Key Points
Three or more mismatches at the Low Expression Loci may adversely affect clinical outcome after 7/8 matched transplantation.
Match grade at the Low Expression HLA Loci may be considered to select 7/8 donors with potentially lower posttransplant risks.
Abstract
A single mismatch in highly expressed HLA-A, -B, -C, and -DRB1 loci (HEL) is associated with worse outcomes in hematopoietic stem cell transplantation, while less is known about the cumulative impact of mismatches in the lesser expressed HLA loci DRB3/4/5, DQ, and DP (LEL). We studied whether accumulation of LEL mismatches is associated with deleterious effects in 3853 unrelated donor transplants stratified according to number of matches in the HEL. In the 8/8 matched HEL group, LEL mismatches were not associated with any adverse outcome. Mismatches at HLA-DRB1 were associated with occurrence of multiple LEL mismatches. In the 7/8 HEL group, patients with 3 or more LEL mismatches scored in the graft-versus-host vector had a significantly higher risk of mortality (1.45 and 1.43) and transplant-related mortality (1.68 and 1.54) than the subgroups with 0 or 1 LEL mismatches. No single LEL locus had a more pronounced effect on clinical outcome. Three or more LEL mismatches are associated with lower survival after 7/8 HEL matched transplantation. Prospective evaluation of matching for HLA-DRB3/4/5, -DQ, and -DP loci is warranted to reduce posttransplant risks in donor-recipient pairs matched for 7/8 HEL.
Introduction
Allogeneic hematopoietic stem cell transplantation is an effective treatment of a broad range of hematologic, immune, metabolic, and malignant disorders. Best outcomes are obtained when the donor is an HLA-identical sibling.1-3 Previous studies have shown that matching for all alleles of the HLA-A, -B, -C, and -DRB1 loci (8/8 match) was associated with the highest survival rates in transplantation with unrelated donors (URDs).4-7 A single mismatch at any of these loci (7/8 match) was associated with higher transplant-related mortality (TRM). The evaluation of these studies led the National Marrow Donor Program (NMDP) and the Center for International Blood and Marrow Transplant Research (CIBMTR) to provide guidelines defining the minimal requirements for appropriate HLA typing resolution and matching criteria for URD.8,9 The recommendations state: “whenever possible, donors who are high-resolution matched at HLA-A, -B, -C, and -DRB1 should be sought, but unavailability of such a donor is not a contraindication for transplantation. If a mismatch is unavoidable, a single-locus mismatched donor (HLA-A, -B, -C, or -DRB1) can be used with acceptable risks of TRM.”
In retrospective registry studies, an isolated mismatch in DQ and DP loci was not associated with mortality.4-7 One study showed that the addition of a mismatch in HLA-DQB1 to another mismatch was associated with worse survival.10 HLA-DPB1 mismatching has been associated with a higher risk of acute graft-versus-host disease (aGvHD) and a decreased risk of relapse, without a significant effect on overall survival.4,5,11 More recently, a study that categorized HLA-DPB1 mismatch according to T-cell-epitopes12 showed that the nonpermissive mismatches were associated with increased risks of overall mortality, TRM, and severe aGvHD compared with permissive mismatches or DP matches.13 These findings indicate that not all mismatches at the same locus have an equivalent effect on outcomes. The impact of mismatches in DRB3, DRB4, and DRB5 loci has not been investigated extensively in hematopoietic stem cell transplantation.
The HLA loci may be classified into 2 categories according to their expression14-30 or to the impact of mismatches in transplant outcome.4-7,10-13 The HLA loci A, B, C, and DRB1 are called high expression loci (HEL), because their products are abundant on the cell surface and/or mismatches are strongly associated with transplant outcome. HLA-DRB3/4/5, -DQ, and -DP loci, whose products are expressed at low levels, may be categorized as low expression loci (LEL).14-30 Examination of the distribution of HLA alleles in many world populations shows different patterns for the HEL and LEL. The HLA-A, -B, -C, and -DRB1 loci contain many alleles that are evenly distributed, and, consequently, a large proportion of the subjects of a given population are heterozygous at these loci.31-33 In contrast, HLA-DQA1, -DQB1, -DPA1, and -DPB1 loci are comprised of only a few alleles that, combined, account for a large proportion of the gene pool; the reduced genetic diversity results in many subjects being homozygous at these loci. Similarly, DRB3, DRB4, and DRB5 have low diversity, deletions are present in some common haplotypes, and many individuals carry only one allele of DRB3/4/5. In humans, HLA-DRB3, -DRB4, and -DRB5 genes behave as alleles of a single locus,27,28 because the presence of one of these genes at the haplotype level excludes the presence of the other 2 genes. The DP molecules present allelic variations in both the α and β subunits, so that up to 4 different HLA-DP molecules may be present in an individual from pairing of subunits. The DQ subunits also have genetic variations in DQA1 and DQB1, although some alleles of DQA1 cannot pair efficiently with some alleles of DQB1.34
Mismatches in HLA-DRB3, -DRB4, -DRB5, -DQ, and -DP are able to elicit allo-recognition in vitro,15,17,22,24,27 but the effect of isolated mismatches in these HLA class II loci in transplant outcome has been negligible or difficult to prove.4,5 If the allelic products of the LEL have a weak individual effect on outcome, we hypothesized that their effect may be demonstrable only in combination with mismatches in other loci.
Materials and methods
Patients
The cohort was previously described,5 with the exclusion of 4 transplants in which HLA typing for all LEL loci could not be performed in either the patient or the donor. The study included 3853 patients reported to the NMDP who underwent transplantation between 1988 and 2004 for acute lymphoblastic leukemia, acute myeloid leukemia, chronic myeloid leukemia, and myelodysplastic syndrome. Research was approved and conducted under the supervision of the NMDP Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki. Approximately 4% of surviving patients would not provide consent for use of research data. To adjust for the potential bias introduced by exclusion of nonconsenting surviving patients, a corrective action plan modeling process randomly excluded the same percentage of deceased patients (n = 392), using a biased coin randomization with exclusion probabilities based on characteristics associated with not providing consent for use of the data in survivors. All studies performed by NMDP apply this adjustment factor, as previously described.4,5
HLA typing and criteria for scoring mismatches
High-resolution typing was performed for HLA-A, -B, -C, -DRB1, -DRB3, -DRB4, -DRB5, -DQA1, -DQB1, -DPA1, and -DPB1, as previously described.5 Up to 2 mismatches could be identified per locus. Mismatches at homozygous alleles were considered as single mismatches. The highest level mismatch (antigen or allele) per locus was assigned as the overall mismatch for a given locus. According to the match grades defined above, there can be up to 8 allele matches/mismatches in the HLA-HEL and 6 allele matches/mismatches in the HLA-LEL. The directionality of mismatches in the graft-versus-host (GvH), host-versus-graft (HvG) vectors, as well as the overall mismatch, were evaluated for all outcomes. The number of transplants and their characteristics utilizing the GvH vector are shown in supplemental Table 1. Mismatches in HLA loci were scored as previously described4,5 ; additional analyses were performed according to the number of mismatched DQ and DP heterodimers (supplemental Materials).
Definitions and outcomes
The primary outcome of the analysis was overall survival, defined as time from graft infusion (d 0) to death from any cause. A number of secondary end points were also analyzed. Primary graft failure was defined as failure to achieve an absolute neutrophil count >500 × 106/L by d 28 that was maintained for 3 consecutive measurements. Data about secondary graft failure were not available. aGvHD grades 3 and 4 were defined by the Glucksberg scale.35 Extensive chronic GvHD was defined according to the Seattle criteria.36 Clinical relapse of the primary disease was defined by the CIBMTR criteria.37 Disease-free survival (DFS) is survival without recurrence of the primary disease. TRM is death in continuous complete remission of the primary disease.
Biostatistical methods
Probabilities for overall survival and DFS were calculated using the Kaplan-Meier estimator. Survival curves were compared using the log-rank test. Neutrophil engraftment was considered a dichotomous outcome and analyzed by logistic regression. Values for other outcomes were estimated using the cumulative incidence function.5 Death was considered a competing risk for all of the end points except overall survival and DFS. Relapse was also considered a competing event for TRM. Patients were censored when they underwent a second HCT procedure or, if alive, at last follow-up. To analyze the association between number and type of HLA mismatches and clinical outcomes, multivariate proportional hazards models were created to allow pairs mismatched at specific loci to be compared with HLA-matched pairs. This biostatistical approach allowed precise estimates of the association between number and type of HLA mismatches or locus-specific mismatches and outcomes without confounding any additional HLA mismatches present. Models were stratified by the 3 HEL categories of 8/8, 7/8, and <7/8. The LEL mismatch was classified as 0, 1, 2, and >2 LEL MM. Because of multiple testing, a significant P value was considered less than P = .01 for the main effect of HLA matching. All models were tested for significant clinical covariates, including disease, disease stage, Karnofsky performance status, donor-patient cytomegalovirus serology, patient race, patient age, T-cell depletion, use of total body irradiation, graft source (peripheral blood or bone marrow), donor age, patient-donor sex match, and year of transplantation. Models included any clinical factors that were related to a given outcome at P < .05. All variables were tested for affirmation of the proportional hazards assumption and to look for interactions with HLA matching. No significant interactions were identified. All variables satisfied the proportional hazards assumption except Karnofsky performance, so the analyses were stratified for this variable. Center effect was tested and was not present.
Results
Table 1 shows the population characteristics and distribution of cumulative mismatches. Of the studied donor-recipient pairs, only 240 (6.2%) were matched at all HEL and LEL loci. Greater mismatching in LEL was associated with mismatching in HEL (P < .0001); only 5.8% of the transplants matching in 8/8 HEL alleles had 3 or more LEL mismatches, compared with 11.1% in transplants matched for 7/8 HEL and 20.5% in those matched for <7/8 HEL. Three or more LEL mismatches were more common in 7/8 pairs mismatched in HLA-DRB1 (33.3%) compared with 7/8 pairs mismatched in HLA-A, -B, or -C loci (8%) (P < .0001).
Characteristics of patients with acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, and myelodysplastic syndrome where donor/recipient pairs have high-resolution typing for HLA-A, -B, -C, -DRB1, -DQ, and -DP through the NMDP and where recipient received a myeloablative conditioning regimen
| Variable . | 8/8 for HLA-A, -B,-C and -DRB1 n (%) . | 7/8 for HLA-A, -B,-C and -DRB1 n (%) . | <7/8 for HLA-A, -B,-C and -DRB1 n (%) . | P value . |
|---|---|---|---|---|
| Number of patients | 1837 | 985 | 1031 | |
| Number of centers | 105 | 100 | 97 | |
| Age, median (range), y | 35 (<1-65) | 31 (<1-65) | 28 (<1-59) | <.0001 |
| Age at transplant | <.0001 | |||
| ≤20 y | 361 (20) | 271 (27) | 347 (34) | |
| 21–50 y | 1258 (68) | 626 (64) | 633 (61) | |
| >50 y | 217 (12) | 88 (9) | 51 (5) | |
| Race | <.0001 | |||
| White | 1712 (93) | 858 (87) | 768 (74) | |
| Black | 44 (2) | 50 (5) | 95 (9) | |
| Hispanic | 54 (3) | 53 (5) | 113 (11) | |
| Other | 27 (1) | 24 (2) | 54 (5) | |
| Male sex | 1041 (57) | 537 (55) | 605 (59) | .17 |
| Karnofsky prior to transplant > 90 | 1325 (72) | 713 (72) | 750 (73) | .24 |
| HEL matching | <.0001 | |||
| HLA-A, -B, -C and -DRB1 matched | 1837 (100) | 0 | 0 | |
| HLA-A, -B, or -C mismatched only | 0 | 868 (88) | 740 (72) | |
| HLA-DRB1 mismatched only | 0 | 117 (12) | 6 (<1) | |
| Mismatched at DRB1 and at one of HLA-A, -B and/or -C | 0 | 0 | 285 (28) | |
| Number of patients | 1837 | 985 | 1031 | |
| LEL | <.0001 | |||
| DQ, DP, and DRB3/4/5 | ||||
| 6/6 Match | 240 (13) | 99 (10) | 85 (8) | |
| 5/6 Match | 906 (49) | 411 (42) | 364 (35) | |
| 4/6 Match | 583 (32) | 366 (37) | 371 (36) | |
| <4/6 Match | 108 (6) | 109 (11) | 211 (21) | |
| DRB3/4/5 | <.0001 | |||
| Matched | 1709 (93) | 873 (89) | 853 (83) | |
| 1 mismatch | 127 (7) | 110 (11) | 172 (17) | |
| 2 mismatches | 1 (<1) | 2 (<1) | 6 (<1) | |
| DQ | <.0001 | |||
| Matched | 1675 (91) | 795 (81) | 731 (71) | |
| 1 mismatch | 156 (8) | 184 (19) | 274 (27) | |
| 2 mismatches | 6 (<1) | 6 (1) | 26 (2) | |
| DP | .0006 | |||
| Matched | 265 (14) | 126 (13) | 115 (11) | |
| 1 mismatch | 1028 (56) | 535 (54) | 532 (52) | |
| 2 mismatches | 544 (30) | 324 (33) | 384 (37) | |
| Diagnosis | .0002 | |||
| Acute myeloid leukemia | 496 (27) | 294 (30) | 265 (26) | |
| Acute lymphoblastic leukemia | 410 (22) | 253 (26) | 289 (28) | |
| Chronic myeloid leukemia | 756 (41) | 366 (37) | 416 (40) | |
| Myelodysplastic syndrome | 175 (10) | 72 (7) | 61 (6) | |
| Disease status at transplant | .0009 | |||
| Early | 833 (45) | 378 (38) | 389 (38) | |
| Intermediate | 673 (37) | 410 (42) | 448 (43) | |
| Advanced | 327 (18) | 195 (20) | 192 (19) | |
| Other | 4 (<1) | 2 (<1) | 2 (<1) | |
| Conditioning regimen: total body irradiation based | 1490 (81) | 807 (82) | 909 (88) | <.0001 |
| GvHD prophylaxis | <.0001 | |||
| Tacrolimus + (MTX or MMF or steroids) ± other | 365 (20) | 186 (19) | 141 (14) | |
| Tacrolimus ± other | 3 (<1) | 6 (1) | 3 (<1) | |
| CsA + MTX ± other | 1075 (59) | 514 (52) | 554 (54) | |
| CsA ± other (No MTX) | 68 (4) | 30 (3) | 38 (4) | |
| MMF ± other | 4 (<1) | 1 (<1) | 0 | |
| MTX ± other (No CSA) | 13 (1) | 5 (1) | 13 (1) | |
| T-cell depletion | 307 (17) | 241 (24) | 282 (27) | |
| Other | 2 (<1) | 2 (<1) | 0 | |
| Graft type | <.0001 | |||
| Bone marrow | 1695 (92) | 916 (93) | 1000 (97) | |
| PBSC | 142 (8) | 69 (7) | 31 (3) | |
| Donor/recipient sex match | <.0001 | |||
| Male/male | 717 (39) | 331 (34) | 333 (32) | |
| Male/female | 443 (24) | 237 (24) | 214 (21) | |
| Female/male | 324 (18) | 206 (21) | 272 (26) | |
| Female/female | 353 (19) | 211 (21) | 212 (21) | |
| Donor/recipient cytomegalovirus match | .02 | |||
| Negative/negative | 667 (36) | 341 (35) | 305 (30) | |
| Negative/positive | 515 (28) | 266 (27) | 304 (29) | |
| Positive/negative | 297 (16) | 160 (16) | 174 (17) | |
| Positive/positive | 303 (16) | 192 (19) | 218 (21) | |
| Unknown | 55 (3) | 26 (3) | 30 (3) | |
| Donor age, median (range), y | 36 (18-60) | 36 (19-59) | 36 (18-60) | .23 |
| 18-29 | 478 (25) | 243 (25) | 271 (26) | |
| 30-39 | 726 (40) | 376 (38) | 384 (37) | |
| 40-49 | 508 (28) | 284 (29) | 305 (30) | |
| 50 and older | 125 (7) | 82 (8) | 71 (7) | |
| Time from diagnosis to transplant, mo median (range) | 11 (0.3-232) | 13 (0.3-309) | 15 (0.4-200) | <.0001 |
| Year of transplant | <.0001 | |||
| 1988-1993 | 335 (18) | 176 (18) | 282 (27) | |
| 1994-1998 | 753 (41) | 403 (41) | 455 (44) | |
| 1999-2003 | 749 (41) | 406 (41) | 294 (29) | |
| Median follow-up of survivors, mo | 73 (3-194) | 63 (6-191) | 86 (4-192) | .003* |
| Variable . | 8/8 for HLA-A, -B,-C and -DRB1 n (%) . | 7/8 for HLA-A, -B,-C and -DRB1 n (%) . | <7/8 for HLA-A, -B,-C and -DRB1 n (%) . | P value . |
|---|---|---|---|---|
| Number of patients | 1837 | 985 | 1031 | |
| Number of centers | 105 | 100 | 97 | |
| Age, median (range), y | 35 (<1-65) | 31 (<1-65) | 28 (<1-59) | <.0001 |
| Age at transplant | <.0001 | |||
| ≤20 y | 361 (20) | 271 (27) | 347 (34) | |
| 21–50 y | 1258 (68) | 626 (64) | 633 (61) | |
| >50 y | 217 (12) | 88 (9) | 51 (5) | |
| Race | <.0001 | |||
| White | 1712 (93) | 858 (87) | 768 (74) | |
| Black | 44 (2) | 50 (5) | 95 (9) | |
| Hispanic | 54 (3) | 53 (5) | 113 (11) | |
| Other | 27 (1) | 24 (2) | 54 (5) | |
| Male sex | 1041 (57) | 537 (55) | 605 (59) | .17 |
| Karnofsky prior to transplant > 90 | 1325 (72) | 713 (72) | 750 (73) | .24 |
| HEL matching | <.0001 | |||
| HLA-A, -B, -C and -DRB1 matched | 1837 (100) | 0 | 0 | |
| HLA-A, -B, or -C mismatched only | 0 | 868 (88) | 740 (72) | |
| HLA-DRB1 mismatched only | 0 | 117 (12) | 6 (<1) | |
| Mismatched at DRB1 and at one of HLA-A, -B and/or -C | 0 | 0 | 285 (28) | |
| Number of patients | 1837 | 985 | 1031 | |
| LEL | <.0001 | |||
| DQ, DP, and DRB3/4/5 | ||||
| 6/6 Match | 240 (13) | 99 (10) | 85 (8) | |
| 5/6 Match | 906 (49) | 411 (42) | 364 (35) | |
| 4/6 Match | 583 (32) | 366 (37) | 371 (36) | |
| <4/6 Match | 108 (6) | 109 (11) | 211 (21) | |
| DRB3/4/5 | <.0001 | |||
| Matched | 1709 (93) | 873 (89) | 853 (83) | |
| 1 mismatch | 127 (7) | 110 (11) | 172 (17) | |
| 2 mismatches | 1 (<1) | 2 (<1) | 6 (<1) | |
| DQ | <.0001 | |||
| Matched | 1675 (91) | 795 (81) | 731 (71) | |
| 1 mismatch | 156 (8) | 184 (19) | 274 (27) | |
| 2 mismatches | 6 (<1) | 6 (1) | 26 (2) | |
| DP | .0006 | |||
| Matched | 265 (14) | 126 (13) | 115 (11) | |
| 1 mismatch | 1028 (56) | 535 (54) | 532 (52) | |
| 2 mismatches | 544 (30) | 324 (33) | 384 (37) | |
| Diagnosis | .0002 | |||
| Acute myeloid leukemia | 496 (27) | 294 (30) | 265 (26) | |
| Acute lymphoblastic leukemia | 410 (22) | 253 (26) | 289 (28) | |
| Chronic myeloid leukemia | 756 (41) | 366 (37) | 416 (40) | |
| Myelodysplastic syndrome | 175 (10) | 72 (7) | 61 (6) | |
| Disease status at transplant | .0009 | |||
| Early | 833 (45) | 378 (38) | 389 (38) | |
| Intermediate | 673 (37) | 410 (42) | 448 (43) | |
| Advanced | 327 (18) | 195 (20) | 192 (19) | |
| Other | 4 (<1) | 2 (<1) | 2 (<1) | |
| Conditioning regimen: total body irradiation based | 1490 (81) | 807 (82) | 909 (88) | <.0001 |
| GvHD prophylaxis | <.0001 | |||
| Tacrolimus + (MTX or MMF or steroids) ± other | 365 (20) | 186 (19) | 141 (14) | |
| Tacrolimus ± other | 3 (<1) | 6 (1) | 3 (<1) | |
| CsA + MTX ± other | 1075 (59) | 514 (52) | 554 (54) | |
| CsA ± other (No MTX) | 68 (4) | 30 (3) | 38 (4) | |
| MMF ± other | 4 (<1) | 1 (<1) | 0 | |
| MTX ± other (No CSA) | 13 (1) | 5 (1) | 13 (1) | |
| T-cell depletion | 307 (17) | 241 (24) | 282 (27) | |
| Other | 2 (<1) | 2 (<1) | 0 | |
| Graft type | <.0001 | |||
| Bone marrow | 1695 (92) | 916 (93) | 1000 (97) | |
| PBSC | 142 (8) | 69 (7) | 31 (3) | |
| Donor/recipient sex match | <.0001 | |||
| Male/male | 717 (39) | 331 (34) | 333 (32) | |
| Male/female | 443 (24) | 237 (24) | 214 (21) | |
| Female/male | 324 (18) | 206 (21) | 272 (26) | |
| Female/female | 353 (19) | 211 (21) | 212 (21) | |
| Donor/recipient cytomegalovirus match | .02 | |||
| Negative/negative | 667 (36) | 341 (35) | 305 (30) | |
| Negative/positive | 515 (28) | 266 (27) | 304 (29) | |
| Positive/negative | 297 (16) | 160 (16) | 174 (17) | |
| Positive/positive | 303 (16) | 192 (19) | 218 (21) | |
| Unknown | 55 (3) | 26 (3) | 30 (3) | |
| Donor age, median (range), y | 36 (18-60) | 36 (19-59) | 36 (18-60) | .23 |
| 18-29 | 478 (25) | 243 (25) | 271 (26) | |
| 30-39 | 726 (40) | 376 (38) | 384 (37) | |
| 40-49 | 508 (28) | 284 (29) | 305 (30) | |
| 50 and older | 125 (7) | 82 (8) | 71 (7) | |
| Time from diagnosis to transplant, mo median (range) | 11 (0.3-232) | 13 (0.3-309) | 15 (0.4-200) | <.0001 |
| Year of transplant | <.0001 | |||
| 1988-1993 | 335 (18) | 176 (18) | 282 (27) | |
| 1994-1998 | 753 (41) | 403 (41) | 455 (44) | |
| 1999-2003 | 749 (41) | 406 (41) | 294 (29) | |
| Median follow-up of survivors, mo | 73 (3-194) | 63 (6-191) | 86 (4-192) | .003* |
Mismatching at HLA-DRB3/4/5
Table 1 shows that the number of mismatches in HLA-DRB3/4/5 increases with the occurrence of HEL mismatches. Among the single DRB3/4/5 mismatches, DRB3 presented only allele level mismatches. The majority of the DRB4 mismatches were defined by the presence or absence of an expressed allele of this locus that resulted from the presence or absence of the null allele DRB4*01:03N in either the patient or the donor (29 of 31 in the 8/8 group and 25 of 25 in the 7/8 group). There were no mismatches in DRB5 in the 8/8 group; in the 7/8 group, 5 of the 7 mismatches occurred at the locus level and resulted from a deletion of the DRB5 locus in some haplotypes. In the 8/8 and 7/8 groups, there were only 3 transplants with mismatches in both DRB3 and DRB4.
Mismatches in DRB3/4/5 scored in either direction were not associated with survival, DFS, relapse, TRM, or grade 3-4 aGvHD in any of the groups stratified according to HEL match grade.
Mismatching at HLA-DQ
Mismatches in HLA-DQ were more common in the transplants presenting a single mismatch at DRB1 (53.6%) than in those with a single mismatch at the class I loci (17.5%) or matched in 8/8 alleles (8.8%). Almost all mismatches in HLA-DQ were due to mismatches only at HLA-DQB1 or combined mismatches in HLA-DQA1 and -DQB1. Only 4 of 3016 transplants that matched in DRB1 and DQB1 were mismatched in DQA1. The majority of the DQ mismatches were single (Table 1). As noted above, the HLA-DQ mismatches were less common in the 8/8 group than in the 7/8 group.
In the multivariate analysis of the 8/8 group, a single mismatch in HLA-DQ scored in either direction was not associated with any adverse outcome. There were no significant differences in any of the outcomes between the groups presenting different numbers of DQ-heterodimer mismatches compared with each other or with the 8/8-DQ matched groups in either the HvG or GvH vectors.
Among the 7/8 transplants with a single DQ mismatch, 111, 54, and 19 transplants had 1, 2, and 3 heterodimer mismatches, respectively. The presence of one DQ-heterodimer mismatch in the GvH vector, compared with the 7/8 transplants matched in DQ, showed a significant association with decreased survival (relative risk [RR] = 1.37; 95% confidence interval [CI], 1.08-1.73; P < .01); a similar but not significant trend was observed for TRM for the same comparison (RR = 1.38; 95% CI, 1.05-1.79; P < .02). Although not statistically significant, the groups with 2 and 3 heterodimer mismatches showed similar trends to the group with one DQ heterodimer mismatch for the association with TRM (RR = 1.13; 95% CI, 0.77-1.65, NS and RR = 1.43; 95% CI, 0.73-2.81, NS, respectively) compared with the 7/8 transplants matched in DQ.
There were no associations between the number of mismatches in HLA-DQ heterodimers and any of the outcomes when the mismatches were graded in the HvG vector or as the highest in either (overall) direction.
Mismatching at HLA-DP
Most of the mismatches in HLA-DP were due to mismatches in DPB1 alone or to the combination of mismatches in DPA1 and DPB1; only 5.2% of the 534 transplants matched in DPB1 were mismatched in DPA1.
The effect of mismatches in DP was evaluated according to match grades in 10 alleles of HLA-A, -B, -C, -DRB1, and -DQB1 loci.5 HLA-DP mismatching was significantly associated with increased risk of grade 3-4 aGvHD (RR = 1.43; 95% CI, 1.16-1.76; P < .001). The 10/10, 9/10, or <9/10 groups were stratified according to the number of DP mismatches; within each of these categories, there were no significant associations with aGvHD, survival, DFS, TRM, and relapse according to the number of mismatched DP heterodimers. Similar DP effects were observed when matching at HLA-DQ was omitted.
Total number of mismatches in LEL
LEL mismatches were summed as the number of mismatches in 3 operationally defined loci with a maximum of 6 possible allele matches. In the pairs with 3 or more LEL mismatches, 80% to 85% were mismatched at 3 LELs. Far fewer were mismatched at 4 LELs, and transplants with 5 or 6 LEL mismatches were rare (<1% in the 8/8 and 7/8 categories).
Tables 2 and 3 and Figures 1 and 2 show outcomes with match grades scored according to the GvH vector; the characteristics are shown in supplemental Table 1. Table 2 shows the univariate analyses of potential associations between HEL matches, LEL mismatches scored according to the GvH vector, and outcomes.
Univariate results for 3-4 acute GvHD, 1-y TRM, and 5-y overall survival according to the number of HEL matches (HLA-A, B, C, and DRB1) and LEL (DRB3/4/5, DQ, and DP) mismatches scored in the GvH vector
| Number of HEL matches . | Number of LEL mismatches, % . | P value . | |||
|---|---|---|---|---|---|
| 0 mm . | 1 mm . | 2 mm . | >2 mm . | ||
| 3-4 Acute GvHD by d 100 | |||||
| 8/8 | 23 (19-27) | 29 (26-32) | 30 (26-34) | 32 (24-42) | .03 |
| 7/8 | 34 (28-42) | 34 (29-39) | 39 (33-44) | 43 (34-53) | .27 |
| 6/8 | 43 (33-52) | 42 (36-49) | 47 (41-54) | 40 (29-51) | .6 |
| TRM at 1 y | |||||
| 8/8 | 30 (26-35) | 36 (33-39) | 39 (36-44) | 39 (30-49) | .02 |
| 7/8 | 39 (32-46) | 45 (40-50) | 46 (41-51)% | 61 (51-70) | .005 |
| 6/8 | 59 (50-69)% | 48 (42-55) | 59 (52-65) | 67 (56-77) | .02 |
| Overall survival at 5 y | |||||
| 8/8 | 38 (34-43) | 37 (33-40) | 37 (33-41) | 37 (28-47) | .94 |
| 7/8 | 31 (24-38) | 33 (28-38) | 27 (22-32) | 25 (17-34) | .24 |
| 6/8 | 17 (10-25) | 26 (20-32) | 19 (14-24) | 18 (11-28) | .19 |
| Number of HEL matches . | Number of LEL mismatches, % . | P value . | |||
|---|---|---|---|---|---|
| 0 mm . | 1 mm . | 2 mm . | >2 mm . | ||
| 3-4 Acute GvHD by d 100 | |||||
| 8/8 | 23 (19-27) | 29 (26-32) | 30 (26-34) | 32 (24-42) | .03 |
| 7/8 | 34 (28-42) | 34 (29-39) | 39 (33-44) | 43 (34-53) | .27 |
| 6/8 | 43 (33-52) | 42 (36-49) | 47 (41-54) | 40 (29-51) | .6 |
| TRM at 1 y | |||||
| 8/8 | 30 (26-35) | 36 (33-39) | 39 (36-44) | 39 (30-49) | .02 |
| 7/8 | 39 (32-46) | 45 (40-50) | 46 (41-51)% | 61 (51-70) | .005 |
| 6/8 | 59 (50-69)% | 48 (42-55) | 59 (52-65) | 67 (56-77) | .02 |
| Overall survival at 5 y | |||||
| 8/8 | 38 (34-43) | 37 (33-40) | 37 (33-41) | 37 (28-47) | .94 |
| 7/8 | 31 (24-38) | 33 (28-38) | 27 (22-32) | 25 (17-34) | .24 |
| 6/8 | 17 (10-25) | 26 (20-32) | 19 (14-24) | 18 (11-28) | .19 |
Note: supplemental Table 1 shows the number of transplants in each category when scored in the GvH vector. Numbers in each group are for the survival model. Other models may have had fewer evaluable patients, as described in “Biostatistical methods.” Table shows incidence of the events (%) and CI.
