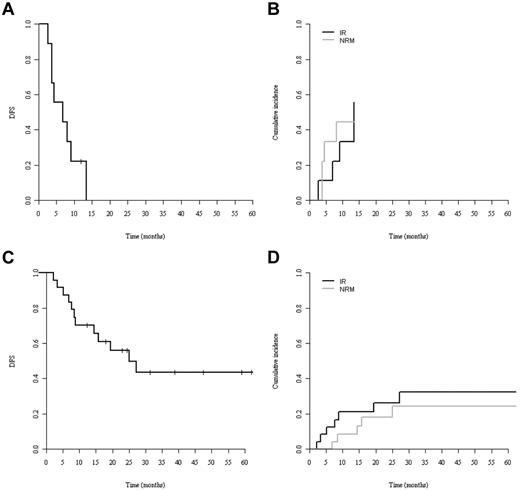
By univariate comparison, patients allografted in CR1 tended to show better 3-year DFS (36%; 95% CI, 1%-61%) and OS (52%; 95% CI, 24%-79%) compared with patients allografted in more advanced disease: DFS: 26% (95% CI, 1%-50%) and OS: 29% (95% CI, 4%-54%) without reaching statistical significance (P = .12 for DFS and P = .06 for OS; Figure 1C-D). Patients older and younger than 41 years of age had neither significantly different DFS nor OS (Figure 1E-F). Only a single patient survived disease free after RIC (Figure 2A). Although the poor outcome of RIC was largely because of a high NRM rate (Figure 2B), no RIC patient had prolonged disease control because all experienced early relapse or early death. In contrast, the DFS curve of the MAC group reached a plateau at 40% with 5 patients surviving disease free beyond 2 years after transplantation (Figure 2C).
Survival of MAC and RIC patients. (A) DFS. (B) NRM (gray line) and relapse (IR; black line) cumulative incidences of patients receiving RIC. (C) DFS. (D) NRM (gray line) and relapse (black line) cumulative incidences of patients receiving MAC.
Survival of MAC and RIC patients. (A) DFS. (B) NRM (gray line) and relapse (IR; black line) cumulative incidences of patients receiving RIC. (C) DFS. (D) NRM (gray line) and relapse (black line) cumulative incidences of patients receiving MAC.
If survival analysis was restricted to those patients who were allografted in CR1 with MAC (n = 19), the 3-year DFS and OS were 45% and 60%, respectively. Age, donor source, and chronic GVHD did not significantly affect outcomes. The main prognostic factors and their association with outcomes are listed in Table 5.
Univariate analysis for outcomes of patients with BPDCN
| Variable . | NRM . | Relapse . | DFS . | OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | RR . | 95% CI . | P . | RR . | 95% CI . | P . | |
| Age at allo-SCT, y | ||||||||||||
| < 41 | 1 | 0.27-3.23 | .90 | 1 | 0.27-2.67 | .77 | 1 | 0.39-2.33 | .92 | 1 | 0.37-2.55 | .95 |
| ≥ 41 | 0.93 | 0.84 | 0.96 | 0.97 | ||||||||
| Status at allo-SCT | ||||||||||||
| CR1 | 1 | 0.16-2.44 | .49 | 1 | 1.02-9.99 | .05 | 1 | 0.82-4.86 | .13 | 1 | 0.94-6.75 | .06 |
| > CR1 or refractory | 0.63 | 3.19 | 2 | 2.53 | ||||||||
| Donor type | ||||||||||||
| Related | 1 | 0.33-7.72 | .55 | 1 | 0.34-4.52 | .74 | 1 | 0.56-4.25 | .40 | 1 | 0.61-5.77 | .27 |
| Unrelated | 1.61 | 1.24 | 1.53 | 1.88 | ||||||||
| Conditioning regimens | ||||||||||||
| RIC | 1 | 0.09-1.16 | .08 | 1 | 0.17-1.85 | .35 | 1 | 0.07-0.58 | .003 | 1 | 0.07-0.63 | .005 |
| MAC | 0.32 | 0.57 | 0.21 | 0.22 | ||||||||
| Stem cell source | ||||||||||||
| BM | 1 | .70 | 1 | .80 | 1 | .93 | 1 | .89 | ||||
| PBSCs | 0.77 | 0.20-2.99 | 1.24 | 0.32-4.82 | 1.09 | 0.40-2.98 | 1.26 | 0.43-3.72 | ||||
| Cord blood | 1.33 | 0.24-7.42 | 0.46 | 0.05-4.06 | 0.86 | 0.21-3.44 | 0.99 | 0.19-5.13 | ||||
| Chronic GVHD | ||||||||||||
| Absence | 1 | .59 | 1 | .54 | 1 | .46 | 1 | .83 | ||||
| Presence | 1.49 | 0.35-6.43 | 1.61 | 0.35-7.41 | 1.55 | 0.54-4.44 | 1.12 | 0.40-3.16 | ||||
| Variable . | NRM . | Relapse . | DFS . | OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P . | RR . | 95% CI . | P . | RR . | 95% CI . | P . | RR . | 95% CI . | P . | |
| Age at allo-SCT, y | ||||||||||||
| < 41 | 1 | 0.27-3.23 | .90 | 1 | 0.27-2.67 | .77 | 1 | 0.39-2.33 | .92 | 1 | 0.37-2.55 | .95 |
| ≥ 41 | 0.93 | 0.84 | 0.96 | 0.97 | ||||||||
| Status at allo-SCT | ||||||||||||
| CR1 | 1 | 0.16-2.44 | .49 | 1 | 1.02-9.99 | .05 | 1 | 0.82-4.86 | .13 | 1 | 0.94-6.75 | .06 |
| > CR1 or refractory | 0.63 | 3.19 | 2 | 2.53 | ||||||||
| Donor type | ||||||||||||
| Related | 1 | 0.33-7.72 | .55 | 1 | 0.34-4.52 | .74 | 1 | 0.56-4.25 | .40 | 1 | 0.61-5.77 | .27 |
| Unrelated | 1.61 | 1.24 | 1.53 | 1.88 | ||||||||
| Conditioning regimens | ||||||||||||
| RIC | 1 | 0.09-1.16 | .08 | 1 | 0.17-1.85 | .35 | 1 | 0.07-0.58 | .003 | 1 | 0.07-0.63 | .005 |
| MAC | 0.32 | 0.57 | 0.21 | 0.22 | ||||||||
| Stem cell source | ||||||||||||
| BM | 1 | .70 | 1 | .80 | 1 | .93 | 1 | .89 | ||||
| PBSCs | 0.77 | 0.20-2.99 | 1.24 | 0.32-4.82 | 1.09 | 0.40-2.98 | 1.26 | 0.43-3.72 | ||||
| Cord blood | 1.33 | 0.24-7.42 | 0.46 | 0.05-4.06 | 0.86 | 0.21-3.44 | 0.99 | 0.19-5.13 | ||||
| Chronic GVHD | ||||||||||||
| Absence | 1 | .59 | 1 | .54 | 1 | .46 | 1 | .83 | ||||
| Presence | 1.49 | 0.35-6.43 | 1.61 | 0.35-7.41 | 1.55 | 0.54-4.44 | 1.12 | 0.40-3.16 | ||||
PBSCs indicates peripheral blood stem cells; and RR, relative risk.
Discussion
