To the editor:
In an interesting article, Hunt et al described the effect of the addition of the CC chemokine receptor 5 (CCR5) inhibitor maraviroc to the antiretroviral regimen of 23 HIV-1–infected individuals who incompletely restored their CD4 counts under therapy.1 They observed that the administration of maraviroc, by blocking ligand-induced CCR5 internalization, resulted in an increase in the plasma level of the CCR5-binding chemokine macrophage inflammatory protein-1β/CC chemokine ligand 4 (MIP-1β/CCL4). They proposed that such free CCR5 ligands might activate T cells, monocytes/macrophages, and neutrophils. Actually, they noticed an increase in markers of T-cell and monocyte/macrophage activation, as well as in peripheral blood neutrophil counts. They hypothesized then that this overactivation could be responsible for the reduction they observed in plasma lipopolysaccharide, a bacterial product that takes advantage of HIV-induced immune barrier damage to translocate from the gut into the organism.
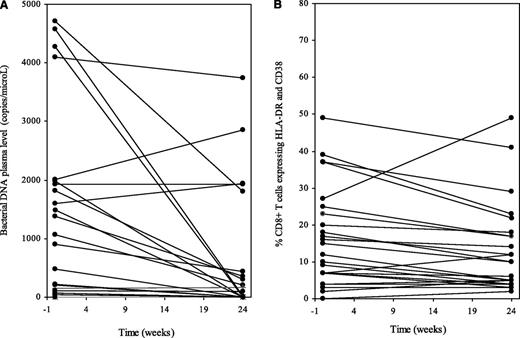
In a recent article, we also described the effect of treatment intensification with maraviroc in 60 nonimmunologic responders.2 We have now quantified bacterial DNA in the plasma of 24 of these patients by polymerase chain reaction performed on the bacterial ribosomal 16S RNA gene (16S rDNA).3 Before intensification, 16S rDNA plasma levels were linked to the plasma levels of the inflammatory chemokines MIP-1β/CCL4 (Spearman correlation = 0.464, P = .008) and RANTES (regulated on activation normal T-cell expressed and secreted)/CCL5 (Spearman correlation = 0.607, P < .001). In line with Hunt et al’s data, the level of 16S rDNA in the plasma decreased after 6 months of maraviroc (P < .001, Figure 1A). Yet, the percentage of fresh CD8+ T cells coexpressing HLA-DR and CD38 tended to decrease during maraviroc intensification (from 10.2 [interquartile range, 6-22] to 10.0 [4-18], P = .06, Figure 1B). Plasma levels of soluble CD14 did not increase (from 13 281 [interquartile range, 10 605-16 632] to 13 636 [10 646-16 249] pg/mL, P = .950).
Effect of the CCR5 antagonist maraviroc on circulating bacterial DNA level and on immune activation. Bacterial 16S ribosomal DNA plasma levels (A) and percentage of CD8+ T cells coexpressing HLA-DR and CD38 (B) in nonimmunologic responders to antiretroviral therapy to whom the CCR5 antagonist maraviroc was added for 6 months.
Effect of the CCR5 antagonist maraviroc on circulating bacterial DNA level and on immune activation. Bacterial 16S ribosomal DNA plasma levels (A) and percentage of CD8+ T cells coexpressing HLA-DR and CD38 (B) in nonimmunologic responders to antiretroviral therapy to whom the CCR5 antagonist maraviroc was added for 6 months.
Thus, in our study, the maraviroc-induced decrease in circulating levels of bacterial products was not associated with an increase in immune activation. This raises the interesting hypothesis that, given its fair gut-associated lymphoid tissue penetration,4 maraviroc might have a direct effect on microbial translocation. Although our trial was not placebo-controlled, we do not believe that the drastic drop in circulating bacterial DNA we observed between week 0 and week 24 was the consequence of an improvement in treatment adherence because we did not observe any decrease in the plasma 16S rDNA between enrollment and intensification start (data not shown). Additional work is needed to clarify the mechanism responsible for the effect of maraviroc on the plasma load of bacterial compounds and the versatile effect of maraviroc on T-cell and monocyte activation. Apart from technical issues, the latter might depend on the baseline level of immune activation, as suggested by Hunt et al.1 Of note, in their study, the preintensification duration of treatment was 4 times shorter, and the percentage of HLA-DR+CD38+CD8+ T cells at week 0 was 50% higher than in our study.
Authorship
Acknowledgments: The authors are grateful to all of the investigators who participated in the MARIMUNO assay.
Contribution: C.P. and B.A. were in charge of the immunological part of the study; J.-P.L. quantified 16S rDNA; C.B., J.G., C.L.-C., L.C., and J.R. were in charge of the study in their clinical centers; S.T. was the project manager; P.F. performed the statistical analysis; and P.C. was the principal investigator of this substudy of the MARIMUNO-ANRS 145 study and wrote the manuscript.
Conflict-of-interest disclosure: C.P., J.R., and P.C. have received honoraria for lectures from ViiV Healthcare. J.R. and P.C. have been advisory board members for ViiV Healthcare. P.C. received a research grant from ViiV Healthcare. J.G. has received honoraria and support for travel expenses from ViiV Healthcare. The remaining authors declare no competing financial interests.
Correspondence: Pierre Corbeau, Institute for Human Genetics, CNRS UPR1142, 141 rue de la Cardonille, 34396 Montpellier, France; e-mail: pierre.corbeau@igh.cnrs.fr.