Multivariate model of HLA mismatches scored in the GvH vector at the HEL (HLA-A, B, C, DRB1) and LEL (DRB3/4/5, DQ, DP) HLA loci
| . | Grades 3-4 aGvHD . | Relapse . | TRM . | Overall survival . | ||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) . | P value . | RR (95% CI) . | P value . | RR (95% CI) . | P value . | RR (95% CI) . | P value . | |
| 8/8 | ||||||||
| 0 mm | 1 | .01* | 1 | .01* | 1 | .17* | 1 | .92* |
| 1 vs 0 | 1.41 (1.11-1.79) | .005 | 0.75 (0.59-0.95) | .02 | 1.19 (0.98-1.44) | .07 | 1.05 (0.90-1.23) | .52 |
| 2 vs 0 | 1.49 (1.16-1.92) | .002 | 0.64 (0.49-0.84) | .001 | 1.23 (1.01-1.50) | .04 | 1.05 (0.89-1.24) | .56 |
| >2 vs 0 | 1.58 (1.05-2.39) | .03 | 0.67 (0.41-1.11) | .12 | 1.30 (0.93-1.81) | .13 | 1.07 (0.80-1.42) | .65 |
| 2 vs 1 | 1.06 (0.87-1.29) | .57 | 0.86 (0.67-1.11) | .24 | 1.03 (0.88-1.22) | .68 | 1.00 (0.87-1.15) | .97 |
| >2 vs 1 | 1.12 (0.77-1.65) | .55 | 0.90 (0.55-1.47) | .68 | 1.09 (0.80-1.49) | .59 | 1.02 (0.77-1.33) | .91 |
| >2 vs 2 | 1.06 (0.72-1.57) | .77 | 1.05 (0.63-1.73) | .86 | 1.05 (0.76-1.45) | .75 | 1.02 (0.77-1.34) | .90 |
| 7/8 | ||||||||
| 0 mm | 1 | .22* | 1 | .47* | 1 | .01* | 1 | .03* |
| 1 vs 0 | 0.95 (0.70-1.30) | .77 | 0.80 (0.54-1.17) | .25 | 1.10 (0.84-1.42) | .49 | 1.01 (0.81-1.26) | .90 |
| 2 vs 0 | 1.17 (0.86-1.58) | .32 | 0.95 (0.65-1.39) | .79 | 1.29 (0.99-1.67) | .06 | 1.19 (0.95-1.49) | .13 |
| >2 vs 0 | 1.30 (0.87-1.96) | .20 | 0.68 (0.36-1.30) | .24 | 1.68 (1.20-2.37) | .003 | 1.45 (1.06-1.96) | .08 |
| 2 vs 1 | 1.22 (0.96-1.56) | .11 | 1.19 (0.85-1.65) | .31 | 1.17 (0.96-1.44) | .13 | 1.17 (0.98-1.40) | .08 |
| >2 vs 1 | 1.37 (0.95-1.96) | .09 | 0.85 (0.46-1.58) | .61 | 1.54 (1.14-2.07) | .005 | 1.43 (1.09-1.87) | .01 |
| >2 vs 2 | 1.12 (0.78-1.61) | .54 | 0.72 (0.39-1.33) | .29 | 1.31 (0.97-1.77) | .08 | 1.22 (0.92-1.60) | .16 |
| . | Grades 3-4 aGvHD . | Relapse . | TRM . | Overall survival . | ||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) . | P value . | RR (95% CI) . | P value . | RR (95% CI) . | P value . | RR (95% CI) . | P value . | |
| 8/8 | ||||||||
| 0 mm | 1 | .01* | 1 | .01* | 1 | .17* | 1 | .92* |
| 1 vs 0 | 1.41 (1.11-1.79) | .005 | 0.75 (0.59-0.95) | .02 | 1.19 (0.98-1.44) | .07 | 1.05 (0.90-1.23) | .52 |
| 2 vs 0 | 1.49 (1.16-1.92) | .002 | 0.64 (0.49-0.84) | .001 | 1.23 (1.01-1.50) | .04 | 1.05 (0.89-1.24) | .56 |
| >2 vs 0 | 1.58 (1.05-2.39) | .03 | 0.67 (0.41-1.11) | .12 | 1.30 (0.93-1.81) | .13 | 1.07 (0.80-1.42) | .65 |
| 2 vs 1 | 1.06 (0.87-1.29) | .57 | 0.86 (0.67-1.11) | .24 | 1.03 (0.88-1.22) | .68 | 1.00 (0.87-1.15) | .97 |
| >2 vs 1 | 1.12 (0.77-1.65) | .55 | 0.90 (0.55-1.47) | .68 | 1.09 (0.80-1.49) | .59 | 1.02 (0.77-1.33) | .91 |
| >2 vs 2 | 1.06 (0.72-1.57) | .77 | 1.05 (0.63-1.73) | .86 | 1.05 (0.76-1.45) | .75 | 1.02 (0.77-1.34) | .90 |
| 7/8 | ||||||||
| 0 mm | 1 | .22* | 1 | .47* | 1 | .01* | 1 | .03* |
| 1 vs 0 | 0.95 (0.70-1.30) | .77 | 0.80 (0.54-1.17) | .25 | 1.10 (0.84-1.42) | .49 | 1.01 (0.81-1.26) | .90 |
| 2 vs 0 | 1.17 (0.86-1.58) | .32 | 0.95 (0.65-1.39) | .79 | 1.29 (0.99-1.67) | .06 | 1.19 (0.95-1.49) | .13 |
| >2 vs 0 | 1.30 (0.87-1.96) | .20 | 0.68 (0.36-1.30) | .24 | 1.68 (1.20-2.37) | .003 | 1.45 (1.06-1.96) | .08 |
| 2 vs 1 | 1.22 (0.96-1.56) | .11 | 1.19 (0.85-1.65) | .31 | 1.17 (0.96-1.44) | .13 | 1.17 (0.98-1.40) | .08 |
| >2 vs 1 | 1.37 (0.95-1.96) | .09 | 0.85 (0.46-1.58) | .61 | 1.54 (1.14-2.07) | .005 | 1.43 (1.09-1.87) | .01 |
| >2 vs 2 | 1.12 (0.78-1.61) | .54 | 0.72 (0.39-1.33) | .29 | 1.31 (0.97-1.77) | .08 | 1.22 (0.92-1.60) | .16 |
Note: supplemental Table 1 shows the number of transplants in each category when scored in the GvH vector. Models were adjusted or stratified for the following variables: grades 3-4 acute GvHD, adjusted for T depletion, disease, year of transplant; stratified on Karnofsky score; relapse, adjusted for disease and disease status, cytomegalovirus match, and Karnofsky score; TRM, adjusted for recipient age, disease, cytomegalovirus match, year of transplant, Karnofsky score, and radiation (yes or no); and overall survival, adjusted for recipient age, disease and disease status, cytomegalovirus match, year of transplant; stratified on Karnofsky score.
P values for the all comparisons made for the outcome in each match grade category.
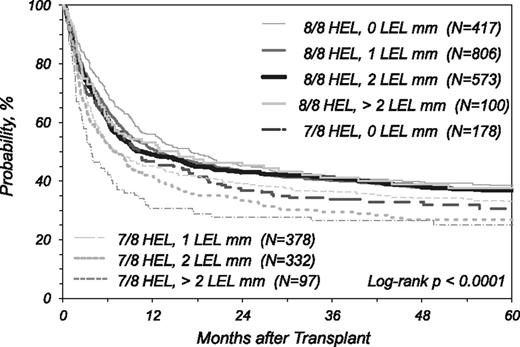
Kaplan Meier estimate of overall survival in patients presenting no mismatch (8/8) or one mismatch (7/8) in the GvH vector in the HEL (HLA-A, -B, -C, and -DRB1 loci) stratified according to the degree of mismatching at HLA-DRB3/4/5, DQ, and DP (LEL) loci.