Because BPDCN is a rare disease that was recognized as a distinct entity only recently, information regarding the optimum therapeutic approach for this aggressive malignancy is limited. It has been suggested that consolidating SCT could be associated with long-term disease control but evidence for its potential feasibility and efficacy is sparse. Reimer et al7 and Dalle et al9 published a case series of BPDCN patients who underwent SCT with 10 patients each 4 auto-SCT and 6 allo-SCT; 1 auto-SCT and 9 allo-SCT, respectively). Long-term survivors were observed, but both studies were restricted to young populations with median ages of 28 and 22 years, respectively. Other shortcomings of these 2 case compilations were the limited number of patients studied and that information on essential transplantation details, such as donor source and conditioning regimens, was not provided.
Whereas the number of autografted patients was too small to draw meaningful conclusions, the present study is the largest so far to examine the role of allo-SCT in BPDCN. For the first time we were able to calculate survival probabilities on a reasonable patient sample including elderly patients and to correlate these with important patient and transplantation characteristics, such as age, remission status at transplantation, conditioning intensity, and donor source. Despite the limitations inherent to a retrospective study, our data indicate that allo-SCT is effective in BPDCN and might provide curative potential for a significant proportion of patients, especially when performed in CR1. This also applies for patients above the age of 40, who represent the majority of the population affected with the disease, and for those receiving an allograft from an unrelated donor.
In the present study, long-term disease control was only observed after MAC, suggesting that MAC allo-SCT is an effective treatment for BPDCN. In contrast, it is difficult to make such a conclusion from our results on RIC in BPDCN, because the number of patients was small, patients treated with RIC were significantly older, and information about comorbidity is not available. The excess number of events in the RIC group was because of NRM rather than relapse. Conversely, a significant contribution of chronic GVHD to disease control could not be demonstrated in our allo-SCT series. Therefore, it remains to be shown if the efficacy of allo-SCT can be attributed to an eventual graft-versus-leukemia effect or if is just a matter of high-dose intensity conferred with MAC regimens. The majority of reported conditioning regimens used in successful allo-SCT for BPDCN have to be considered as MAC.12,18-20 The assumption of a high-dose effect could also explain why long-term survivors have been observed occasionally after auto-SCT by us and others.7,9
The results of the present study on BPDCN provide evidence that high-dose therapy followed by allo-SCT from related and unrelated donors can provide durable disease control in up to 50% of patients affected by this otherwise inevitably fatal condition. Our data suggest that allo-SCT should be attempted in CR1 if possible and should aim at administrating high-dose intensities for conditioning even in elderly patients unless more valid evidence for graft-versus-leukemia efficacy in BPDCN become available.
Presented in part at the 53rd Annual Meeting of the American Society of Hematology, December 11, 2011, San Diego, CA, and at the 38th Annual European Group of Blood and Marrow Transplantation Meeting, April 4, 2012, Geneva, Switzerland.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the data managers and physicians within the EBMT who made this analysis possible.
Authorship
Contribution.: D.R.-W., S.D., and P. D. designed and performed the research, provided patients and samples, collected the data, performed the statistical analysis, analyzed the data, and wrote the manuscript; A.B. performed the statistical analysis and wrote the manuscript; E.P. collected the data; D.B., E.C., A.I.A., W.A., D.W.B., J.C., N.K., G.M., G.R., F.J., C.P., and M.M. provided patients and samples and wrote the manuscript; V.R. and A.S. designed the research, provided patients and samples, and wrote the manuscript; and all authors proofread the manuscript and agreed upon the data presented.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Damien Roos-Weil, MD, AP-HP, Hôpital Pitié-Salpêtrière, Département d'Hématologie Clinique, 47-83 Bd de l'Hôpital, 75651 Paris Cedex, France; e-mail: damien.roosweil@psl.aphp.fr.
References
Author notes
D.R.-W. and S.D. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal