Key Points
Maraviroc intensification unexpectedly increases T-cell activation in peripheral blood and rectal mucosa during treated HIV infection.
Maraviroc appears to redistribute CD8+ T cells from the gut to peripheral blood during treated HIV infection.
Abstract
The CCR5 inhibitor maraviroc has been hypothesized to decrease T-cell activation in HIV-infected individuals, but its independent immunologic effects have not been established in a placebo-controlled trial. We randomized 45 HIV-infected subjects with CD4 counts <350 cells per mm3 and plasma HIV RNA levels <48 copies per mL on antiretroviral therapy (ART) to add maraviroc vs placebo to their regimen for 24 weeks followed by 12 weeks on ART alone. Compared with placebo-treated subjects, maraviroc-treated subjects unexpectedly experienced a greater median increase in % CD38+HLA-DR+ peripheral blood CD8+ T cells at week 24 (+2.2% vs −0.7%, P = .014), and less of a decline in activated CD4+ T cells (P < .001). The % CD38+HLA-DR+ CD4+ and CD8+ T cells increased nearly twofold in rectal tissue (both P < .001), and plasma CC chemokine receptor type 5 (CCR5) ligand (macrophage-inflammatory protein 1β) levels increased 2.4-fold during maraviroc intensification (P < .001). During maraviroc intensification, plasma lipopolysaccharide declined, whereas sCD14 levels and neutrophils tended to increase in blood and rectal tissue. Although the mechanisms explaining these findings remain unclear, CCR5 ligand-mediated activation of T cells, macrophages, and neutrophils via alternative chemokine receptors should be explored. These results may have relevance for trials of maraviroc for HIV preexposure prophylaxis and graft-versus-host disease. This trial was registered at www.clinicaltrials.gov as #NCT00735072.
Introduction
Despite effective antiretroviral therapy (ART), HIV-infected individuals, particularly those with incomplete CD4+ T-cell recovery on ART, continue to have at least a 10-year shorter life expectancy than the general population and remain at higher risk for morbidities associated with aging.1-4 Because immune activation and inflammation persist in most ART-suppressed HIV-infected individuals and predict morbidity and mortality in this setting,5-10 reducing persistent immune activation has emerged as a major priority.
Several lines of evidence suggested that inhibition of CC chemokine receptor type 5 (CCR5) might be a promising approach to reduce persistent immune activation in this setting. First, CCR5 signaling may facilitate trafficking of T cells to areas of inflammation and may lower the threshold for cellular activation.11,12 HIV-infected individuals heterozygous for the CCR5Δ32 mutation also experience slower progression to AIDS and death.13 Furthermore, natural hosts of nonpathogenic simian immunodeficiency virus infection have low CCR5 expression on central memory CD4+ T cells, which has been proposed as a mechanism to explain their lack of immune activation during chronic infection.14-16 Lastly, viremic HIV-infected subjects initiating CCR5 antagonist-containing ART experience greater CD4+ T-cell recovery during early therapy than those randomized to comparator regimens,17,18 an effect hypothesized to be explained by either redistribution of CD4+ T cells into peripheral blood (as a consequence of inhibition of chemotaxis to lymphoid tissues) or a direct effect of CCR5 inhibitors on T-cell activation.18
To assess the direct immunomodulatory effects of maraviroc in vivo, independent of its antiviral effects, we performed a randomized placebo-controlled trial of maraviroc intensification among HIV-infected subjects maintaining ART-mediated viral suppression. We focused on individuals with incomplete CD4+ T-cell recovery (CD4 count <350 cells per mm3) as they tend to have the highest levels of persistent immune activation and are at highest risk for morbidity and mortality. Our a priori hypothesis was that 24 weeks of maraviroc intensification would reduce CD8+ T-cell activation in this setting. We also performed serial rectal biopsies on a subset to determine the effects of maraviroc intensification on gut-associated lymphoid tissue (GALT).
Methods
Trial design, sites, and study subjects
Enrolled subjects were randomized to add either maraviroc or matching placebo to their existing suppressive ART regimen for 24 weeks, followed by 12 weeks of observation on ART alone. The primary outcome was the week 24 change in the % activated (CD38+HLA-DR+) CD8+ T cells. Consenting subjects also participated in a serial rectal biopsy substudy to evaluate the effects of maraviroc intensification on GALT.
Subjects were recruited from 4 study sites (University of California, San Francisco [UCSF]; Stanford University Medical Center; Case Western Reserve University Medical Center; and the Ruth M. Rothstein CORE Center at Rush University) between September 2008 and December 2009. Chronically HIV-infected adults maintaining plasma HIV RNA levels below the limit of detection of the locally available clinical assay for ≥1 year on stable ART and with persistent CD4+ T-cell counts <350 cells per mm3 were eligible. Detectable episodes of viremia <500 copies per mL were allowed in the prior year if they were flanked by confirmed undetectable values. Patients were ineligible if they experienced an increase in CD4+ T-cell count >100 cells per mm3 in the last year and reported <90% adherence to ART; had any serious acute illness in the preceding 3 months; had previously received a CCR5 inhibitor; were pregnant or breastfeeding; or had any of the following laboratory abnormalities: absolute neutrophil count <1000 cells per mm3, platelet count <50 000 cells per mm3, hemoglobin <8 mg/dL, creatinine clearance <40 mL/min, and serum transaminases >2.5 times the upper limit of normal.
The study was approved by the institutional review boards at UCSF, Stanford, Case Western Reserve University Medical Center, and Rush University, and all subjects provided written informed consent in accordance with the Declaration of Helsinki.
Study procedures
Enrolled subjects were randomized (1:1 ratio, stratified by site, block sizes of 2) to receive dose-adjusted maraviroc or placebo, twice daily for 24 weeks, followed by a 12-week observation period. Subjects, clinicians, and laboratory personnel were blinded to treatment assignment. Subjects receiving a protease inhibitor–containing regimen (other than tipranavir) or any other strong cytochrome p450 3A4 (CYP3A4) inhibitor received the 150-mg twice-daily dose. All others received the 600-mg twice-daily dose (if receiving a strong CYP3A4 inducer including efavirenz or etravirine) or the 300-mg twice-daily dose (if receiving neither an inducer nor an inhibitor of CYP3A4). Study visits occurred at weeks 1, 2, 4, 8, 12, 16, 20, 24, 28, and 36. CD4+ and CD8+ T-cell counts, liver function tests, complete blood count with differential, and renal function tests were measured at each study visit. Plasma HIV RNA levels (by clinical assay performed at each site) were measured every 12 weeks during the study. Peripheral blood mononuclear cells (PBMCs), plasma, and serum were cryopreserved at baseline and weeks 4, 24, and 36, for immunology and virology testing.
