Key Points
Tandem autologous/reduced-intensity allogeneic transplantation is superior to autologous transplantation alone in multiple myeloma.
Abstract
Long-term follow-up of prospective studies comparing allogeneic transplantation to autologous transplantation in multiple myeloma is few and controversial. This is an update at a median follow-up of 96 months of the European Group for Blood and Marrow Transplantation Non–Myeloablative Allogeneic stem cell transplantation in Multiple Myeloma (NMAM)2000 study that prospectively compares tandem autologous/reduced intensity conditioning allogeneic transplantation (auto/RICallo) to autologous transplantation alone (auto). There are 357 myeloma patients up to age 69 years enrolled. Patients with an HLA-identical sibling were allocated to auto/RICallo (n = 108) and those without to auto alone (n = 249). At 96 months progression-free survival (PFS) and overall survival (OS) were 22% and 49% vs 12% (P = .027) and 36% (P = .030) with auto/RICallo and auto respectively. The corresponding relapse/progression rate (RL) was 60% vs 82% (P = .0002). Non-relapse mortality at 36 months was 13% vs 3% (P = .0004). In patients with the del(13) abnormality corresponding PFS and OS were 21% and 47% vs 5% (P = .026), and 31% (P = .154). Long-term outcome in patients with multiple myeloma was better with auto/RICallo as compared with auto only and the auto/RICallo approach seemed to overcome the poor prognostic impact of del(13) observed after autologous transplantation. Follow up longer than 5 years is necessary for correct interpretation of the value of auto/RICallo in multiple myeloma.
Introduction
Despite improvements in survival of patients with multiple myeloma by treatment with new drugs such as thalidomide, bortezomib, and lenalidomide, documented cures of the disease are lacking. Allogeneic hematopoietic stem cell transplantation has been studied, but results have been controversial.1-7 Recently, we published the first results of a prospective Non–Myeloablative Allogeneic stem cell transplantation in Multiple Myeloma study (NMAM2000) comparing tandem autologous (auto)/reduced intensity conditioning allogeneic transplantation (RICallo) with autologous transplantation—single (auto) or tandem(auto/auto).8 Although superior progression-free survival (PFS), overall survival (OS), and relapse rate (RL) using the tandem auto/RICallo treatment modality was documented using appropriate tests for crossing curves, interpretation of the results has been controversial. This update of the study, after a median follow-up of 96 months, supports and strengthens the previous conclusion that the tandem auto/RICallo approach prolongs PFS and OS long term due to lower progression/relapse rate. This is true both using an intention to treat analysis and an analysis that compares only those patients who received treatment according to protocol. Considering this study started in the era predating “novel” agents, our results suggest that reports of the “death” of allogeneic transplantation7 are greatly exaggerated.
Patients and methods
Patients
The study design was presented previously.8 Briefly, patients were included in this study from February 2001 to January 2005. Three hundred and fifty-seven patients up to age 69 years who had a response better than progression to first-line induction treatment were enrolled. All patients had undergone HLA typing and 108 of them had an HLA-identical sibling and were assigned to the auto/RICallo treatment arm. Two hundred and forty-nine patients without a matched sibling were allocated to the auto arm. Single or tandem auto was optional in the auto arm. Two patients in the auto/RICallo arm did not have an HLA-identical donor but did have a sibling donor with 1 HLA mismatch. They were mistakenly treated according to the auto/allo arm protocol and were included in this arm in the intention to treat analysis. Patient characteristics at inclusion were evenly distributed with the exception of age at diagnosis, which was slightly higher in the auto group (median 57 years vs 54 years in the auto/RICallo group) as previously presented. Median time of follow-up after inclusion at the first auto was 96 months (range 47–127 months) as compared with 61 months in our previous report. The study was approved by the Karolinska Institute’s ethical committee (Internal Review Board) and conducted in accordance with the Declaration of Helsinki.
