MicroRNAs (miRNAs) are established posttranscriptional regulators of the protein content inside of a cell. In this issue of Blood, He et al identify a provocative new function of exogenous miRNAs in promoting natural killer–cell activation by stimulating Toll-like receptor–1 signals.1
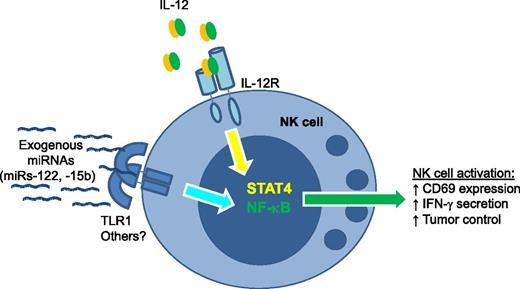
NK-cell activation by exogenous miRNAs depends on TLR1. Specific miRNA sequences, including miR-122 and miR-15b, act in a TLR1-dependent fashion to induce nuclear factor-κB signals in human NK cells. This signal works in concert with IL-12 (canonically signaling via STAT4) to induce NK-cell activation. miRNA–TLR1-based NK-cell coactivation results in increased CD69 surface expression, augmented IFN-γ secretion, and enhanced lymphoma cell line control in mice in vivo.
NK-cell activation by exogenous miRNAs depends on TLR1. Specific miRNA sequences, including miR-122 and miR-15b, act in a TLR1-dependent fashion to induce nuclear factor-κB signals in human NK cells. This signal works in concert with IL-12 (canonically signaling via STAT4) to induce NK-cell activation. miRNA–TLR1-based NK-cell coactivation results in increased CD69 surface expression, augmented IFN-γ secretion, and enhanced lymphoma cell line control in mice in vivo.
miRNAs are a familiar mechanism used by hematopoietic and immune cells to regulate protein output by messenger RNAs (mRNAs).2 These small regulatory RNAs are transcribed as primary miRNAs from encoded genes, undergo complex biogenesis and nuclear export, and ultimately end up in the RNA-induced silencing complex destined to target specific mRNAs. Typically this is mediated via mRNA degradation or by inhibiting translation.3 Hundreds of unique miRNA genes and sequences have been cataloged, with thousands predicted in the human genome. Most work in the miRNA field, including that of natural killer (NK) cells,4 focuses on their regulatory capacity inside the cell—how miRNA expression is controlled, what mRNAs are targeted by a given miRNA, and the impact of coordinated mRNA repression on responses at the cellular and molecular levels. However, studies have repeatedly identified miRNA sequences in extracellular fluids, including the plasma, serum, and cerebral spinal fluid. Most of these studies have focused on the potential of free miRNAs as biomarkers of disease progression or recurrence, including many hematologic malignancies.5 What else might these small RNAs be doing in the circulation? Are they simply markers of the cells that transcribe them, or could they be used by the host to recognize and respond to the presence of disease? Few studies have explored the functional impact of miRNA molecules that are found to routinely circulate in humans outside the cell.
The Yu and Caligiuri laboratories asked just that question: What is the potential for “free” miRNAs to activate immune cells, in this case, NK cells?1 These investigators synthesized miRNAs commonly identified in the circulation, added these exogenous miRNAs to the culture medium of human NK cells, and observed dose-dependent NK cell activation when miRNAs (specifically, miRs-122, -15b, -21, and -155) were combined with interleukin (IL)-12. While induction of the nonspecific activation marker CD69 and interferon-gamma (IFN-γ) secretion were costimulated by exogenous miRNAs, there appeared to be a minimal impact on promoting NK-cell cytotoxicity against tumor targets. Next, mice were injected with miRs-15b or -122, and NK cells exhibited increased CD69 expression, but again this resulted in only very modest changes in degranulation or cytotoxicity. Notably, exogenous miRNAs failed to activate T cells, providing a clear negative control for these in vivo experiments. The authors went on to challenge mice with a luciferase-expressing A20 lymphoma cell line with or without pretreatment via intravenous injection of liposomal miR-122. Bioluminescent imaging of tumor-challenged mice revealed a reduction in A20 cell burden in miR-122 compared with vehicle injected controls; an effect that was diminished if NK cells were depleted. Thus, exogenous provision of miR-122 promoted NK-cell–dependent antitumor responses in vivo.
What is the mechanism whereby NK cells sensed exogenous miRNAs? Based on known target specificities of TLRs, the authors hypothesized that this receptor family was contributing to NK-cell activation. The mRNAs of TLRs 1, 3, and 6 were identified in NK cells, and unexpectedly the blockade of TLR1 by anti-TLR1 monoclonal antibodies partially reduced miR-122–costimulated IFN-γ production as well as downstream phosphorylation of the p65 component of the nuclear factor-κB pathway. These data suggest that NK cells use TLR1 to gauge extracellular miRNAs, perhaps as an additional sensor of inflammation (see figure). While novel, these findings are in line with another recent report of miRNAs binding to TLRs, specifically miRs-21 and -29a to murine TLR7 and human TLR8,6 postulated to contribute to a protumor microenvironment. For NK cells, it will be highly informative to analyze mice with an NK-cell–specific loss of TLR1 to further confirm the mechanistic link between miR-122 costimulation and this TLR.
These findings raise new questions regarding the miRNA–TLR link for NK-cell activation. What is the dimerization structure of the NK-cell–expressed TLR1 receptor for miRNAs? How does TLR1 recognize specific miRNA sequences? Previously reported TLR1 ligands have vastly differing structural makeup. In addition, the proposed recognition falls outside of the known specificities of TLR1.7 These facts raise intriguing possibility of alternative TLR1-based recognition. Further, it will be important to study how miRNA-based TLR activation integrates into the larger picture of NK-cell activation, which includes both cytokine signals as well as inhibitory and activating NK receptors. Nonetheless, this report highlights the translational potential that exists for the use of exogenous miRNAs as NK-cell activators in vivo.
In summary, this study advances a new mode of NK-cell activation by stimulating TLR1 signals via specific exogenous miRNAs. Such findings lead to many new questions in immunobiology in general and NK cell biology specifically and point toward potential novel approaches to activate NK cells in vivo to respond to malignant target cells.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal