Key Points
Bortezomib consolidation after ASCT improves PFS in myeloma.
Improvement of response is seen with bortezomib consolidation after ASCT in myeloma.
Abstract
The Nordic Myeloma Study Group conducted an open randomized trial to compare bortezomib as consolidation therapy given after high-dose therapy and autologous stem cell transplantation (ASCT) with no consolidation in bortezomib-naive patients with newly diagnosed multiple myeloma. Overall, 370 patients were centrally randomly assigned 3 months after ASCT to receive 20 doses of bortezomib given during 21 weeks or no consolidation. The hypothesis was that consolidation therapy would prolong progression-free survival (PFS). The PFS after randomization was 27 months for the bortezomib group compared with 20 months for the control group (P = .05). Fifty-one of 90 patients in the treatment group compared with 32 of 90 controls improved their response after randomization (P = .007). No difference in overall survival was seen. Fatigue was reported more commonly by the bortezomib-treated patients in self-reported quality-of-life (QOL) questionnaires, whereas no other major differences in QOL were recorded between the groups. Consolidation therapy seemed to be beneficial for patients not achieving at least a very good partial response (VGPR) but not for patients in the ≥ VGPR category at randomization. Consolidation with bortezomib after ASCT in bortezomib-naive patients improves PFS without interfering with QOL. This trial was registered at www.clinicaltrials.gov as #NCT00417911.
Introduction
Treatment with high-dose melphalan followed by autologous stem cell transplantation (ASCT) has improved survival in patients with multiple myeloma1-4 and remains the gold standard for younger patients even in the era of new drugs.4 In a previous study, we showed that a reduced initial therapy induced less toxicity but with no reduction in treatment efficacy.5 Building on these results, we now aim to explore if consolidation therapy after ASCT could improve treatment results. The proteasome inhibitor bortezomib has also proved to be very efficient as a relapse treatment for patients who have previously undergone ASCT.6 In this open, multicenter phase 3 randomized trial, we compared the effect of bortezomib consolidation initiated 3 months after ASCT with no consolidation, which was standard procedure within the Nordic countries at the time the study began. Importantly, patients included in this trial did not receive bortezomib as part of induction therapy. The primary objective of the study was to determine whether the addition of bortezomib consolidation would improve progression-free survival (PFS).
Knowing that many patients have a high quality of life (QOL) during the first period of disease control7 and that consolidation might interfere with this, we also focused on toxicity and QOL during the study period.
Methods
Study design and patients
The study was undertaken at 23 centers in Denmark, Estonia, Finland, Iceland, Norway, and Sweden. Patients were enrolled between October 2005 and April 2009. The clinical data cutoff was April 2010 when the last randomized patient had been followed for 12 months. An extra update for overall survival (OS) was performed in April 2011. The primary end point was PFS, and secondary end points were response rate, OS, QOL, and tolerability.
Myeloma patients with newly diagnosed symptomatic and measurable disease were eligible for inclusion in this trial. All patients had received initial therapy followed by stem cell collection and ASCT. The regimen used for initial therapy was not mandated. However, the patients had to be bortezomib naive at the time of inclusion. The most common initial treatment was Cy-Dex (cyclophosphamide and high-dose steroids), used for 169 out of 183 in the control group and 161 out of 187 in the consolidation group. Eight patients in both groups received a combination of thalidomide and steroids, and the remaining patients received vincristine, adriamycin and dexamethasone or similar combinations. Patients were included at the time of ASCT but randomized 3 months later.
Exclusion criteria were neuropathy > grade 2 according to National Cancer Institute Common Toxicity Criteria, severe heart disease including myocardial infarction within 6 months before enrollment, heart failure, New York Heart Association ≥ Class III or cardiac amyloidosis, history of hypotension, or previous exposure to bortezomib.
All patients signed a written informed consent before inclusion. The study was approved by the ethical committees and health authorities in all participating countries and conducted in accordance with the 1975 Declaration of Helsinki and the Guidelines for Good Clinical Practice.
Randomization
Patients were randomly assigned in a 1:1 ratio 3 months after ASCT to receive 20 doses of bortezomib during 21 weeks starting no later than within 2 weeks after randomization or to no further treatment. Stratification factors were age (<60 years vs ≥60 years) and single vs double ASCT. The clinical investigators at each site called the research unit at Lunds University Hospital where randomization was performed using a computerized system.
Consolidation therapy
Bortezomib was given as a single drug intravenously in 6 cycles. In the first 2 cycles, bortezomib was given twice weekly on days 1, 4, 8, and 11 in a 3-week schedule, followed by 4 cycles in which bortezomib was given once weekly on days 1, 8, and 15 in a 4-week schedule. The starting dose was 1.3 mg/m2, but subsequent doses could be reduced due to neuropathy and/or hematologic toxicity according to the standard prespecified dose-modification algorithm. No doses were postponed. If, for any reason, a dose could not be administered, it was reported as reduced to 0. In total, a maximum of 20 doses were given during 21 weeks. No corticosteroids, apart from a dose equivalent to <50 mg prednisone daily for no more than 1 week due to other medical conditions, or any other antineoplastic drugs were allowed. A total of 6 patients, 1 control and 5 bortezomib-treated patients, did receive steroids: 3 due to chronic obstructive lung disease and 1 each for vasculitis, hemolytic anemia, and high fever. In 1 case, the dose was higher than permitted, and that patient was censored at the time steroid treatment was started. Bisphosphonates were administered according to national guidelines.
Diagnostic, response, and relapse criteria
Symptomatic myeloma was defined using the criteria of the International Myeloma Working Group.8 Disease response and relapse were defined according to the European Group for Blood and Marrow Transplantation9 incorporating near complete response (nCR)10 and very good partial response (VGPR).11 Because immunofixation data were lacking in a proportion of patients, nCR and CR are reported together. Patients with no measurable M protein but for whom no bone marrow examination confirming CR was performed were included in the VGPR group.
Follow-up evaluation
All patients were evaluated with serum and urine electrophoresis monthly for the first year and then every second month until disease progression. A bone marrow examination needed to be done to confirm CR. All data were reviewed according to Good Clinical Practice criteria by an independent academic contract research organization. After disease progression, patients were followed for survival. Survival time was measured from randomization, that is, 3 months after ASCT until disease progression or death from any cause (PFS) or death from any cause (OS).
Health-related quality of life
Health-related quality of life (HRQOL) was assessed prospectively by use of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30, version 3.0).12 Scoring of the EORTC QLQ-C30 was performed according to published methods.13 The questionnaire has previously been shown to be reliable and valid for myeloma patients.14
The questionnaires were filled in at baseline (time of randomization), at 8 weeks after randomization, and then every 3 months until 2 years and every 6 months until 3.5 years. The baseline questionnaires were administered by a physician or nurse and were filled in by the patients before randomization. The subsequent sets of questionnaires were mailed to the patients’ homes with a stamped return envelope.
Statistical analysis
The hypothesis of this study was that consolidation treatment with bortezomib would prolong median PFS by 12 months. To demonstrate this with a power of 80% and a significance level of 5% (2-sided test), 396 patients needed to be included, of whom at least 80% would be randomized (ie, at least 159 patients per arm).
The proportions of patients with a given characteristic were compared using Fisher’s exact test for variables with frequency scale and Mann-Whitney U test for the remaining variables. A Mann-Whitney U test was also used to calculate the significance of differences in the EORTC QLQ-C30 score between the control group and bortezomib group. Based on previous work with myeloma patients, the minimal important difference in an HRQOL scale score was classified as a difference of at least 6 points (0-100 scale).15
PFS and OS rates were calculated according to the Kaplan-Meier method, and survival comparisons between groups were made by the log-rank test. PFS and survival were calculated from randomization (ie, 3 months after high-dose therapy and ∼6 months after start of induction therapy). The statistical analyses were performed in SPSS Statistics 18 for Windows (IBM Corporation, Somers, NY).
Results
Patients and baseline characteristics
Figure 1 shows the study profile. Four hundred and three patients were included at the time of ASCT. A total of 4 patients were excluded due to nonsecreting disease (2) or not fulfilling diagnostic criteria (2). Of the remaining 399 patients, 29 were not randomized due to withdrawn consent (17), neuropathy (4), progressive disease before randomization (4), logistic reasons (2), early death (1), or severe infection (1). Of the remaining 370 patients, 187 were randomized to bortezomib consolidation therapy and 183 to the control group.
Flow diagram of 403 patients included in the Nordic Myeloma Study Group bortezomib consolidation study.
Flow diagram of 403 patients included in the Nordic Myeloma Study Group bortezomib consolidation study.
Baseline characteristics are shown in Table 1. All variables were equally distributed between the 2 groups.
Baseline characteristics
| Variable . | Bortezomib (n = 187) . | Control (n = 183) . |
|---|---|---|
| Age, years* | 59.1 (9.90) | 58.7 (8.80) |
| Male† | 59 (111) | 60 (109) |
| Double ASCT† | 5 (9) | 3 (6) |
| Myeloma subtype† | n = 187 | n = 183 |
| IgG | 55 (103) | 63 (116) |
| IgA | 26 (50) | 21 (39) |
| Light chain | 18 (33) | 14 (25) |
| Other | 1 (1) | 2 (3) |
| ISS disease stage† | n = 125 | n = 141 |
| I | 38 (48) | 41 (58) |
| II | 38 (48) | 31 (44) |
| III | 23 (29) | 28 (39) |
| β2-microglubilin (mg/L)‡ | 3.0/4.9 | 3.4/5.1 |
| Albumin (g/L)‡ | 36.8/35.8 | 37.0/36.2 |
| Creatinine (μmol/L)‡ | 82.0/118.1 | 83.0/123.1 |
| Hemoglobin (g/L)‡ | 112.0/114.1 | 110.0/111.7 |
| Platelets (×109/L)‡ | 249.0/252.9 | 243.0/253.9 |
| Fluorescence in situ hybridization analysis for cytogenetic abnormalities† | n = 73 | n = 66 |
| Absence of del(13q), t(4;14), or del(17p) | 75.3 (55) | 63.7 (42) |
| Presence of del(13q)§ | 21.9 (16) | 16.7 (11) |
| Presence of t(4;14) and/or del(17p) | 19.2 (14) | 19.7 (13) |
| Variable . | Bortezomib (n = 187) . | Control (n = 183) . |
|---|---|---|
| Age, years* | 59.1 (9.90) | 58.7 (8.80) |
| Male† | 59 (111) | 60 (109) |
| Double ASCT† | 5 (9) | 3 (6) |
| Myeloma subtype† | n = 187 | n = 183 |
| IgG | 55 (103) | 63 (116) |
| IgA | 26 (50) | 21 (39) |
| Light chain | 18 (33) | 14 (25) |
| Other | 1 (1) | 2 (3) |
| ISS disease stage† | n = 125 | n = 141 |
| I | 38 (48) | 41 (58) |
| II | 38 (48) | 31 (44) |
| III | 23 (29) | 28 (39) |
| β2-microglubilin (mg/L)‡ | 3.0/4.9 | 3.4/5.1 |
| Albumin (g/L)‡ | 36.8/35.8 | 37.0/36.2 |
| Creatinine (μmol/L)‡ | 82.0/118.1 | 83.0/123.1 |
| Hemoglobin (g/L)‡ | 112.0/114.1 | 110.0/111.7 |
| Platelets (×109/L)‡ | 249.0/252.9 | 243.0/253.9 |
| Fluorescence in situ hybridization analysis for cytogenetic abnormalities† | n = 73 | n = 66 |
| Absence of del(13q), t(4;14), or del(17p) | 75.3 (55) | 63.7 (42) |
| Presence of del(13q)§ | 21.9 (16) | 16.7 (11) |
| Presence of t(4;14) and/or del(17p) | 19.2 (14) | 19.7 (13) |
ISS, International Staging System.
Median (interquartile range).
% (n).
Median/mean.
Regardless of absence or presence of t(4;14) and/or del(17p).
Completion of consolidation treatment
The median number of bortezomib injections received was 19, and the median given dose was 90% (calculated as the total given dose divided by total planned dose for each patient).
Survival
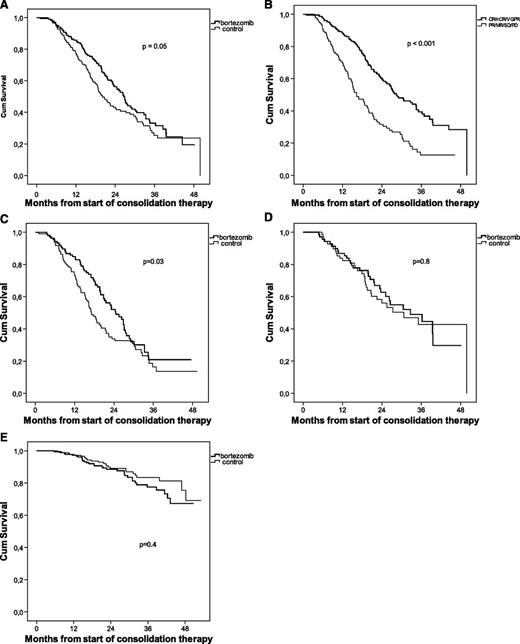
The overall median follow-up time was 38 months. The median PFS was prolonged for patients randomized to bortezomib consolidation (27 months vs 20 months, P = .05) (Figure 2A).