Laboratory measurements
Plasma HIV RNA levels by single-copy assay (SCA) were assessed on batched cryopreserved plasma. A median of 7.5 mL of plasma (interquartile range [IQR], 6.5-8 mL) was assessed for each sample with a detection limit of <0.3 copies per mL.19 For longitudinal analysis, undetectable values were assigned the lower limit of detection.
T-cell immunophenotyping was performed on cryopreserved PBMCs that were thawed and washed with complete medium, then washed in PBS and stained with LIVE/DEAD Violet (Invitrogen). The activation subset was stained with fluorochrome-labeled monoclonal antibodies against CD4 (Alexa Fluor 700; BD Pharmigen), CD8 (antigen-presenting cell [APC]; BD), HLA-DR (fluorescein isothiocyanate [FITC]; BD), CD38 (phycoerythrin [PE]; BD), CCR5 (clone 2D7 APC–Cyanin [Cy] 7; BD Pharmigen), CD45-RA (FITC; BD Pharmingen), and CCR7 (PE-Cy7; BD Pharmingen). Stained cells were analyzed on a BD LSRII flow cytometer with BD FACSDiva software. Viable cells were sorted by forward and side scatter to identify the lymphocyte population. Markers were analyzed in comparison with gating determined using isotype control antibodies.
Cryopreserved plasma was assessed for lipopolysaccharide (LPS) (QCL-1000; Lonza),20 interleukin 6 (R&D Systems), D-dimer (Diagnostica-Stago), soluble CD14 (sCD14; R&D Systems), soluble CD163 (sCD163; Trillium Diagnostics), and the CCR5 ligand macrophage-inflammatory protein (MIP) 1β (R&D Systems).
Rectal biopsy substudy
At UCSF, consenting subjects underwent flexible sigmoidoscopy with rectal biopsies 2 weeks prior to baseline and at weeks 6 and 22. Thirty 3-mm biopsy pieces of rectal mucosa were obtained at 15 to 20 cm above the anus using a jumbo forceps. Four were formalin-fixed and paraffin-embedded for immunohistochemistry, and 18 were placed immediately in 15 mL of RPMI 1640 with 10% fetal calf serum, piperacillin-tazobactam (500 μg/mL), and amphotericin B (1.25 μg/mL) and transported within 4 to 5 hours to University of California, Davis, where they were processed the same day.21 Rectal mononuclear cells were isolated from biopsy specimens using a protocol optimized for lymphocyte viability and yield.22 Biopsy pieces were digested in 3 rounds of 0.5 mg/mL collagenase type II (Sigma-Aldrich), then tissue was disrupted with a syringe bearing a 16-gauge blunt-end needle and passed through a 70-μm cell strainer. Yields ranged from 4 × 106 to 21 × 106 mononuclear cells from 18 biopsy pieces. Rectal mononuclear cells were stained with antibodies to CD3 (PacBlue; BD), CD4 (APC-Cy5.5; Invitrogen), CD8 (QD605; Invitrogen), CD45RA (Phycoerythrin-Texas Red, ECD; Beckman Coulter), CCR7 (PE-Cy7; BD), CD38 (PE-Cy5; BD), HLA-DR (FITC; BD), CCR5 (PE; BD), CD20 (APC-Cy7; BioLegend), viability (Aqua; Invitrogen) and analyzed with previously described methods.22
Statistical analysis
The primary analysis compared the week 24 change from baseline in the % activated (CD38+HLA-DR+) CD8+ T cells between maraviroc and placebo-treated subjects with a Wilcoxon rank-sum test. Differences between treatment arms in T-cell activation changes (and all other biomarker changes) across all time points were also assessed with linear mixed models, log-transforming the outcome if necessary to satisfy model assumptions (xtmixed in Stata 10, College Station, TX). Changes in slopes before and after week 24 were assessed using linear splines. For all analyses, observations were censored for early discontinuation of ART or study medication.
Sample size determination
The standard deviation of the change in activated CD8+ T cells from baseline to week 8 in our recent trial of valganciclovir in a similar population was 5.6%.25 Assuming this standard deviation and a Type I error of 5%, with 21 subjects in each arm, we expected 80% power to detect a 5-percentage-point difference in the week 24 change in CD8+ T-cell activation between arms, a difference associated with a 1.3-fold greater hazard of subsequent mortality in our recent study of HIV-infected Ugandans with early ART-mediated viral suppression.26 Given 2 premature study drug discontinuations and 1 subject without baseline samples stored, the study was overenrolled by 3 subjects (n = 45 subjects randomized to maraviroc or placebo) to ensure 42 subjects with samples available for the primary end-point analysis.
Results
Subject characteristics
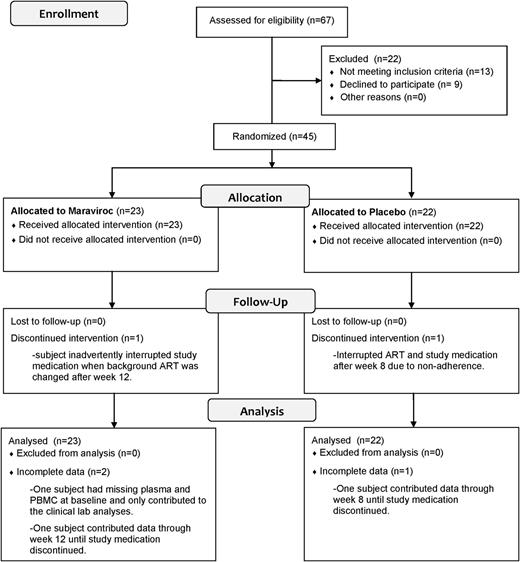
Of 67 screened subjects, 9 refused participation, and 13 were excluded, largely due to incomplete viral suppression or high CD4+ T-cell counts (Figure 1). Baseline characteristics of maraviroc-treated (n = 23) and placebo-treated (n = 22) subjects were comparable between arms (Table 1). Most were men between 40 and 60 years of age and had been receiving a suppressive ART regimen for a median of 30 months. Median CD4+ T-cell count was 205 cells per mm3, and all but 3 subjects had a plasma HIV RNA level <48 copies per mL at the baseline visit (2 placebo-treated subjects with 49 and 72 copies per mL and 1 maraviroc-treated subject with 58 copies per mL).
Enrollment, allocation, and follow-up for trial subjects. The outcomes of the 67 screened subjects are described in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Enrollment, allocation, and follow-up for trial subjects. The outcomes of the 67 screened subjects are described in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Baseline characteristics
| Characteristic . | Placebo median (IQR) n = 22 . | Maraviroc median (IQR) n = 23 . |
|---|---|---|
| Age, years | 50 (43-57) | 50 (46-56) |
| Male gender, no. (%) | 20 (91) | 23 (100) |
| Ethnicity, no. (%) | ||
| White/European American | 14 (64) | 13 (57) |
| Black/African American | 4 (18) | 6 (26) |
| Hispanic/Latino | 3 (14) | 4 (17) |
| Asian | 1 (5) | 0 (0) |
| CD4+ T-cell count, cells per mm3 | 202 (161-256) | 206 (131-260) |
| Self-reported nadir CD4 count, cells per mm3 | 21 (8-41) | 30 (3-90) |
| Plasma HIV RNA level, copies per mL | <48 | <48 |
| Duration of current ART regimen, months | 31 (15-43) | 30 (21-42) |
| Hepatitis C virus antibody positive, no. (%) | 3 (14) | 4 (21) |
| Serum AST level, mg/dL | 27 (22-31) | 25 (17-30) |
| Serum ALT level, mg/dL | 32 (24-41) | 34 (22-41) |
| Characteristic . | Placebo median (IQR) n = 22 . | Maraviroc median (IQR) n = 23 . |
|---|---|---|
| Age, years | 50 (43-57) | 50 (46-56) |
| Male gender, no. (%) | 20 (91) | 23 (100) |
| Ethnicity, no. (%) | ||
| White/European American | 14 (64) | 13 (57) |
| Black/African American | 4 (18) | 6 (26) |
| Hispanic/Latino | 3 (14) | 4 (17) |
| Asian | 1 (5) | 0 (0) |
| CD4+ T-cell count, cells per mm3 | 202 (161-256) | 206 (131-260) |
| Self-reported nadir CD4 count, cells per mm3 | 21 (8-41) | 30 (3-90) |
| Plasma HIV RNA level, copies per mL | <48 | <48 |
| Duration of current ART regimen, months | 31 (15-43) | 30 (21-42) |
| Hepatitis C virus antibody positive, no. (%) | 3 (14) | 4 (21) |
| Serum AST level, mg/dL | 27 (22-31) | 25 (17-30) |
| Serum ALT level, mg/dL | 32 (24-41) | 34 (22-41) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Forty-two of 45 (93%) subjects completed 24 weeks of study medication and had specimens to contribute to the primary analysis. One placebo-treated subject discontinued study medication due to adherence issues, 1 maraviroc-treated subject inadvertently stopped study medication during a change in the subject’s background HIV regimen, and 1 maraviroc-treated subject did not have available samples from the baseline visit.
Changes in low-level viremia
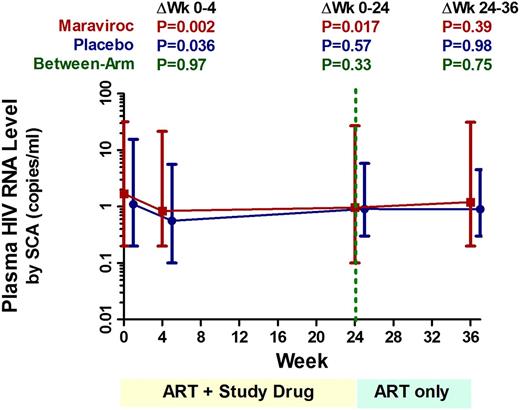
Thirty-five subjects (78%) had adequate plasma volume available for the longitudinal plasma HIV RNA–level assessment by SCA. At baseline, 11 of 17 (65%) placebo-treated subjects and 13 of 18 (72%) maraviroc-treated subjects had detectable viremia (median 2.9 and 2.2 copies per mL, respectively). By week 4 of therapy, the extent of low-level viremia declined by a mean 48% in the placebo arm (95% confidence interval [CI], −4% to −72%; P = .036) and 52% in the maraviroc arm (95% CI, −22% to −68%; P = .002), but there was no evidence for a difference between arms at any time point (Figure 2).
Changes in low-level viremia during maraviroc intensification. Changes in low-level viremia (by SCA) were assessed with linear mixed models for maraviroc-treated (red) and placebo-treated (blue) subjects. Undetectable values were assigned a value equal to the lowest limit of detection for the assay. Estimated mean levels at each time point are plotted with 95% CIs. Mean changes over each indicated interval are also compared both within and between each arm, with P values provided for each comparison.
Changes in low-level viremia during maraviroc intensification. Changes in low-level viremia (by SCA) were assessed with linear mixed models for maraviroc-treated (red) and placebo-treated (blue) subjects. Undetectable values were assigned a value equal to the lowest limit of detection for the assay. Estimated mean levels at each time point are plotted with 95% CIs. Mean changes over each indicated interval are also compared both within and between each arm, with P values provided for each comparison.
Changes in peripheral blood T-cell counts
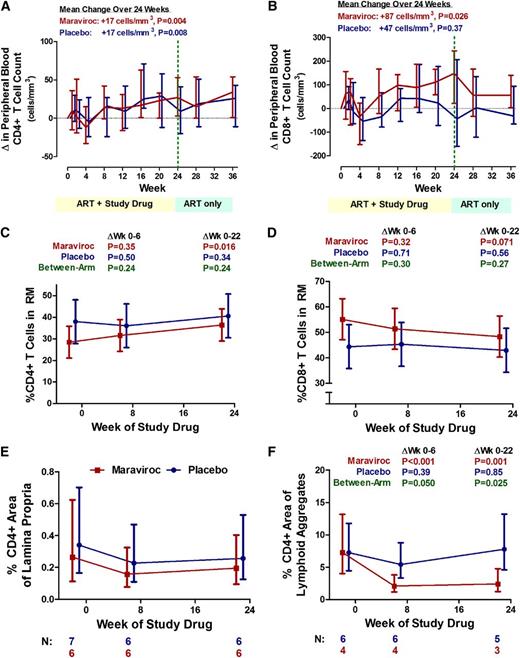
Over 24 weeks, CD4+ T-cell counts increased by a mean +17 cells per mm3 in the placebo arm (95% CI, +7 to +27 cells per mm3; P = .008) and +17 cells per mm3 in the maraviroc arm (95% CI, +7 to +28 cells per mm3; P = .004), with no evidence for a difference between arms (P = .97), or before and after week 24 in the maraviroc arm (P = .56; Figure 3A). Although there was no evidence for a change in CD8+ T-cell counts through week 24 in the placebo arm (P = .37), CD8+ T-cell counts increased by a mean +87 cells per mm3 over 24 weeks in the maraviroc arm (95% CI, +10 to +164 cells per mm3; P = .026) and tended to decline after discontinuing maraviroc at week 24, although this was not statistically significant (P = .27; Figure 3B).