Kaplan Meier estimate of overall survival in patients presenting no mismatch (8/8) or one mismatch (7/8) in the GvH vector in the HEL (HLA-A, -B, -C, and -DRB1 loci) stratified according to the degree of mismatching at HLA-DRB3/4/5, DQ, and DP (LEL) loci.
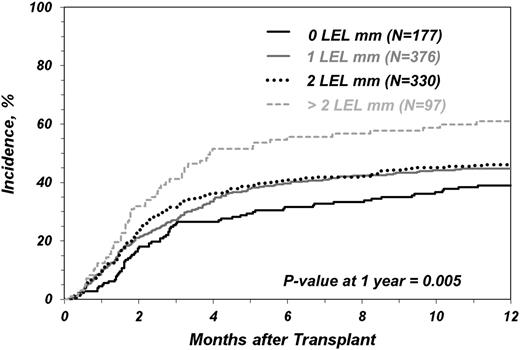
Incidence of TRM as a function of degree of mismatching at HLA-DRB3/4/5, DQ and DP (LEL) loci in transplants matched in 7/8 alleles of HLA HLA-A, -B, -C, and -DRB1 loci.
Incidence of TRM as a function of degree of mismatching at HLA-DRB3/4/5, DQ and DP (LEL) loci in transplants matched in 7/8 alleles of HLA HLA-A, -B, -C, and -DRB1 loci.
Table 3 shows the results of multivariate analysis according to the same HEL and LEL groups. In the 8/8 group, mismatching at the LEL loci was associated with higher incidence of grade 3-4 aGvHD with 1 or 2 LEL mismatches compared with no LEL mismatches (Table 3). A smaller number of cases had >2 LEL mismatches and the P value did not meet the >.01 threshold for significance, but the RR is similar. For relapse, the presence of 2 LEL mismatches was significantly associated with reduced incidence of relapse compared with transplants fully matched at LEL in the GvH vector. In the 8/8 groups, single or accumulated LEL mismatches were not associated with TRM or survival. Figure 1 illustrates overall survival according to the degree of matching in HEL (8/8 and 7/8) and LEL; in the 8/8 category, the survival 5 y after transplantation was virtually identical and nonsignificantly different in the multivariate analysis in the groups presenting a different number of LEL mismatches. This figure also illustrates that any of the 8/8 groups had superior survival over the 7/8 groups (Figure 1 legend).
Table 3 shows a significant difference in survival in the 7/8 groups with >2 LEL mismatches (n = 97) compared with the group with 1 LEL mismatch (n = 378). The risk for mortality was higher, but not significant for the category with >2 LEL mismatches, compared with the categories with zero (n = 178) or 2 (n = 332) LEL mismatches.
Table 3 also shows that in the 7/8 group, >2 LEL mismatches in the GVH vector (n = 97, 11%) were associated with a higher risk of TRM compared with transplants with zero or one mismatch in the LEL loci. In the 7/8 groups, single or accumulated LEL mismatches were not associated with grade 3-4 aGvHD or relapse. Rescoring the LEL mismatches according to overall or the HvG vector did not show any significant association with any of the transplant outcomes examined in the 7/8 category. The rate of neutrophil engraftment at d 28 in the 8/8 (90% to 91%) or 7/8 (87% to 88%) categories was virtually identical among the different LEL groups. Similarly, there was no association between the number of LEL mismatches and cGVHD.
Figure 2 shows the association of LEL mismatches with the cumulative incidence of TRM in the 7/8 HEL matched pairs. The unadjusted estimated incidence of TRM at 1 y for 7/8 transplants with >2 LEL mismatches scored in the GvH vector was 61% compared with incidences of 39%, 45%, and 46% in the 7/8 transplants with 0, 1, and 2 LEL mismatches, respectively (P = .005) (Table 2). These results are slightly different from those presented in Table 3, which were adjusted for clinical covariates. Risk for TRM was higher for any of the 7/8 groups compared with any of the 8/8 groups (supplemental Table 2).
Additional multivariate subanalyses were conducted in 4 transplant groups mismatched at HLA-A, -B, -C, and -DRB1 loci to examine the effect of the LEL mismatches in TRM. In each of the mismatched HEL groups, the transplants with >2 LEL mismatches presented higher risks (ranging from 1.09 to 4.35) for TRM than the transplants with 2, 1, and 0 LEL mismatches (data not shown). A similar trend was observed in the subanalyses examining overall survival in the HLA-A and -DRB1 mismatched groups; in these groups, the RRs for >2 LEL mismatches ranged from 1.60 to 2.62, compared with the transplants with 2, 1, and 0 LEL mismatches (data not shown).
We investigated whether mismatch at a specific LEL made a more significant contribution to poor outcome. Because all 7/8 transplants with >2 LEL mismatches included 1 or 2 mismatches in HLA-DP, we could only evaluate the effect of a second HLA-DP mismatch against a mismatch at the other LEL. We observed virtually identical risks for all outcomes whether the second mismatch was at HLA-DP or another LEL (data not shown). Therefore, no single LEL appeared to have a more pronounced effect on clinical outcome when mismatched vs the other LEL.
Discussion
Three or more mismatches at LEL were associated with poorer overall survival and TRM in the 7/8 matched HEL group, and therefore could be taken into account in donor selection when a mismatched unrelated transplant is being considered. Our data suggest that if a 7/8 matched transplant is going to be performed, it may be beneficial to use a donor matched at 4 or more LEL. Mismatching for LEL in the 8/8 matched transplants was associated with higher grade 3-4 aGvHD and lower relapse but was not associated with TRM or survival, suggesting that attempts to match for LEL in this setting would not be useful.
The 8/8 groups with a different number of LEL mismatches did not show significant differences in survival; therefore, matching for the LEL loci may not be considered in donor selection if the patient and the potential donors considered are fully matched in the HEL. However, LEL typing may be necessary for donor selection if the patient has humoral sensitization against products of the LEL.38,39
Mismatches in the GvH vector appeared to have a more relevant impact in causing aGvHD, TRM, and mortality than the mismatch in HvG, suggesting that analyses using the overall matching score may have underestimated the biologic importance of LEL. Because LEL have fewer alleles and a lower degree of diversity than HEL, the consideration of matching vector is consequently more important. For example, there were 417 transplants matching in all HEL and LEL when the GvH vector was used (74% increase) compared with the overall bidirectional matching scoring (n = 240).