Analysis of chromosomal aberrations
Cytogenetic analysis of the chromosome 13 deletion (del(13q14)) was performed in 214 patients using fluorescent in situ hybridization (FISH) as previously described.9 The del(13) aberration was present in 92 patients, 29 of whom were in the auto/RICallo group and 63 in the auto group. One hundred and twenty-two patients were negative for del(13), 34 in the auto/allo group and 88 in the auto group. Although del(13) is not an optimal prognostic marker for outcome after auto, at the time this was the only chromosomal aberration that could be adequately analyzed in most centers. It is still of some value since it is often associated with new and better prognostic chromosomal makers, which indicate poor prognosis after auto (del(17p), t(14;16), t(14;20)).9,10
Treatment regimens and response criteria
Prior to inclusion in the study, patients received induction chemotherapy with the VAD (vincristine, doxorubicin, and dexamethasone) regimen or with regimens similar to VAD. Seventy-three percent of patients in the auto/RICallo arm and 67% in the auto arm received the VAD regimen, while a mixture of regimens was used in other patients. Novel drugs such as thalidomide, lenalidomide, and bortezomib were not used prior to relapse/progression. All patients with at least stable disease after the induction therapy were included in the study and received an autologous transplant.
Of the 108 patients allocated to the auto/RICallo arm, 92 received the tandem auto/RICallo transplantation according to protocol (1 additional patient as compared with the previous report was found to have been treated according to protocol).8 The 16 patients who did not receive their planned RICallo were described previously. The RIC regimen consisted of fludarabine 30 mg/m2/day for 3 days + total body irradiation 2 Gy. Prevention of graft-vs-host disease (GVHD) was achieved using cyclosporin and mycophenolate mofetil as previously described.
Patients without a matched sibling donor received either no further treatment (n = 145) or, at the discretion of the center, a second auto as part of a tandem transplantation program. The conditioning for the second autograft was the same as for the first (melphalan 200 mg/m2).
After-progression treatment was optional. In the auto/RICallo group, 18 patients received donor lymphocyte infusions (DLI), 2 erroneously before progression, 4 within 2 months from progression, and 12 later. Nine patients received another allograft and 5 received an autograft. Other patients received a variety of treatments including chemotherapy and new drugs. In the auto group, 44 patients received an additional autograft, 10 within 2 months from progression and 34 later. Two patients received RICallo within 2 months from progression and 11 patients received an allograft later, 2 of them as a tandem auto/RICallo. All other patients received a variety of chemotherapy regimens, radiotherapy, and new drugs such as thalidomide, bortezomib, and lenalidomide. Since the use of novel drugs was optional after progression, meaningful analysis of their impact could not be performed.
The European Group for Blood and Marrow Transplantation (EBMT) response criteria were used as previously described.11
Statistical methods
The primary endpoint was PFS from the time of inclusion in the study (ie, from the date of the first auto). Secondary endpoints were OS, RL, complete remission rate (CR), and nonrelapse mortality incidence (NRM). Detailed definitions and statistical methods used have been described previously.8 Due to crossing survival curves, the standard log-rank test was not valid, and appropriate tests for differences in the long-term outcomes were used, specifically the method based on the “cloglog” transform of survival functions, as suggested by Klein et al.12 The comparison of OS and PFS was reported at 60 months and 96 months (standard timing and the median follow-up, respectively). The median follow-up of OS after progression was 66 months; therefore, only the difference at 60 months from progression was reported. In addition to these tests, the landmark log-rank test with the Z-OLS correction was applied.13 It was checked that results were consistent with the tests at specific time points, but for brevity, P values were not reported.
The main statistical analysis was made as an intention-to-treat (ITT) analysis, ie, all patients enrolled contributed to the analysis of outcome since first auto (108 auto/RICallo, 249 auto). In addition, an exploratory analysis was conducted in 2 subgroups defined by the presence or absence of del(13). Outcomes were also compared after the second transplantation, including only patients who received the type of transplantation planned according to protocol (auto/RICallo, 92; auto/auto, 104).