Analysis of outcome from start of consolidation therapy. Kaplan-Meier estimates of PFS for bortezomib-treated patients vs controls (A), PFS for patients achieving ≥VGPR vs patients achieving <VGPR (B), PFS for patients in the <VGPR category at randomization (C), PFS for patients in the ≥VGPR category at randomization (D), and OS for bortezomib-treated patients vs controls (E).
Analysis of outcome from start of consolidation therapy. Kaplan-Meier estimates of PFS for bortezomib-treated patients vs controls (A), PFS for patients achieving ≥VGPR vs patients achieving <VGPR (B), PFS for patients in the <VGPR category at randomization (C), PFS for patients in the ≥VGPR category at randomization (D), and OS for bortezomib-treated patients vs controls (E).
Patients achieving at least VGPR at any time experienced a significantly longer PFS compared with those who did not (28 months vs 16 months, P < .001) irrespective of whether the patient was randomized to bortezomib treatment or not (Figure 2B). The beneficial effect of bortezomib consolidation was only seen in patients not achieving at least VGPR after ASCT (Figure 2C-D). Further, no difference in PFS was seen in patients in the ≥VGPR category at randomization compared with those improving their response to ≥VGPR during the study (data not shown).
Because only 15 patients underwent a double ASCT, no meaningful statistics could be calculated comparing single and double ASCT. No difference in PFS was seen for patients under the age of 60 years compared with those aged 60 or over (data not shown). After 3 years of follow-up, the OS was similar in both treatment groups, ∼80% (Figure 2E).
Response rate
The response rates at randomization 3 months after ASCT and best response during the study, calculated on an intention-to-treat basis, are presented in Table 2. At randomization there was no difference in response rate between the groups; ∼20% of all randomized patients had achieved CR/nCR, and ∼40% had achieved at least VGPR. Measured as best response achieved during the study, there was a difference with more bortezomib-treated patients achieving at least VGPR (71% vs 57%, P < .01) and a trend toward more patients achieving CR/nCR (45% vs 35%, P = .055). Improvement of response from partial response (PR) to at least VGPR was also more common in patients receiving bortezomib consolidation (51 of 90 patients [57%] vs 32 of 90 [36%], P = .007).
Response rates
| Variables . | Bortezomib . | Control . | Test* . | ||
|---|---|---|---|---|---|
| n . | % (n) . | n . | % (n) . | P value . | |
| At randomization | 179† | 180† | |||
| ≥nCR | 20.1 (36) | 20.6 (37) | |||
| VGPR | 19.6 (35) | 18.3 (33) | |||
| PR | 51.4 (92) | 50.0 (90) | |||
| MR | 7.3 (13) | 8.9 (16) | |||
| SD | 1.7 (3) | 2.2 (4) | |||
| Best response | 182‡ | 183 | |||
| ≥nCR | 45.1 (82) | 35.0 (64) | |||
| VGPR | 25.8 (47) | 22.4 (41) | |||
| PR | 25.3 (46) | 38.3 (70) | |||
| MR | 2.7 (5) | 3.3 (6) | |||
| SD | 0.5 (1) | 0.5 (1) | |||
| PD | 0.5 (1) | 0.5 (1) | |||
| ≥nCR | 45.1 (82) | 35.0 (64) | .055 | ||
| ≥VGPR | 70.9 (129) | 57.4 (105) | .0088 | ||
| Variables . | Bortezomib . | Control . | Test* . | ||
|---|---|---|---|---|---|
| n . | % (n) . | n . | % (n) . | P value . | |
| At randomization | 179† | 180† | |||
| ≥nCR | 20.1 (36) | 20.6 (37) | |||
| VGPR | 19.6 (35) | 18.3 (33) | |||
| PR | 51.4 (92) | 50.0 (90) | |||
| MR | 7.3 (13) | 8.9 (16) | |||
| SD | 1.7 (3) | 2.2 (4) | |||
| Best response | 182‡ | 183 | |||
| ≥nCR | 45.1 (82) | 35.0 (64) | |||
| VGPR | 25.8 (47) | 22.4 (41) | |||
| PR | 25.3 (46) | 38.3 (70) | |||
| MR | 2.7 (5) | 3.3 (6) | |||
| SD | 0.5 (1) | 0.5 (1) | |||
| PD | 0.5 (1) | 0.5 (1) | |||
| ≥nCR | 45.1 (82) | 35.0 (64) | .055 | ||
| ≥VGPR | 70.9 (129) | 57.4 (105) | .0088 | ||
MR, minor response; SD, stabile disease; PD, progressive disease.
