Key Points
Severe thrombocytopenia is rare and major hemorrhage is uncommon in children with persistent and chronic ITP.
In children with persistent or chronic ITP, there is a trend toward reserving drug therapy for those experiencing significant bleeding.
Abstract
Long-term follow-up of children with immune thrombocytopenia (ITP) indicates that the majority undergo remission and severe thrombocytopenia is infrequent. Details regarding bleeding manifestations, however, remain poorly categorized. We report here long-term data from the Intercontinental Cooperative ITP Study Group Registry II focusing on natural history, bleeding manifestations, and management. Data on 1345 subjects were collected at diagnosis and at 28 days, 6, 12, and 24 months thereafter. Median platelet counts were 214 × 109/L (interquartile range [IQR] 227, range 1-748), 211 × 109/L (IQR 192, range 1-594), and 215 × 109/L (IQR 198, range 1-598) at 6, 12, and 24 months, respectively, and a platelet count <20 × 109/L was uncommon (7%, 7%, and 4%, respectively). Remission occurred in 37% of patients between 28 days and 6 months, 16% between 6 and 12 months, and 24% between 12 and 24 months. There were no reports of intracranial hemorrhage, and the most common site of bleeding was skin. In patients with severe thrombocytopenia we observed a trend toward more drug treatment with increasing number of bleeding sites. Our data support that ITP is a benign condition for most affected children and that major hemorrhage, even with prolonged severe thrombocytopenia, is rare.
Introduction
Immune thrombocytopenia (ITP) during childhood is usually characterized by acute onset of thrombocytopenia with ∼75% of children having disease resolution within 6 months. Most children experience only mild bleeding in the form of bruising and petechiae. The risk of severe hemorrhage is related to duration of marked thrombocytopenia and is highly variable. Long-term outcome of children with chronic ITP is primarily defined by retrospective series.1-4 As a group, these studies show that many children achieve spontaneous remission beyond 6 months, with severe thrombocytopenia being uncommon and serious bleeding rare even in children with persistent disease. The Intercontinental Cooperative ITP Study Group (ICIS) published a large prospective registry of children with ITP with rates of chronic ITP, then defined as a platelet count <150 × 109/L at 6 months, ranging from 23% to 47% depending on age, with an additional 25% of children subsequently achieving a normal platelet count between 6 and 12 months.5,6 Data beyond 12 months and information regarding bleeding events, however, were not reported.
ICIS Registry II was designed to obtain prospective data on children diagnosed with ITP regarding timing, location, and severity of hemorrhage at diagnosis and during the subsequent course of the disease. The analysis of hemorrhage at diagnosis and during the subsequent 28 days was previously reported.7 This new analysis describes follow-up ICIS Registry II data at 6, 12, and 24 months following ITP diagnosis with a focus on natural history, bleeding manifestations, and clinical management.
Materials and methods
ICIS Registry II was a prospective cohort study designed to enroll consecutive patients with ITP at each participating center. Children eligible for enrollment were older than 4 months and younger than 20 years of age with newly diagnosed ITP based upon standard criteria at the time (platelet count of <150 × 109/L),8,9 and under the care of an ICIS investigator who agreed to submit data to the ICIS coordinating office in Basel, Switzerland. Each participating center was required to document approval of the study by its institutional review board and written informed consent from the parents of participating patients in accordance with the Declaration of Helsinki. Medical management of the child was at the discretion of the investigator and not defined by the study protocol. Patients enrolled in the registry between January 2001 and December 2007 were included in this analysis and were not enrolled in prior or subsequent ICIS registries.
Data were collected from the medical records at the participating center and submitted to the ICIS coordinating office. Information including demographics, diagnostic platelet count, initial therapy, sites of bleeding, and the severity of bleeding using the scale of Bolton-Maggs and Moon10,11 was reported. Follow-up data collected at 28 days, and 6, 12, and 24 months, following diagnosis included platelet count, medical management since the last visit, hospitalization, death related to ITP, and any change in the patient’s diagnosis. Bleeding severity was retrospectively assessed at 28 days following diagnosis using the severity scale of Bolton-Maggs and Moon.10,11 Beyond 28 days, bleeding manifestations were retrospectively recorded at 6, 12, and 24 months by describing all sites of bleeding since the last research visit, specifically skin, mouth, menorrhagia, gross hematuria, epistaxis, gastrointestinal, intracranial, and other. For this analysis, bleeding severity was based on the number of bleeding sites, presuming that patients with a greater number of bleeding sites had more severe bleeding.
For the purpose of analysis, remission was defined as a platelet count greater than the threshold (either ≥150 × 109/L or ≥100 × 109/L) on all subsequent follow-up visits in the absence of prior splenectomy. For natural history data, to account for any bias regarding missing data or patients lost to follow-up, we included only the 343 patients who had platelet counts recorded at all study visits. The remainder of the reported data represents all enrolled patients.
The majority of the results are descriptive. Nonparametric testing was performed to compare the relationship between platelet count and number of bleeding sites at 6, 12, and 24 months independently. A test for linear trend was performed to determine trends in likelihood of treatment based on number of bleeding sites at different platelet thresholds. An α level of 0.05 was used.
Results
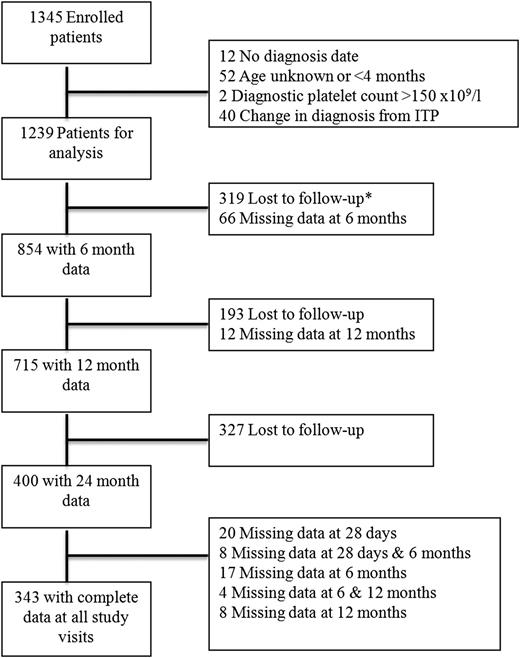
There were 1345 subjects enrolled on ICIS Registry II between January 1, 2001 and December 31, 2007 by 74 pediatric hematology investigators from 22 different countries. At time of this analysis, all patients had completed the 24-month follow-up period. Figure 1 outlines patient enrollment and follow-up. Included in this analysis are 1106 subjects previously described.7 A total of 106 patients were excluded from the analysis (12 without a recorded date of diagnosis, 52 of unknown age or age <4 months, 2 with platelet count >150 × 109/L at diagnosis, and 40 with a recorded change in diagnosis from primary ITP). In the remaining 1239 patients, the mean age was 5.9 years (SD 4.5), and approximately half were male (51.7%). At diagnosis, 388 patients (31.3%) received no drug therapy, 311 (25.1%) intravenous immunoglobulin (IVIG) only, 285 (23%) steroids only, and 83 (6.7%) anti-D only. An additional 172 children (13.9%) received other therapy or combination therapy with the agents listed above.
Patient enrollment and follow-up. *Lost to follow-up indicates patients did not have any subsequent visits.
Patient enrollment and follow-up. *Lost to follow-up indicates patients did not have any subsequent visits.
There were 854 (69%), 715 (58%), and 400 (32%) patients with complete data available for analysis at 6, 12, and 24 months, 343 of whom had platelet counts recorded at 28 days, 6 months, 12 months, and 24 months.
Natural history
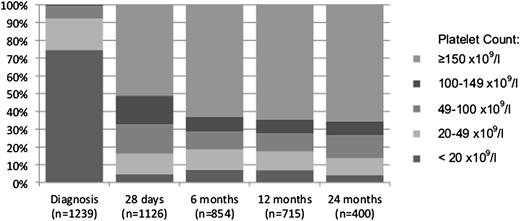
There were no ITP-related deaths. Median and interquartile range (IQR) of platelet counts were 214 × 109/L (IQR 227, range 1-748), 211 × 109/L (IQR 192, range 1-594), and 215 × 109/L (IQR 198, range 1-598) at 6, 12, and 24 months, respectively. Distribution of platelet counts at diagnosis, 28 days, 6, 12, and 24 months are shown in Figure 2. Approximately two-thirds of patients at 6, 12, and 24 months had a platelet count ≥150 × 109/L. Very few patients had severe thrombocytopenia with a platelet count <20 × 109/L at the 3 time points (7%, 7%, and 4%, respectively) or <10 × 109/L (2%, 2%, and 1%, respectively).
Percentage of children with thrombocytopenia. Percentage at diagnosis, at 28 days, and at 6, 12, and 24 months.
Percentage of children with thrombocytopenia. Percentage at diagnosis, at 28 days, and at 6, 12, and 24 months.