Results
ITT analysis of all patients
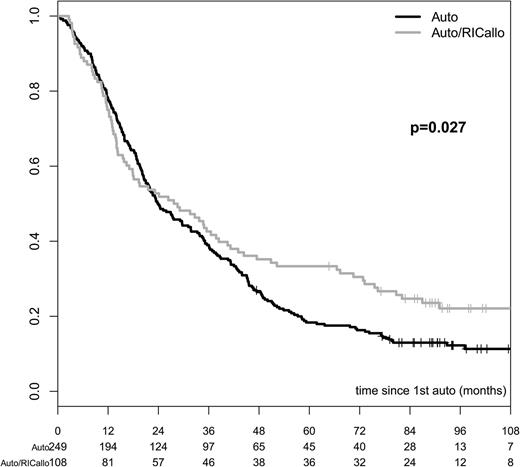
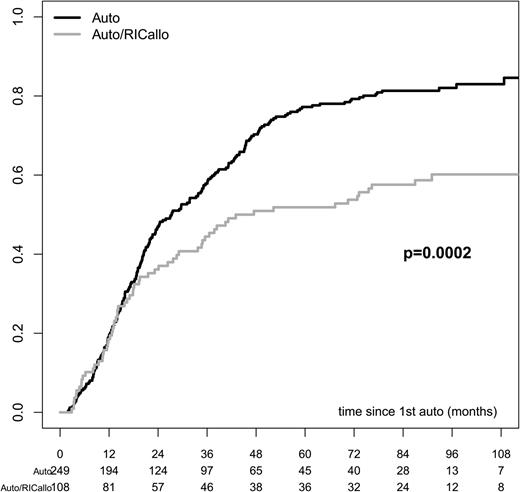
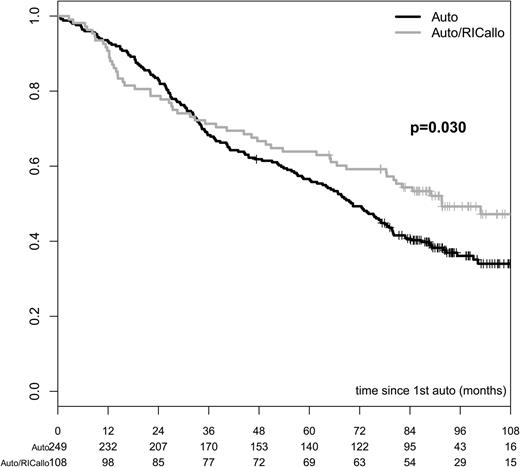
The results of the ITT analysis are shown in Figures 1–3. The PFS was significantly better for the auto/RICallo group: 33% vs 18% (P = .003) at 60 months and 22% vs 12% (P = .027) at 96 months (Figure 1). This benefit for the auto/RICallo group was emerging after 2 to 3 years of follow-up and was related to a significantly lower relapse risk, which was 52% and 60% vs 77% and 82% at 60 and 96 months (overall P = .0002) in the auto/RICallo and auto groups, respectively (Figure 2). Similarly, OS was significantly superior in the auto/RICallo group: 64% and 49% at 60 and 96 months, respectively, vs 57% (P = .204) and 36% (P = .030) in the auto group (Figure 3). NRM was significantly higher in the auto/RICallo group: 13% at 36 months as compared with 3% in the auto group (P = .0001). This difference did not increase significantly with time: 18% vs 6% at 96 months. The CR rate was similar at 12 months, 34% vs 36%, but tended to increase in the auto/RICallo group and was 50% vs 41% at 60 months. For patients who did not obtain CR, the best response status in the auto/RICallo and auto group were partial remission (PR) 43% vs 50%, no response 3% vs 5%, and progressive disease 3% vs 4%, respectively.