Fisher’s exact test, exact significance (2-sided).
Eleven patients had unconfirmed responses at the time of randomization.
Five patients had incomplete follow-up.
Toxicity
Sensory peripheral neuropathy was reported by 57% of patients in the treatment group vs 24% in the control group. Neuropathic pain was reported by 34% and 12%, respectively. Sensory neuropathy > grade 2 on the Common Toxicity Criteria scale was experienced by 5% of bortezomib-treated patients vs 1% for controls (P < .04), whereas neuropathic pain > grade 2 was experienced by 6% vs 1% (P < .006) (Figure 3).
Neurologic toxicity. Number of patients experiencing neuropathic pain (A) and peripheral sensory neuropathy (B) by treatment arm.
Neurologic toxicity. Number of patients experiencing neuropathic pain (A) and peripheral sensory neuropathy (B) by treatment arm.
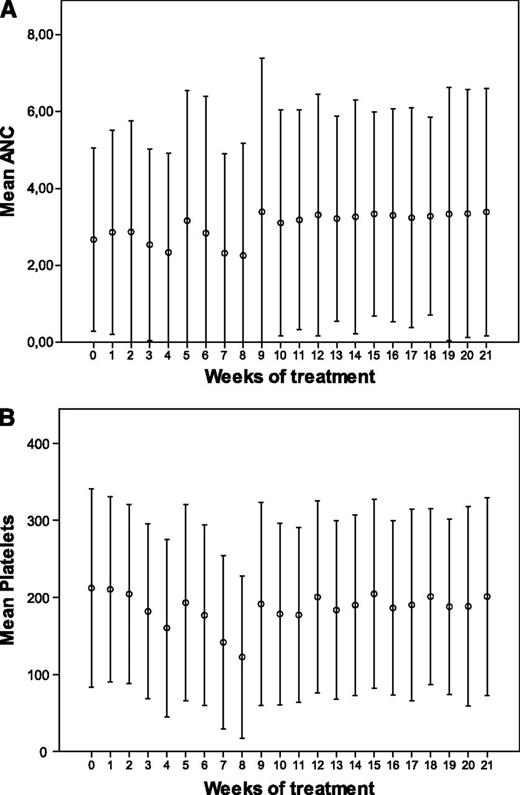
Hematologic toxicity was mild and manageable. It seemed as if the reduction of neutrophil and platelet counts was less pronounced during the last 4 treatment cycles when bortezomib was given once weekly (Figure 4).
Hematologic toxicity. Mean neutrophil (A) and platelet (B) count for patients randomized to bortezomib during the consolidation period. ANC, absolute neutrophil count.
Hematologic toxicity. Mean neutrophil (A) and platelet (B) count for patients randomized to bortezomib during the consolidation period. ANC, absolute neutrophil count.
Two cases of secondary primary malignancy were reported. One of the bortezomib-treated patients developed breast cancer, and 1 patient in the control group developed rectal cancer.
HRQOL
Baseline questionnaires were available for 311 patients (84%). There were no significant differences in HRQOL score between the bortezomib group and the control group at baseline.
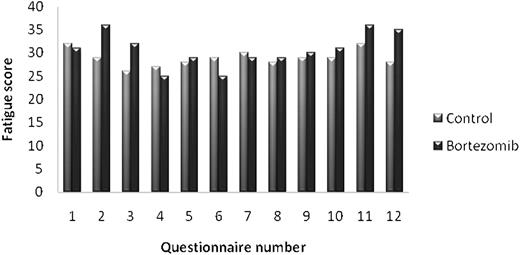
After 8 weeks, there was a statistically significant increase in fatigue and nausea/vomiting in the bortezomib group (P < .01). However, only the fatigue scale reached what we previously had set as a cutoff for clinically relevant changes (6 points on a 0-100 scale) (Figure 5). There were no significant differences in HRQOL scores between the bortezomib group and the control group during the rest of the study period.