In our study and others,4,5 no adverse effects of isolated mismatches in the LEL were seen. This observation is consistent with the hypothesis that each individual LEL has a weak deleterious or no effect by itself. Other data show that the mismatches in alleles of DRB3/4/5, DQ, and DP are weakly stimulatory in primary responses evaluated by mixed lymphocyte reactions in spite of evidence that they may elicit T cell allo-recognition.17,22,24,25
In spite of the association between TRM and multiple LEL mismatches in the 7/8 groups in the present study, there was no significant association between LEL mismatches and the incidence of grade 3-4 aGvHD to explain the higher TRM. Some of the 7/8 groups had a small sample size; therefore, this discrepancy may have resulted from limited statistical power. The present study was not designed to investigate if LEL mismatches increase the severity, prolong the duration of aGvHD, or determine refractoriness to treatment.
The association between HLA-DP mismatching and the incidence of aGvHD was previously reported.4,5,11 In the analysis of the mismatches scored according to the GvH vector, it was observed that in the 7/8 transplants, the presence of an additional mismatch in HLA-DQ, in combination with a mismatch in HEL, was associated with increased risk for patient mortality. This observation is congruent with the nonsignificant trend described in the previous analysis of the same cohort,5 which used an overall match grade for HLA-DQ rather than the GvH vector in the analysis. The impact of the DQ mismatch, paired with other HLA mismatches, on survival, confirms the observations made by Petersdorf et al10 in an early study.
In this and other studies, it was observed that although HLA-DQA140 and HLA-DPA141 are tightly associated with their respective B1 subunits, there is still a margin for discrepancy, particularly if DRB1 is mismatched. Therefore, prospective typing of HLA-DQA1 and -DPA1 loci, in addition to typing of DRB3/4/5, DQB1, and DPB1, is recommended. We found no evidence that the number of DQ or DP heterodimers mattered, so the classical definitions of mismatching may be applied.
The transplants with one mismatch at DRB1 had higher proportions of multiple LEL mismatches compared with the transplants with one mismatch at the class I loci. Subanalyses of groups stratified according to the mismatched locus demonstrated that the effect of the LEL was independent of the HEL type and was not the result of linkage disequilibrium between alleles at the HLA class II loci.
In the design of the present study, we did not use a scoring algorithm that takes into account permissive and nonpermissive HLA-DPB1 mismatches, as defined by Zino et al.12 Fleischhauer et al13 proposed that the avoidance of an URD with a nonpermissive T-cell–epitope mismatch at HLA-DPB1 might provide a practical clinical strategy for lowering the risks of mortality after unrelated-donor hematopoietic cell transplantation without requiring HLA-DPB1 matching for all patients. Interestingly, the classification of the nonpermissive DPB1 mismatch also takes into account the GvH or HvG vector. The alleles DPB1*01:01, 02:01, 02:02, 03:01, 04:01, 04:02, 05:01, and 06:01 correlate with the HLA specificities DPw1 to DPw6 defined by T cells, respectively.28 With the exception of DPB1*03:01, all other DPB1 alleles with a counterpart of a DPw T-cell–defined associated specificity belong to the nonimmunogenic group according to Zino et al.12 These observations indicate that mismatches in many of the common DPB1 alleles may elicit T-cell allo-responses. This conclusion is supported by the findings of studies performed by Rutten et al42,43 that demonstrated that, in vivo, both permissive and nonpermissive HLA-DPB1 mismatches resulted in strong polyclonal immune responses. Thus, studies examining the effect of DP mismatches according to the permissive/nonpermissive categorization, in combination with the occurrence of mismatches in other HLA loci (LEL and HEL), are warranted. It is possible that the evaluation of permissive/nonpermissive in the context of the LEL score proposed in the present study may result in the design of an improved strategy for optimization of donor selection.
In summary, the results obtained in the present study indicate that prospective evaluation of matching for DRB3/4/5, DQ, and DP loci may be warranted to reduce posttransplant risks in donor recipient pairs mismatched in one (7/8) HEL (HLA-A, -B, -C, and -DRB1).
The matching score described here was built on the basis of allele level matches in 8 HEL and 6 LEL and provides an easy way for evaluating and optimizing the selection of an URD with one mismatch in the HEL HLA loci.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases, Grant/Cooperative Agreement 5U01HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, contract HHSH234200637015C with the Health Resources and Services Administration, grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research, and grants from Allos, Inc., Amgen, Inc., Angioblast, an anonymous donation to the Medical College of Wisconsin, Ariad, Be the Match Foundation, Blue Cross and Blue Shield Association, Buchanan Family Foundation, CaridianBCT Celgene Corporation, CellGenix GmbH, Children’s Leukemia Research Association, Fresenius-Biotech North America, Inc., Gamida Cell Teva Joint Venture Ltd., Genentech, Inc., Genzyme Corporation, GlaxoSmithKline, HistoGenetics, Inc., Kiadis Pharma, The Leukemia & Lymphoma Society, The Medical College of Wisconsin, Merck and Co., Inc., Millennium: The Takeda Oncology Co., Milliman USA, Inc., Miltenyi Biotec, Inc., NMDP, Optum Healthcare Solutions, Inc., Osiris Therapeutics, Inc., Otsuka America Pharmaceutical, Inc, RemedyMD, Sanofi, Seattle Genetics, Sigma-Tau Pharmaceuticals, Soligenix, Inc., Stem-Cyte, A Global Cord Blood Therapeutics Co., Stemsoft Software, Inc., Swedish Orphan Biovitrum, Tarix Pharmaceuticals, Teva Neuroscience, Inc., THERAKOS, Inc., and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.
Authorship
Contribution: M.A.F.-V., J.P.K., M.H., S.R.S., P.C., E.W.P., M.S., R.C., S.J.L., and M.d.L. critically revised the research plan; M.A.F.-V., S.R.S., S.J.L., and M.d.L. drafted the manuscript; M.A.F.-V., J.P.K., M.H., S.R.S., C.A., H.N., L.A.B.-L., P.C., N.F., D.L.C., M.M.H., M.O., E.W.P., M.S., R.C., S.J.L., and M.d.L. analyzed and interpreted data and critically revised the manuscript; J.P.K. and M.H. performed statistics; and M.A.F.-V. drafted the research plan.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcelo Fernandez-Vina, Department of Pathology, Stanford Medical School Blood Center, 3373 Hillview Ave, Palo Alto, CA 94304; e-mail: marcelof@stanford.edu.