PFS in patients with multiple myeloma treated with auto/RIC allo or auto alone. PFS was significantly better for the auto/RICallo group: 33% vs 18% (P = .003) at 60 months and 22% vs 12% (P = .027) at 96 months. All patients included—ITT. Gray = auto/RICallo; black = auto.
PFS in patients with multiple myeloma treated with auto/RIC allo or auto alone. PFS was significantly better for the auto/RICallo group: 33% vs 18% (P = .003) at 60 months and 22% vs 12% (P = .027) at 96 months. All patients included—ITT. Gray = auto/RICallo; black = auto.
RL in patients with multiple myeloma treated with auto/RIC allo or auto alone. RL was lower with auto/RICallo: 52% vs 77% at 60 months and 60% vs 82% at 96 months in the auto/RICallo and auto groups, respectively (overall P = .0002) All patients included—ITT. Gray = auto/RICallo; black = auto.
RL in patients with multiple myeloma treated with auto/RIC allo or auto alone. RL was lower with auto/RICallo: 52% vs 77% at 60 months and 60% vs 82% at 96 months in the auto/RICallo and auto groups, respectively (overall P = .0002) All patients included—ITT. Gray = auto/RICallo; black = auto.
OS in patients with multiple myeloma treated with auto/RIC allo or auto alone. OS was better in the auto/RICallo group long term: 49% vs 36% at 96 months in the auto/RICallo and auto groups, respectively (P = .030). All patients included—ITT. Gray = auto/RICallo; black = auto.
OS in patients with multiple myeloma treated with auto/RIC allo or auto alone. OS was better in the auto/RICallo group long term: 49% vs 36% at 96 months in the auto/RICallo and auto groups, respectively (P = .030). All patients included—ITT. Gray = auto/RICallo; black = auto.
Analysis of del(13) status
Considering patients with the del(13) abnormality, PFS was 31% and 21% vs 10% and 5% at 60 (P = .016) and 96 months (P = .026) in the auto/RICallo and auto groups, respectively (Figure 4). Without the del(13) abnormality, the PFS was 41% and 26% vs 23% and 16% at 60 months (P = .054) and 96 months (P = .198) in the auto/RICallo and auto groups, respectively. The advantage for auto/RICallo tended to be more pronounced in patients with the del(13) abnormality but was also seen in patients without the del(13). Thus, the prognostic impact on PFS of the chromosome del(13) abnormality tended to be more pronounced in the auto group (ie, 5% vs 16% PFS in patients with and without the del(13) abnormality at 96 months [P = .004]) in contrast to the auto/RICallo group (ie, 21% vs 26% PFS in patients with and without the del(13) abnormality at 96 months [P = .490]). A similar difference in impact on OS was seen, being more pronounced in the auto group ie, 31% vs 46% in patients with and without the del(13) abnormality at 96 months (P = .055) than in the autoRIC/allo group (ie, 47% vs 55% OS in patients with and without the del(13) abnormality at 96 months [P = .686]). Thus, here too the impact of del(13) seems to have more importance as a poor prognostic factor in the auto group than in the auto/RICallo group, indicating that to some extent allogeneic in contrast to autologous transplantation may overcome a poor prognostic parameter such as del(13).
PFS in patients with multiple myeloma with the del(13) abnormality. PFS was better with auto/RICallo: 31% vs 10% (P = .016) at 60 months and 21% vs 5% at 96 months (P = .026). All patients with del(13) included—ITT. Gray = auto/RICallo; black = auto.
PFS in patients with multiple myeloma with the del(13) abnormality. PFS was better with auto/RICallo: 31% vs 10% (P = .016) at 60 months and 21% vs 5% at 96 months (P = .026). All patients with del(13) included—ITT. Gray = auto/RICallo; black = auto.
Analysis of patients receiving auto/RICallo vs tandem auto/auto according to protocol
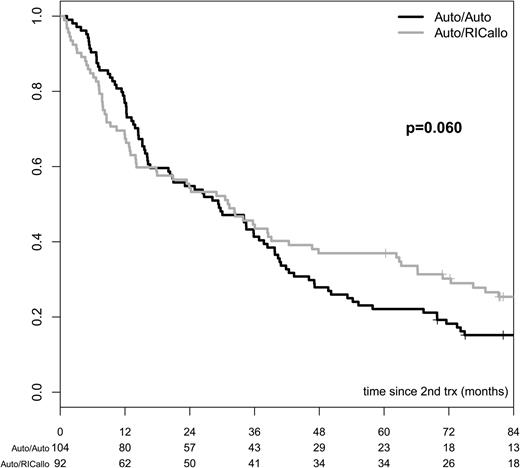
The advantage of auto/RICallo, including only those patients who received the RICallo according to protocol, as compared with auto/auto, including only those patients who received this modality in a planned tandem fashion, was similar to that observed in the ITT analysis (Figures 5 and 6). The PFS from second transplant was 37% and 24% vs 22% and 12% at 60 months (P = .023) and 96 months (P = .060) in the RIC/allo and auto/auto groups, respectively (Figure 5). Thus, the tandem auto/RICallo group tended to be even better considering that the PFS time was calculated from the time of second transplant. The difference in OS between tandem auto/RICallo and tandem auto was similar to that in the ITT analysis. OS was 64% and 52% vs 61% and 35% at 60 months (P = .608) and 96 months (P = .027) in the auto/RICallo vs auto/auto, respectively (Figure 6). The RL and NRM were similar to those in the ITT analysis. Non-relapse mortality was 12% vs 2% at 24 months (P = .003) and the relapse rate was 56% vs 82% at 96 months (P = .001).
PFS in patients with multiple myeloma receiving second transplant (RICallo or second auto) according to protocol. PFS was better with auto/RICallo than with auto/auto: 37% vs 22% at 60 months (P = .023) and 24% vs 12% at 96 months (P = .060). Gray = auto/RICallo; black = auto.
PFS in patients with multiple myeloma receiving second transplant (RICallo or second auto) according to protocol. PFS was better with auto/RICallo than with auto/auto: 37% vs 22% at 60 months (P = .023) and 24% vs 12% at 96 months (P = .060). Gray = auto/RICallo; black = auto.
OS in patients with multiple myeloma receiving second transplant (RICallo or second auto) according to protocol. OS was better with auto/RICallo than with auto/auto: 64% vs 61% at 60 months (P = .608) and 52% vs 35% at 96 months (P = .027). Gray = auto/RICallo; black = auto.
OS in patients with multiple myeloma receiving second transplant (RICallo or second auto) according to protocol. OS was better with auto/RICallo than with auto/auto: 64% vs 61% at 60 months (P = .608) and 52% vs 35% at 96 months (P = .027). Gray = auto/RICallo; black = auto.
Overall survival from disease progression
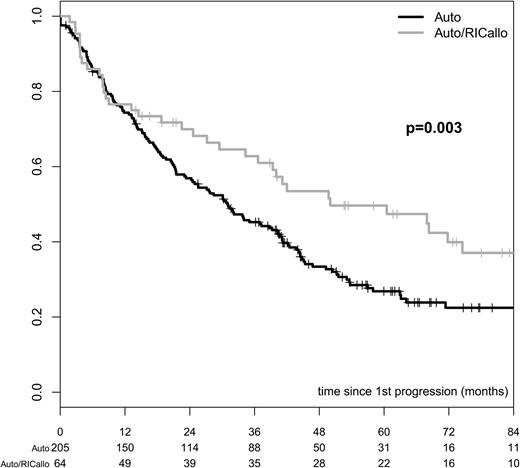
At the time of follow-up, 64 patients in the auto/RICallo group and 205 patients in the auto group had progressed. ITT analysis showed that OS at 60 months from progression was 50% in the auto/RICallo group and 27% in the auto group (P = .003; Figure 7). Comparing only those patients who had received auto/RICallo (n = 51) or auto/auto (n = 84) according to protocol, the corresponding values at 60 months were 48% and 26% (P = .019; Figure 5). Of the 16 patients in the auto/RICallo group receiving DLI after progression, 7 responded, 4 entered CR, and 3 entered PR. Of the 4 patients who entered CR, 2 were still in CR at follow-up at 87 months and 125 months, while 2 died at 86 months and 93 months after inclusion. Of the 3 patients entering PR, 2 were still in PR at follow-up at 84 months and 108 months and 1 patient entered CR after a second RICallo and was alive at 101 months.
OS from the time of first relapse/progression in patients with multiple myeloma treated with auto/RICallo or auto alone. The survival from first relapse/progression was significantly longer in the auto/RICallo arm than in the auto arm: 50% with auto/RICallo vs 27% with auto at 60 months from progression (P = .003). All patients who reached first relapse/progression included. Gray = auto/RICallo; black = auto.
OS from the time of first relapse/progression in patients with multiple myeloma treated with auto/RICallo or auto alone. The survival from first relapse/progression was significantly longer in the auto/RICallo arm than in the auto arm: 50% with auto/RICallo vs 27% with auto at 60 months from progression (P = .003). All patients who reached first relapse/progression included. Gray = auto/RICallo; black = auto.
GVHD
Acute GVHD in 92 patients who received tandem RICallo was as previously described, ie, grade I in 11%, grade II in 9%, grade III in 9%, and grade IV in 2%. Sixty-seven percent of patients had no acute GVHD. Fifty-four percent of the patients developed chronic GVHD, which was limited in 31% and extensive in 23%. As previously described, patients with acute GVHD had a higher NRM. The cumulative NRM at 60 months in patients with and without acute GVHD was 36% and 4%, respectively (P < .001). In a landmark analysis from 12 months, there were no significant differences in OS, PFS, relapse incidence, or NRM between patients with and without chronic GVHD that occurred within the first 12 months.
Discussion
The importance of long-term follow-up for the correct evaluation of auto/RICallo vs auto is clearly demonstrated in this study. The additional 35 months of follow-up of the NMAM2000 study in comparison with our previous presentation8 shows that nearly twice as many patients are progression free after 8 years with the auto/RICallo procedure compared with the auto procedure (22% vs 12%) and 49% are surviving after the auto/RICallo procedure compared with 36% with auto at this time. In patients who received the auto/ RICallo transplant according to protocol and compared with those who received 2 auto transplants in a planned approach, the differences were similar. Thus our long-term results show superiority for the auto/RICallo procedure irrespective of whether the data were analyzed on an ITT basis or according to protocol.
The study also shows that patients with or without the del(13) abnormality had similar outcome when treated with auto/RICallo and better outcome than those with auto. This is in contrast to the outcome with auto, which was poorer in patients with the del(13) abnormality than in those without, corroborating with other studies.9,10 Although it is now known that del(13) is not a prognostic marker by itself, it is frequently associated with other more important prognostic chromosomal markers such as del(17p), t(4;14), and t(14;16). Therefore, most studies that analyze only del(13) show that patients with the aberration tend to have poorer prognosis than those without,9,10 as in our study. This adds to evidence that shows that del(13) is a surrogate marker for other chromosomal aberrations that indicate poor prognosis with auto. Our results suggest that allogeneic transplantation may overcome this poor prognosis following autologous transplantation and corroborates with recent retrospective findings.14
An interesting observation is that despite the fact that a variety of treatments were administered following progression, OS after relapse/progression was significantly superior in the auto/RICallo group. The previously well-documented graft vs myeloma effect15,16 may persist in patients after auto/RICallo at progression and contribute to this difference. The use of DLI in a fraction of auto/RICallo patients may also play a role. The difference in clinical outcome was seen despite a higher frequency of additional autologous transplants performed in the auto group, while the effect of a difference in the use of new drugs could not be adequately analyzed.