Discussion
This randomized, multicenter phase 3 trial in newly diagnosed myeloma patients showed that consolidation with bortezomib after ASCT did improve response and resulted in a statistically significant 7-month prolongation of PFS in bortezomib-naive patients. However, the study hypothesis of a 12-month prolongation of PFS was not confirmed. Our data indicate that the prolongation of PFS was mediated by an increased proportion of patients achieving an improvement in the quality of the response after bortezomib consolidation. Supporting this theory are the findings that the proportion of patients improving their grade of response was significantly higher for the treatment group and that the beneficial effect of bortezomib consolidation on PFS only was seen in patients not achieving at least VGPR after ASCT. Finally, there was no difference in PFS for patients in the ≥VGPR category after ASCT compared with those who achieved it later during the study.
No difference in OS was seen, and this could be due to the fact that treatment at progression today is very effective and there are many treatment options. More patients in the control group did receive bortezomib-containing combinations after first relapse, 48 vs 19. Apart from this, there were small differences in second-line therapy; a second ASCT was given to 17 controls and 18 patients receiving bortezomib consolidation; thalidomide, 25 vs 27; lenalidomide, 2 vs 9; chemotherapy combinations, 10 vs 13; and radiation alone, 10 vs 4. A landmark analysis starting at the time of relapse did not show any difference in estimated OS (4.0 years for controls vs 3.9 years for patients randomized to consolidation).
The role of consolidation and maintenance therapy is still unclear in myeloma therapy. The earliest experiences with chemotherapy were disappointing,16,17 and the beneficial effect of corticosteroids as single-drug maintenance18 has been questioned.19 Meta-analyses of interferon α have shown a positive effect but at the cost of substantial tolerability problems.20 Several studies have shown prolonged PFS, and some even prolonged OS when thalidomide has been given as consolidation or maintenance after ASCT but with substantial toxicity, mainly neurologic.21 An interesting finding in the French study, Intergroup Francophone du Myélome (IFM) 99-02, is that the beneficial effect of thalidomide was only seen in patients achieving less than VGPR after ASCT.22 Hence, the effect of thalidomide in the IFM study and of bortezomib in our study both seem to have been mediated by improving the degree of treatment response. In contrast, data from the British study, Medical Research Council IX, indicate that the effect of thalidomide given after ASCT has an effect of maintenance more than consolidation because the beneficial effect of thalidomide treatment did not differ due to response after ASCT.23 Similar results were seen in 2 studies using lenalidomide as maintenance therapy after ASCT.24,25 Regardless of response after ASCT, this treatment approach seemed to be beneficial.
In recent years, several studies and meta-analyses indicate that achievement of complete response or at least VGPR after ASCT is associated with a better outcome.26,27 These data support the consolidation concept (ie, to apply a short course of treatment in order to reduce the number of residual tumor cells), which is shown by improved response status in patients with insufficient response after ASCT.
Consolidation with bortezomib after ASCT in combination with other drugs has been reported. In a recently published update, it was shown that the combination of bortezomib, thalidomide, and dexamethasone is superior to thalidomide and dexamethasone alone, given as consolidation therapy after double ASCT. In this study, the same combinations are also used as initial therapy before ASCT. Using a landmark analysis, Cavo et al28 showed that the superior results of the triplet combination over the double are further improved by consolidation therapy and the beneficial effect is most evident in patients not achieving at least nCR after ASCT. Even if it is dubious to compare results between studies, PFS in the Italian study is clearly longer than in our study, indicating that using different drugs in combination can be very effective as consolidation therapy. When our study was planned, there were already data suggesting that corticosteroids did improve the effect of bortezomib in the relapse setting.10,29 However, because studies have implied that steroids do have an effect of their own given as consolidation therapy, we chose to give bortezomib as a single drug, avoiding confusion about the results.