Changes in CD4+ and CD8+ T-cell counts in peripheral blood and rectal tissue during maraviroc intensification. Median changes from baseline in peripheral blood CD4+ (A) and CD8+ (B) T-cell counts are plotted over time for maraviroc-treated (red) and placebo-treated (blue) subjects. Vertical error bars represent IQR at each time point. The mean slopes of change over the indicated intervals (with P values) are also reported. Estimated mean changes (with 95% CI) in % CD4+ (C) and % CD8+ (D) T cells in rectal tissue (by flow cytometry on extracted cells), as well as the % CD4+ area (by immunohistochemistry) in both the lamina propria (E) and lymphoid aggregates (F) of rectal tissue, are also plotted over time for maraviroc-treated (red) and placebo-treated (blue) subjects. P values are provided for the changes within each indicated interval both within arms and between arms. Because only a subset of subjects had baseline rectal tissue with adequate representation of lamina propria and lymphoid aggregates, the number of subjects contributing to each time point is noted below the figure.
Changes in CD4+ and CD8+ T-cell counts in peripheral blood and rectal tissue during maraviroc intensification. Median changes from baseline in peripheral blood CD4+ (A) and CD8+ (B) T-cell counts are plotted over time for maraviroc-treated (red) and placebo-treated (blue) subjects. Vertical error bars represent IQR at each time point. The mean slopes of change over the indicated intervals (with P values) are also reported. Estimated mean changes (with 95% CI) in % CD4+ (C) and % CD8+ (D) T cells in rectal tissue (by flow cytometry on extracted cells), as well as the % CD4+ area (by immunohistochemistry) in both the lamina propria (E) and lymphoid aggregates (F) of rectal tissue, are also plotted over time for maraviroc-treated (red) and placebo-treated (blue) subjects. P values are provided for the changes within each indicated interval both within arms and between arms. Because only a subset of subjects had baseline rectal tissue with adequate representation of lamina propria and lymphoid aggregates, the number of subjects contributing to each time point is noted below the figure.
Changes in rectal T-cell frequency
Because an increase in peripheral blood CD8+ T-cell counts could be explained by either an expansion of CD8+ T cells or a redistribution phenomenon (ie, inhibition of trafficking of CCR5+ CD8+ T cells from peripheral blood to sites of inflammation including the GALT), we assessed rectal CD8+ T-cell frequencies in the 15 subjects participating in the rectal biopsy substudy. Although there was no evidence for a change in the placebo arm, the % CD8+ T cells in rectal tissue tended to decline in the maraviroc arm through week 22 (mean −7%, P for trend = .071), whereas the % CD4+ T cells tended to increase (mean +8%, P = .016).
To confirm that the apparent decline in rectal CD8+ T cells did not simply reflect an increase in CD4+ T-cell density in maraviroc-treated subjects, we assessed rectal CD4+ T-cell density via immunohistochemistry. The lamina propria % CD4+ area tended to decline through week 22 by a similar degree in both arms (Figure 3E), but maraviroc-treated subjects actually experienced a greater decline in the lymphoid aggregate % CD4 area (mean 63% reduction, P = .001) than placebo-treated subjects through week 22 (P = .025; Figure 3F). Collectively, these results support the hypothesis that maraviroc blocks the trafficking of CD8+ T cells from peripheral blood to the gut mucosa and may also decrease T-cell trafficking to lymphoid aggregates within the gut during treated HIV infection.
Changes in peripheral blood T-cell activation
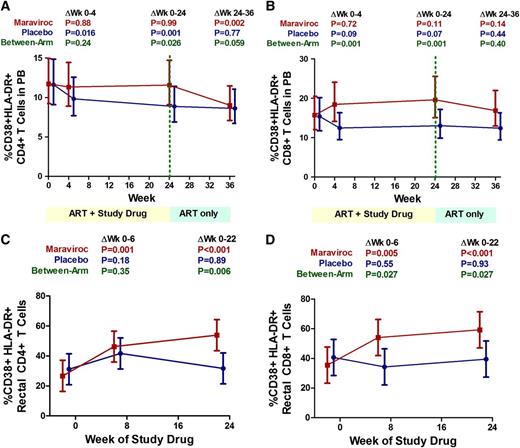
At baseline, the placebo-treated subjects tended to have a lower median % activated (CD38+HLA-DR+) CD8+ T cells (14.1%; IQR, 10% to 19%) than maraviroc-treated subjects (20.4%; IQR, 17% to 25%; P = .08). Although the frequency of activated CD8+ T cells at week 24 tended to decline from baseline in the placebo arm (median change, −0.7%; IQR, −3.5% to +0.6%; P = .13), maraviroc-treated subjects experienced a median +2.2% increase in the % activated CD8+ T cells at week 24 (IQR, −0.6% to + 4.1%; P = .050), significantly greater than the placebo arm (P = .014). Relative changes in CD8+ T-cell activation were also assessed across all time points with linear mixed models. Compared with placebo-treated subjects, maraviroc-treated subjects experienced a greater increase in CD8+ T-cell activation at both week 4 (P = .001) and week 24 (P = .001; Figure 4B).
Changes in peripheral blood and rectal T-cell activation during maraviroc intensification. Estimated mean changes (with 95% CI) in the frequency of activated (CD38+HLA-DR+) CD4+ (A) and CD8+ (B) T cells in peripheral blood are plotted for placebo-treated (blue) and maraviroc-treated (red) subjects. Estimated mean changes (with 95% CI) in the frequency of activated CD4+ (C) and CD8+ (D) T cells in rectal tissue are also plotted for both arms. P values are provided for the changes within each indicated interval both within arms and between arms.
Changes in peripheral blood and rectal T-cell activation during maraviroc intensification. Estimated mean changes (with 95% CI) in the frequency of activated (CD38+HLA-DR+) CD4+ (A) and CD8+ (B) T cells in peripheral blood are plotted for placebo-treated (blue) and maraviroc-treated (red) subjects. Estimated mean changes (with 95% CI) in the frequency of activated CD4+ (C) and CD8+ (D) T cells in rectal tissue are also plotted for both arms. P values are provided for the changes within each indicated interval both within arms and between arms.
We also compared activated CD4+ T-cell changes between arms. At baseline, the median % activated CD4+ T cells was comparable between placebo-treated (11.5%; IQR, 7.5% to 15.7%) and maraviroc-treated subjects (11.8%; IQR, 7.5% to 18.2%; P = .94). From baseline to week 24, the placebo arm experienced a mean 25% relative decline in the % activated CD4+ T cells (P < .001), whereas there was no evidence for a change in the maraviroc arm (P = .98, P for between-arm difference = .026; Figure 4A). Rather, maraviroc-treated subjects experienced a mean 22% relative decline in activated CD4+ T cells after maraviroc was discontinued at week 24 (P = .002). Thus, maraviroc appeared to prevent the decline in CD4+ T-cell activation observed in the placebo arm.
Maraviroc-mediated increases in peripheral blood T-cell activation appeared to be driven by increases in HLA-DR expression, with minimal evidence for differences in CD38 expression between arms (supplemental Table 1; see the Blood Web site). Although the primary analysis was performed in batches on cryopreserved PBMCs, the 15 subjects participating in the rectal biopsy substudy also had T-cell activation measured on PBMCs isolated from freshly obtained whole blood. Although underpowered in this smaller subset, we observed similar trends in peripheral blood T-cell activation changes between arms when assessed on fresh PBMCs (supplemental Table 2).
Changes in rectal T-cell activation
We next assessed the impact of maraviroc intensification on rectal T-cell activation among the 15 subjects in the rectal biopsy substudy. We hypothesized that the immunologic effects of CCR5 inhibition would be more dramatic in GALT because a much larger fraction of CD4+ and CD8+ T cells in GALT express CCR5 than in peripheral blood, regardless of maturational state (supplemental Figure 3).
Compared with placebo-treated subjects, maraviroc-treated subjects had a similar median baseline % activated (CD38+HLA-DR+) CD4+ T cells (26.8% vs 32.1%, P = .65) and CD8+ T cells (44.1% vs 41.3%, P = .75) in rectal mucosa. There was no evidence for a change in the % activated CD4+ or CD8+ T cells in rectal tissue among placebo-treated subjects at any time point (P ≥ .18; Figure 4C-D). However, maraviroc-treated subjects experienced a mean 27-percentage-point increase in the % activated CD4+ T cells (95% CI, +16% to +39%; P < .001) and a mean 24-percentage-point increase in the % activated CD8+ T cells in rectal tissue (95% CI, +11 to +37%; P < .001). These maraviroc-mediated changes correspond to an approximately twofold increase in CD4+ and a 1.7-fold increase in CD8+ rectal T-cell activation and were significantly greater than those observed in the placebo arm (P = .011 and P = .041, respectively).
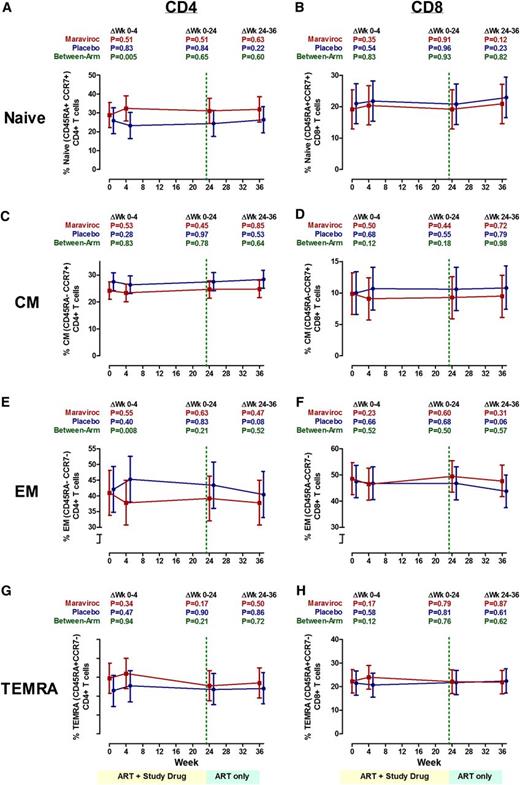
Changes in frequencies of T-cell maturational phenotypes
Because maraviroc appeared to affect T-cell trafficking, we assessed whether a change in maturational phenotype frequencies might explain the maraviroc-mediated increases in peripheral blood and rectal T-cell activation. However, we observed no evidence for a change in naïve (CD45RA+CCR7+), central memory (CD45RA–CCR7+), effector memory (CD45RA–CCR7–), or terminally differentiated effector memory (CD45RA+CCR7–) CD4+ or CD8+ T cells in the peripheral blood of maraviroc-treated subjects at any time point during the study (Figure 5). Although there appeared to be a greater increase in % naive CD4+ T cells and a greater decrease in % effector memory CD4+ T cells in the maraviroc arm at week 4 than in the placebo arm, these differences were no longer apparent at week 24, and the changes within each arm were not significant. There was also no evidence for a difference in rectal T-cell maturational subset changes between maraviroc- and placebo-treated subjects through 22 weeks (P > .33 for all; supplemental Table 4).
Changes in the frequencies of T-cell maturational phenotypes during maraviroc intensification. Estimated mean changes in naive (CD45RA+CCR7+) (A-B), central memory (CD45RA–CCR7+) (C-D), effector memory (CD45RA–CCR7–) (E-F), and terminally differentiated effector memory (TEMRA) (CD45RA+CCR7–) (G-H) CD4+ (A,C,E,G) and CD8+ (B,D,F,H) T-cell frequencies in peripheral blood (with 95% CI) are plotted for maraviroc-treated (red) and placebo-treated (blue) subjects over time. P values are provided for the changes within each indicated interval both within arms and between arms.
Changes in the frequencies of T-cell maturational phenotypes during maraviroc intensification. Estimated mean changes in naive (CD45RA+CCR7+) (A-B), central memory (CD45RA–CCR7+) (C-D), effector memory (CD45RA–CCR7–) (E-F), and terminally differentiated effector memory (TEMRA) (CD45RA+CCR7–) (G-H) CD4+ (A,C,E,G) and CD8+ (B,D,F,H) T-cell frequencies in peripheral blood (with 95% CI) are plotted for maraviroc-treated (red) and placebo-treated (blue) subjects over time. P values are provided for the changes within each indicated interval both within arms and between arms.