Of 3 prospective studies1-3 with somewhat different trial designs published before our first report of the NMAM2000 study, 2 supported the better outcome with auto/RICallo as compared with tandem auto, as discussed previously.2,3 Since that time, 2 additional studies that included large numbers of patients have been published, ie, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 study5 and the Haemato Oncology Foundation for Adults in the Netherlands (HOVON)-50 study.4 At first glance, both seem to contradict our results.
However, the BMT CTN study had a significantly shorter follow-up time. Endpoints were PFS and OS at 36 months. At this time, results are comparable to ours, ie, OS was 77% with auto RICallo and 80% with auto/auto in the BMT CTN study as compared with 71% vs 68% respectively in our study. Also, PFS with auto/RICallo is exactly the same in the 2 studies, ie, 43%, while the auto/auto procedure in the BMT CTN study tends to be somewhat better, ie, 46% vs 39% in our study. The nonrelapse mortality was similar in the 2 groups, ie, 13% in our study vs 11% in the BMT CTN study with the auto/RICallo procedure and 3% vs 4% with auto/auto, respectively. Thus, up until 36 months, our results are similar to those of the BMT CTN study, but our longer follow-up clearly demonstrated the advantage of auto/RICallo.
The HOVON study4 had a somewhat different approach; it used conditioning with total body irradiation 2 Gy without fludarabine and maintenance therapy with thalidomide in some of the auto patients. OS at 6 years was 55% in patients with or without a donor as compared with 59% after auto/RICallo and 49% after auto/auto in our study. However, PFS at 6 years was 28% in patients with a donor compared with 22% without a donor in the HOVON study. The corresponding figures in our study were 30% and 16%. Although not significant in the HOVON study (it was significant in ours), the tendency seems to be the same, corroborating with a significantly lower relapse/progression rate in the auto/RICallo arm in both studies. Thus, the main difference between the 2 studies seems to be the better outcome with the auto/auto procedure in the HOVON study. Patient selection or treatment differences could play a role. Nearly half of the patients in the HOVON study had a sibling donor, while this usually only is the case in about one third. Although there was no significant difference in prognostic parameters at diagnosis, there were a few more patients in CR or very good PR at inclusion (40% vs 36%) and fewer patients with the del(13) abnormality assessed by FISH (13% vs 21%) than in the no donor group. These minor differences seem insufficient to be responsible for the better results in the auto group compared with ours. Although it was not proven to be effective, a fraction of the auto patients received maintenance treatment with thalidomide, which is contrary to our patients. Thus, although the authors claim no advantage for the auto/RICallo procedure, it has to be pointed out that the relapse and progression rate at 6 years was 77% in the auto group (79% in the NMAM 2000 study) and 55% in the auto/RICallo group (54% in the NMAM 2000 study) at 6 years. Here, the results seem to be practically identical and to the advantage of the auto/RICallo procedure.
Although our report and the Italian study indicate better outcome with the auto/RIC allo transplant procedure than with only auto transplantation and no study has shown worse outcome with the auto/RICallo approach, the early higher NRM and the risk of chronic GVHD has raised some questions as to the value of this treatment modality.7,17 These results as well as ours indicate that certain high-risk factors at diagnosis can be overcome by auto/RICallo and that may be a reason to restrict studies on auto/RICallo to poor-risk patients. It also appears that selection of donors could be improved. To date, prospective studies have been based on the availability of an HLA identical sibling donor. In a recent study it was shown that selection of donors with KIR haplotype B could significantly improve results.18
An important question is whether allogeneic transplants should be postponed until progression and relapse. Currently this is not clear. The present study does not provide an answer to this question. However, other studies,19-21 including a retrospective EBMT study that is in progress, indicate that allogeneic transplantation might be an option. Still, these studies are not prospective and do not compare results with those of new drug treatment or autologous transplantation. A recently published EBMT study22 showed very good results with the VTD (bortezomib + thalidomide + dexamethasone) combination following progression.