A weakness in the present study is that a repeat bone marrow examination was not always performed in order to confirm or reject CR. Patients with no measurable M protein but for whom no bone marrow examination confirming CR was performed were downgraded to VGPR. This means that patients in the VGPR response category are a heterogeneous group containing both patients in true CR as well as patients with only a 90% reduction of the original M protein. Unfortunately, cytogenetics was not available for more than a proportion of the patients. The reason for this was that fluorescence in situ hybridization analysis was not standard at the time of the study in the Scandinavian countries, and in addition, the study was performed at smaller local hospitals with limited access to cytogenetic techniques.
An interesting and important finding that limits the difference between the 2 patient groups in this study is that ∼17% of the controls also improved their response. However, in the clinic, it is not a rare finding that patients do improve their response up to 1 year after ASCT.
Some of the strengths of the study are that bortezomib was given as a single drug, meaning that observed effects can only be attributed to this substance. Further, the QOL data show that the additional therapy did not interfere with QOL, which is very important when focusing on PFS. The risk of neurotoxicity might be reduced with a more restrictive dose-modification algorithm, and our study showed that the hematologic toxicity was more pronounced when bortezomib was given twice weekly compared with once weekly. The only significant differences in QOL, fatigue and nausea/vomiting after 7 weeks, also disappeared when the less frequent dose schedule was used. For elderly patients, it has already been suggested that administration of bortezomib once weekly makes the treatment more tolerable.30 Also, modification of administration might be beneficial because now there are data showing that by administering bortezomib subcutaneously instead of intravenously the incidence and severity of neuropathy can be significantly reduced.31
In conclusion, our study shows that consolidation with bortezomib after ASCT improves PFS and indicates that this is due to improvement of response. The treatment was well tolerated as indicated by no important interference with QOL, low frequency of severe toxicity, and that most patients received their treatment without any dose reduction. The relatively short treatment period might have been a reason for the good tolerability. Results from other studies imply that efficacy might be enhanced if bortezomib is given in combination with other agents and that tolerability could be even further improved enhancing the possibility of long-term treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Region Skanes Research Center for monitoring and data handling.
This work was supported by grants from Johnson & Johnson, the Nordic Cancer Union, and the Boras foundation for cancer research.
Authorship
Contribution: U.-H.M., P.G., O.H., S.L., and J.W. designed and directed the study and edited the manuscript; and E.L., K.R., H.S., N.A., L.A., C.B., I.M.D., K.F., T.G.-D., H.G., A.G., N.G., E.H., K.C., A.K.K., H.N., R.L., N.F.A., I.T., and A.W. contributed patients to the study and edited the manuscript.
Conflict-of-interest disclosure: U.-H.M, P.G., K.R., and A.W. have received honoraria from Janssen and Celgene. J.W. serves on an advisory board for Celgene. The remaining authors declare no competing financial interests.
A complete list of the members of the Nordic Myeloma Study Group appears in “Appendix.”
Correspondence: Ulf-Henrik Mellqvist, Section of Hematology, Sahlgrenska University Hospital, SE-413 45 Gothenburg, Sweden; e-mail: ulf-henrik.mellqvist@vgregion.se.
Appendix: study group members
The members of the Nordic Myeloma Study Group are: Henrik Gregersen (Aalborg, Denmark); Niels Frost Andersen (Aarhus, Denmark); Peter Gimsing and Nilsaage Toffner-Clausen (Copenhagen, Denmark); Niels Abildgaard (Odense, Denmark); Edward Laane (Tallinn, Estonia); Raija Silvennoinen (Tampere, Finland); Kari Remes (Turku, Finland); Hlif Steingrimsdottir (Reykjavik, Iceland); Roald Lindås (Bergen, Norway); Tobias Gedde-Dahl, Nina Guldbrandsen, and Ann Kristin Kvam (Oslo, Norway); Einar Haukås (Stavanger, Norway); Inger Marie Dahl (Tromso, Norway); Oyvind Hjertner and Anders Waage (Trondheim, Norway); Cecilie Blimark, Ulf-Henrik Mellqvist, and Jan Westin (Gothenburg, Sweden); Lucia Ahlberg (Linkoping, Sweden); Stig Lenhoff (Lund, Sweden); Ingemar Turesson (Malmo, Sweden); Olle Linder (Orebro, Sweden); Astrid Gruber and Hareth Nahi (Stockholm, Sweden); Karin Forsberg (Umea, Sweden); and Kristina Carlson (Uppsala, Sweden).