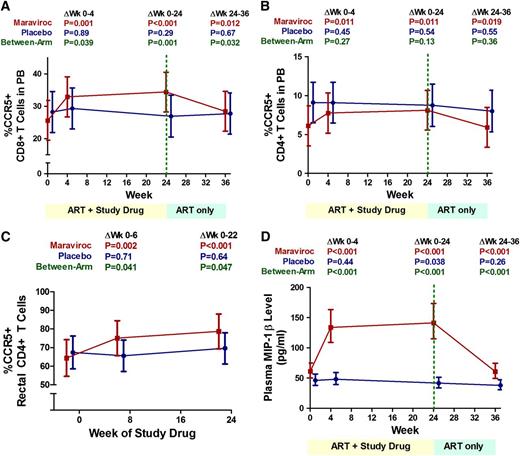
Changes in T-cell CCR5 expression and plasma CCR5 ligand levels
Because maraviroc intensification had the opposite effect on T-cell activation in vivo than we had initially hypothesized, we sought to explore potential mechanisms. It is unlikely that maraviroc was acting as a partial agonist because no signaling through CCR5 was detected in T cells cultured with maraviroc in prior in vitro studies.27 We thus explored indirect mechanisms whereby maraviroc intensification might increase T-cell activation. Because CCR5 receptor-ligand complexes are typically internalized by the cell after binding, the blockade of the receptor-ligand interaction by maraviroc and other CCR5 inhibitors prevents the internalization of both CCR5 and its natural ligands, resulting in an increase in CCR5 on the cell surface as well as an increase in circulating CCR5 ligands.28,29 Although increases in CCR5 ligands (MIP-1α, MIP-1β, and regulated on activation normal T expressed and secreted [RANTES]) are unlikely to affect signaling through CCR5 while maraviroc is bound, these ligands also bind other chemokine receptors including CCR3 and CCR4 on T cells, and CCR1 on monocytes and neutrophils.30-32 We hypothesized that an increase in CCR5 ligand concentrations during maraviroc therapy might result in increased CCR3- and CCR4-mediated activation of T cells and CCR1-mediated activation of monocytes and neutrophils.33-35
Although insufficient PBMCs remained to assess these effects directly, we did assess changes in surface CCR5 expression on T cells and plasma levels of the CCR5 ligand MIP-1β. Although there was no evidence for a change in any of these measurements in the placebo arm, maraviroc intensification resulted in a mean 9-percentage-point increase in the % CCR5+ CD8+ T cells (95% CI, +5% to +14%; P < .001; Figure 6A) and a mean 2.4-percentage-point increase in the % CCR5+ CD4+ T cells in peripheral blood (95% CI, +0.5% to +4.3%; P = .012; Figure 6B). In rectal tissue, maraviroc intensification also increased the % CCR5+ CD4+ T cells (P < .001; Figure 6C) as well as the % CCR5+ B cells at week 22 (P < .001, not shown). CCR5 median fluorescence intensity increased approximately twofold on CCR5+ CD4+ and CD8+ T cells both in peripheral blood and rectal tissue by week 6 of maraviroc intensification (P < .001 for all), although these increases were attenuated by week 22, with only rectal CD4+ T cells exhibiting higher CCR5 fluorescence intensity than baseline at week 22 (P = .028). Maraviroc intensification also resulted in a mean 2.4-fold increase in serum levels of the CCR5 ligand MIP-1β through week 24 (95% CI, 2.0- to 3.0-fold increase; P < .001) that reversed after maraviroc cessation (P < .001; Figure 6D).
Changes in the frequency of CCR5+ T cells and plasma MIP-1β levels during maraviroc intensification. Changes in the % CCR5+ CD8+ T cells (A) and CD4+ T cells (B) in peripheral blood, the % CCR5+ CD4+ T cells in rectal tissue (C), and plasma levels of the CCR5 ligand MIP-1β (D) were assessed in placebo-treated (blue) and maraviroc-treated (red) subjects with linear mixed models. Estimated mean values are plotted over time with 95% CIs. P values are provided for the changes within each indicated interval both within arms and between arms.
Changes in the frequency of CCR5+ T cells and plasma MIP-1β levels during maraviroc intensification. Changes in the % CCR5+ CD8+ T cells (A) and CD4+ T cells (B) in peripheral blood, the % CCR5+ CD4+ T cells in rectal tissue (C), and plasma levels of the CCR5 ligand MIP-1β (D) were assessed in placebo-treated (blue) and maraviroc-treated (red) subjects with linear mixed models. Estimated mean values are plotted over time with 95% CIs. P values are provided for the changes within each indicated interval both within arms and between arms.
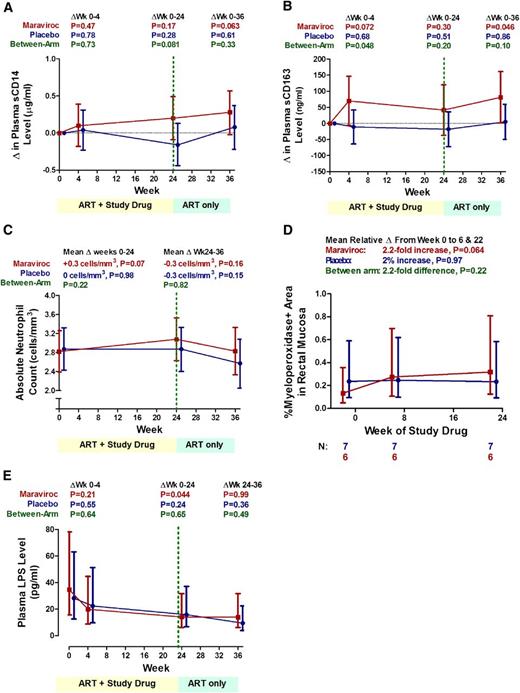
Changes in monocyte activation, neutrophil levels, and plasma LPS levels
If maraviroc-mediated increases in CCR5 ligands were activating monocytes and neutrophils via CCR1, we expected to observe increases in monocyte activation and neutrophil levels in peripheral blood and gut of maraviroc-treated subjects. Although there was no evidence for a change in the placebo arm, sCD14 tended to increase through week 36 in the maraviroc arm (P = .063), and to a greater degree than the placebo arm at week 24 (P = .081; Figure 7A). Similarly, although there was no evidence for a change in the placebo arm, plasma sCD163 tended to increase by a mean 70 ng/mL at week 4 (P = .072) and 79 ng/mL at week 36 (P = .046) in the maraviroc arm, approaching statistical significance compared with the placebo arm at week 4 (P = .048; Figure 7B). However, there was no evidence for a difference in sCD163 levels between arms at week 24.
Changes in monocyte activation, neutrophil levels, and plasma LPS levels during maraviroc intensification. Changes in plasma sCD14 (A), sCD163 levels (B), peripheral blood absolute neutrophil counts (C), rectal tissue myeloperoxidase density (a surrogate marker for neutrophil density) (D), and plasma LPS levels (E) are plotted over time for both maraviroc-treated (red) and placebo-treated subjects (blue). Mean changes from baseline (A-B) or estimated mean values (C-E) with 95% CI from linear mixed models are plotted. P values are provided for the changes within each indicated interval both within arms and between arms. Given more frequent observations, changes in peripheral blood neutrophil counts were modeled as a linear spline with a change point at week 24.