Our study was designed when the new drugs were not commonly used. Numerous studies have later proven their efficacy.23-27 Combinations of new drugs have been used for induction before autologous transplantation,27 and recent studies have shown improved PFS28 and perhaps survival26 using maintenance therapy following autologous transplantation. However, most of these studies have considerably shorter follow-up than the median of 96 months in our study and long-term survival can therefore not be adequately compared to our results with auto/RICallo. In a recent study using new drugs both for induction (bortezomib, lenalidomide, and/or thalidomide) and prospectively lenalidomide for maintenance, including only patients responding to the autologous transplantation, results do not appear superior to our results with autoRICallo at 4 years, and long-term results are lacking.26 Recently it has been shown that use of bortezomib in association with allotransplantation may reduce GVHD without significantly hampering graft-versus-myeloma (GVM).29,30 Also, lenalidomide appears to be very effective in treating relapse following allogeneic transplantation.31 Thus new drugs may be effectively used in combination with allotransplantation and improve results with this transplant modality even further.
Our conclusion is that the tandem auto/ RICallo transplantation modality is a promising therapeutic option for younger patients with multiple myeloma and poor prognostic features. Here, the moderately higher early NRM may be acceptable, while it may be of less value to patients with good prognostic parameters, particularly when considering the improved treatment results currently available for new drugs such as bortezomib, lenalidomide, and others. However, it is also likely that combinations of these drugs and early allogeneic transplantation may further improve the results of auto/RICallo transplants. Carefully designed studies with long follow-up need to be performed in both newly diagnosed and relapsed patients. Such studies may prove that allogeneic transplantation for myeloma should not be abandoned.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the Swedish Cancer Fund and the Stockholm Cancer Society. We thank the following investigators in all participating centers who are not included as coauthors: Vittorio Montefusco, Istituto Nazionale Tumori, Milan, Italy; Pellegrino Musto, IRCCS, Centro Riferimento Oncologico Basilicata, Rionero in Vulture, Italy; Lene Knudsen, Copenhagen University Hospital Herlev, Denmark; Kari Remes, University Central Hospital, Turku, Finland; Kristina Carlson, Akademiska University Hospital, Uppsala, Sweden; Jean-Francois Rossi, University Hospital, Montpellier, France; Andreas Sengelov, Copenhagen University Hospital Rigshospitalet, Denmark; Ulf-Henrik Mellqvist, Sahlgrenska University Hospital, Gothenburg, Sweden; Gareth Morgan, Royal Marsden Hospital, Sutton, United Kingdom; Inge-Marie Dahl, Tromso University Hospital, Norway; Lorentz Brink Oslo, Norway; Andrea Junghaus Leipzig, Germany; Elli Koivunen, University Hospital, Tampere, Finland; and Anders Waage, Trondheim University Hospital, Norway.
Authorship
Contribution: G.G. designed the study, performed research, and wrote the manuscript; S.I. participated in designing the study, performed the statistics, and participated in writing the manuscript; B.B. participated in designing the study, performed research, and participated in writing the manuscript; U.H., A.G., H. Greinix, L.V., F.N., A.M.C., M.B., A.B., G.M., P.C., S.S., K.F., A.v.B., H. Goldschmidt, T.d.W., C.M., L.G., and N.K. performed research and participated in writing the manuscript; D.N. participated in designing the study, performed research, and participated in writing the manuscript.
Conflict-of-interest disclosure: B.B. is presently employed by the Novartis Company and was employed by the Karolinska University Hospital during the design of the study and in the course of the patient inclusion. The remaining authors declare no competing financial interests.
Correspondence: Gösta Gahrton, Karolinska Institutet, Huddinge, Department of Medicine, Karolinska University Hospital, Huddinge, SE-14186 Stockholm, Sweden; e-mail: gosta.gahrton@ki.se.