Changes in monocyte activation, neutrophil levels, and plasma LPS levels during maraviroc intensification. Changes in plasma sCD14 (A), sCD163 levels (B), peripheral blood absolute neutrophil counts (C), rectal tissue myeloperoxidase density (a surrogate marker for neutrophil density) (D), and plasma LPS levels (E) are plotted over time for both maraviroc-treated (red) and placebo-treated subjects (blue). Mean changes from baseline (A-B) or estimated mean values (C-E) with 95% CI from linear mixed models are plotted. P values are provided for the changes within each indicated interval both within arms and between arms. Given more frequent observations, changes in peripheral blood neutrophil counts were modeled as a linear spline with a change point at week 24.
Although there was no evidence for a change in the placebo arm, maraviroc-treated patients tended to experience an increase in peripheral blood neutrophil counts through week 24 (mean +0.3 cell per mm3, P = .07) and a decline in the 12 weeks after discontinuing maraviroc (mean −0.3 cell per mm3, P = .16, P for interaction for slopes before and after week 24 = .09; Figure 7C). However, the difference between arms was not statistically significant. Although we observed no evidence for a change in rectal neutrophil density (% meyloperoxidase+ area) in placebo-treated subjects (P = .97), maraviroc-treated subjects tended to experience a mean 2.2-fold increase in rectal neutrophil density at postbaseline time points (P = .064), although the differences between arms was not statistically significant (Figure 7D).
If maraviroc-mediated increases in CCR5 ligands were increasing monocyte and neutrophil activation via CCR1, we also expected to observe a greater decline in plasma LPS levels in the maraviroc arm due to increased clearance by these cells. Indeed, LPS declined by a mean 59% from baseline to week 24 in the maraviroc arm (95% CI, −3% to −83%; P = .044; Figure 7E), although comparable, albeit not statistically significant, changes were observed in the placebo arm. Although power was limited, there was also no evidence for a difference in interleukin 6 or D-dimer changes between arms (supplemental Table 5).
Adverse events
Overall, the study medication was well tolerated. Two maraviroc-treated and 1 placebo-treated subjects had grade 3 elevations in indirect serum bilirubin levels during treatment, but all were receiving atazanavir, and none represented significant changes from baseline. One maraviroc-treated subject developed an isolated grade 3 triglyceride elevation, but this was similar to values prior to study entry. One maraviroc-treated subject developed fevers without a clear source for ∼2 weeks prior to his week 20 visit. However, the fevers resolved spontaneously, and the patient had continued on study medications without recurrence.
Discussion
Although observational studies and theoretical considerations have suggested that CCR5 blockade might have beneficial immunologic effects in HIV-infected individuals, the immunologic effects of CCR5 inhibition, independent of its antiviral effects, have yet to be characterized in a placebo-controlled trial. We performed a randomized placebo-controlled trial of maraviroc intensification among HIV-infected individuals with incomplete CD4+ T-cell recovery during ART-mediated viral suppression. Maraviroc intensification did not affect the rate of CD4+ T-cell recovery but increased CD8+ T-cell counts in peripheral blood and decreased CD8+ T cells in rectal tissue, compatible with a redistribution effect. Contrary to our initial hypothesis, maraviroc intensification caused a nearly twofold increase in rectal T-cell activation and more modest but statistically significant increases in peripheral blood T-cell activation. Maraviroc intensification also increased plasma levels of the CCR5 ligand MIP-1β by 2.4-fold and tended to increase monocyte activation and neutrophil levels. Although the clinical implications and the mechanisms explaining these effects remain unclear, these results suggest that CCR5 inhibition can have unanticipated effects in vivo that can only be fully characterized by carefully designed clinical trials.
Our primary results differ from 4 recent uncontrolled trials, each of which reported that adding maraviroc to suppressive ART decreased T-cell activation.36-39 However, none of these studies was placebo-controlled, and subjects often improve their ART adherence when entering clinical trials, which may have explained the reduction in low-level viremia experienced by placebo-treated subjects in our own trial and in another recently reported intensification study.40 The apparent reduction in T-cell activation during maraviroc intensification reported in the uncontrolled AIDS clinical trials group study (A5256) was also primarily driven by declines in CD38 expression, whereas HLA-DR expression actually increased significantly (which is largely consistent with our results), and neither of those changes were confirmed when retesting cryopreserved PBMCs from that trial.36 The only other placebo-controlled trial of maraviroc intensification did not find any evidence for a difference in T-cell activation between placebo- and maraviroc-treated subjects, although final results have not yet been published.41 Lastly, although treatment-naive individuals randomized to maraviroc-based ART tended to experience a somewhat earlier decline in T-cell CD38 expression than those randomized to efavirenz-based ART in the recent Maraviroc vs Efavirenz in treatment-naive patients (MERIT) trial, these early changes were not statistically significant, there was no evidence for a difference in CD38 expression at week 48, and HLA-DR was not assessed.42
The mechanisms by which maraviroc increased T-cell activation in our trial remain unclear. A partial agonist effect of maraviroc on CCR5+ T cells is unlikely because maraviroc had no effect on intracellular signaling in T cells in prior in vitro studies.27,43 Maraviroc also did not appear to alter the distribution of T-cell maturational subsets, so T-cell redistribution is unlikely to explain the observed maraviroc-mediated increases in T-cell activation. We hypothesize that the maraviroc-associated increases in T-cell activation may be mediated by the dramatic increase in plasma CCR5 ligands observed during maraviroc intensification. As other groups have reported, maraviroc and other CCR5 antagonists inhibit the binding of the natural CCR5 ligands MIP-1α, MIP-1β, and RANTES to CCR5.28,29 Because CCR5 receptor-ligand complexes are typically internalized by the cell after initial binding, the blockade of the receptor-ligand interaction prevents the internalization of both CCR5 and its ligands, resulting in an increase in CCR5 on the cell surface as well as an increase in circulating levels of CCR5 ligands,28,29 as we observed in our trial. Presumably, this increase in CCR5 ligands would not have a physiological consequence on CCR5-mediated signaling because the increases in ligand are occurring as a consequence of CCR5 blockade. However, RANTES may also signal through CCR3 and CCR4 on T cells, whereas MIP-1α, MIP-1β, and RANTES may all signal through CCR1 on neutrophils and monocytes/macrophages.30-32 Such an effect might explain the maraviroc-mediated increase in microglial activation recently reported in an in vitro study.44 Further studies are necessary to assess whether maraviroc-mediated increases in CCR5 ligands activate T cells via CCR3 and/or CCR4, and monocytes and neutrophils via CCR1.33-35
Although we didn’t have enough remaining samples to evaluate these effects directly, we made several observations that support the hypothesis that maraviroc-mediated increases in CCR5 ligands might be activating monocytes, macrophages, and neutrophils via CCR1. For example, maraviroc tended to increase the monocyte/macrophage activation markers sCD14 and sCD163 and tended to increase neutrophils in peripheral blood and rectal tissue. Although most of these changes were modest in magnitude and not formally statistically significant, they generate intriguing hypotheses that can be addressed in future studies.
It is also important to note that the observed maraviroc-associated increases in T-cell activation were much more dramatic in GALT than in peripheral blood. This is perhaps not surprising because T cells are more likely to express CCR5 in GALT. During maraviroc intensification, we observed a twofold increase in MIP-1β levels in peripheral blood, where only a small fraction of T cells express CCR5. Although we could not quantify the levels of CCR5 ligands in rectal tissue, it is reasonable to hypothesize that there would be an even greater increase in CCR5 ligands in GALT because CCR5 ligand-receptor internalization would presumably be blocked on a much larger number of cells. The maraviroc-mediated increase in activated CD4+ T cells in rectal tissue might also have important implications for studies of CCR5 inhibitors as preexposure prophylaxis against HIV infection. Although CCR5 inhibition should prevent HIV entry via CCR5, the increased frequency of activated CD4+ T-cell targets in rectal tissue might increase the risk of HIV acquisition through unprotected anal receptive sex, particularly among individuals with suboptimal adherence or those exposed to CXC chemokine receptor type 4–using viruses. However, the effect of maraviroc on rectal T-cell activation may well be different in HIV-uninfected individuals, who have much lower levels of microbial translocation and background T-cell activation, a hypothesis to be explored in an HIV Prevention Trials Network study. That the overall effect of maraviroc on rectal T-cell activation may depend on the clinical context is further underscored by a recent trial demonstrating that maraviroc decreased gut-associated graft-versus-host disease in HIV-uninfected stem cell transplant recipients, an effect presumably mediated by decreased trafficking of donor-derived T cells to the gut.44
It is also notable that although CD8+ T-cell counts increased during maraviroc intensification, we saw no evidence for a difference in the rate of CD4+ T-cell recovery between maraviroc and placebo arms. This appears to be inconsistent with studies of maraviroc-containing regimens in viremic and/or treatment naive individuals, where an increase of 25 to 30 cells per mm3 in CD4+ T-cell counts was consistently observed in the first 3 months of therapy with CCR5 inhibitor-containing regimens relative to comparator arms.17 One possibility is that maraviroc-mediated redistribution of T cells may require a threshold level of CCR5 expression on T cells. Because CCR5 expression declines with suppressive ART, CCR5 expression levels on CD4+ T cells in ART-suppressed individuals may be below a threshold where CCR5-mediated signaling significantly contributes to T-cell trafficking. CCR5 expression is consistently higher on CD8+ T cells than CD4+ T cells, even during treatment-mediated viral suppression, which may explain why maraviroc appeared to prevent CD8+ but not CD4+ T-cell redistribution. Even still, maraviroc intensification may cause subtle redistribution effects of CD4+ T cells within the gut (where CCR5 expression levels remain high even during ART) as suggested by the significant reduction in the lymphoid aggregate CD4+ area observed in our trial.
Lastly, it is important to acknowledge that the clinical implications of the maraviroc-mediated increases in T-cell activation that we observed remain unclear. Maraviroc was well tolerated in large clinical trials of treatment-naive and treatment-experienced patients.18,45 Ongoing trials of maraviroc powered to assess its impact on clinical events will be helpful to clarify the clinical implications of our findings.
In summary, we found that maraviroc intensification causes a nearly twofold increase in T-cell activation in rectal tissue and more modest but statistically significant increases in peripheral blood. Although the clinical implications and the precise mechanisms explaining these effects remain unclear, these data suggest that chemokine receptor inhibition can have unexpected effects in vivo. Ongoing clinical end-point studies will be required to determine the clinical significance of these findings and to further explore the direct immunologic effects of maraviroc-mediated increases in CCR5 ligands in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the study subjects who volunteered for this trial as well as the study clinicians and staff who contributed to subject recruitment, retention, and follow-up (including Elilta Hagos, Rebecca Hoh, Jane Baum, Debbie Slamowitz, Julia Lee, and Robert Shafer among others); study medication management and dispensing (Joy Madamba, Marty Hamilton, Michael Banchy, and Hamid Bouiri); data and specimen management (Melissa Krone, Tricia Walton, Michelle Gallagher, Mieoak Bahk, Kirsten Brady, and Cindy Padilla); and laboratory assays (Kathy Medvik and Carey Shive). The authors also thank the members of the data and safety monitoring board (Richard W. Price, Annie Luetkemeyer, and Susa Coffey); the Cleveland Immunopathogenesis Consortium for helpful discussions about pathophysiological mechanisms; and in particular Danny Douek, Jason Brenchley, and Netanya Sandler for discussions about LPS clearance.
This study was funded by investigator-initiated research grants from Pfizer Inc. and the American Foundation for AIDS Research (amfAR; http://www.amfar.org/) (106856-42-RGIM and 107170-44-RGRL). Additional support was provided from the National Institutes of Health (AI36219, AI057020, P01 AI076174, CA157929, AI087035, P30 AI27763, P30AI08215, and UL1 RR024131).
The sponsors of this study had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: P.W.H., N.S.S., and S.G.D. served as the chief investigators; P.W.H., N.S.S., A.L.L., M.M.L., and S.G.D. designed the trial and developed the protocol; P.W.H. and J.N.M. developed the statistical analysis plan; P.W.H., N.S.S., O.A., L.E.G., B.R., M.M.L., and S.G.D. recruited the patients; M.S. supervised the rectal biopsy substudy; T.L.H., V.D., N.T.F., B.M., B.C., T.W.S., B.L.S., S.P., and M.M.L. performed the laboratory investigations; and P.W.H. coordinated the data collection and regulatory requirements, performed the statistical analysis, generated the tables and figures, interpreted the data, and wrote the first draft of the manuscript; and all authors reviewed, revised, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter W. Hunt, UCSF Positive Health Program, SFGH Building 80, Ward 84, 995 Potrero Ave, San Francisco, CA 94110; e-mail: phunt@php.ucsf.edu.