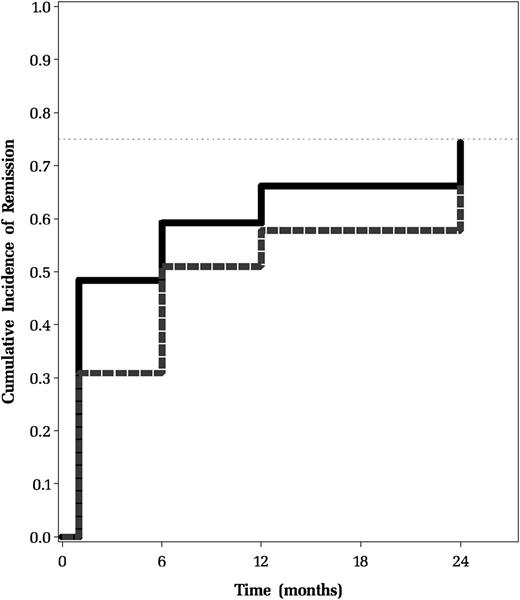
Remission rates were calculated based on the 343 patients who had platelet counts recorded at all visits. The percentage of patients achieving a durable remission based on a platelet count ≥150 × 109/L between 28 days and 6 months was 37% and between 6 and 12 months was 16%. At 12 months after diagnosis, 122 patients (36%) still had a platelet count <150 × 109/L, 29 (24%) of whom had achieved a platelet count ≥150 × 109/L at 24 months. The analysis was also performed using a platelet count of ≥100 × 109/L to define remission. Using this criterion, the percentage of patients achieving a durable remission between 28 days and 6 months was 32% and between 6 and 12 months was 21%. At 12 months after diagnosis, 96 patients (28%) still had a platelet count <100 × 109/L, 28 (29%) of whom had achieved a platelet count ≥100 × 109/L at 24 months. Figure 3 shows comparative cumulative rates of remission over time (28 days to 24 months) in this group using platelet counts of both 150 × 109/L and 100 × 109/L.
Cumulative incidence of remission. Cumulative incidence defined as having a platelet count ≥150 × 109/L on all subsequent follow-up visits without having undergone splenectomy. Data derived from the 343 patients who had platelet counts recorded at all 4 study visits beginning 28 days after diagnosis. Remission was defined as having a platelet count greater than the threshold on all subsequent follow-up visits without having undergone splenectomy.
Cumulative incidence of remission. Cumulative incidence defined as having a platelet count ≥150 × 109/L on all subsequent follow-up visits without having undergone splenectomy. Data derived from the 343 patients who had platelet counts recorded at all 4 study visits beginning 28 days after diagnosis. Remission was defined as having a platelet count greater than the threshold on all subsequent follow-up visits without having undergone splenectomy.
Bleeding manifestations
There were no reports of intracranial hemorrhage in study patients. At each time point, the most common site of bleeding was skin, followed by epistaxis.
The number of bleeding sites reported since the prior visit in relationship to severe thrombocytopenia is shown in Table 1. There was, not surprisingly, an increased percentage of patients with severe thrombocytopenia, defined as a platelet count <20 × 109/L, compared with those with mild thrombocytopenia (20-150 × 109/L) as the number of bleeding sites increased. The median platelet count at 6 months was 260 × 109/L in children who reported no bleeding and 71 × 109/L in children with at least 1 site of bleeding (P < .0001). At 12 and 24 months, these differences remained significant (237 × 109/L vs 73 × 109/L, P < .0001 and 254 × 109/L vs 58 × 109/L, P < .0001).
Number of bleeding sites reported based on platelet count
| No. of bleeding sites reported since last visit . | 6 mo following diagnosis* . | 12 mo following diagnosis* . | 24 mo following diagnosis* . | |||
|---|---|---|---|---|---|---|
| <20 × 109/L (%), n = 61 . | 20-150 × 109/L (%), n = 255 . | <20 × 109/L (%), n = 50 . | 20-150 × 109/L (%), n = 204 . | <20 × 109/L (%), n = 17 . | 20-150 × 109/L (%), n = 121 . | |
| None | 1 (2) | 90 (35) | 12 (24) | 96 (47) | 3 (18) | 65 (54) |
| 1 | 25 (41) | 105 (41) | 18 (36) | 77 (38) | 6 (35) | 35 (29) |
| 2 | 19 (31) | 46 (18) | 10 (20) | 25 (12) | 6 (35) | 15 (12) |
| ≥3 | 16 (26) | 14 (5) | 10 (20) | 6 (3) | 2 (12) | 6 (5) |
| No. of bleeding sites reported since last visit . | 6 mo following diagnosis* . | 12 mo following diagnosis* . | 24 mo following diagnosis* . | |||
|---|---|---|---|---|---|---|
| <20 × 109/L (%), n = 61 . | 20-150 × 109/L (%), n = 255 . | <20 × 109/L (%), n = 50 . | 20-150 × 109/L (%), n = 204 . | <20 × 109/L (%), n = 17 . | 20-150 × 109/L (%), n = 121 . | |
| None | 1 (2) | 90 (35) | 12 (24) | 96 (47) | 3 (18) | 65 (54) |
| 1 | 25 (41) | 105 (41) | 18 (36) | 77 (38) | 6 (35) | 35 (29) |
| 2 | 19 (31) | 46 (18) | 10 (20) | 25 (12) | 6 (35) | 15 (12) |
| ≥3 | 16 (26) | 14 (5) | 10 (20) | 6 (3) | 2 (12) | 6 (5) |
P < .05 for χ2; P < .05 test for trend.
We investigated the relationship between bleeding severity at diagnosis7 and number of bleeding sites reported at 6, 12, and 24 months. In patients with a platelet count <20 × 109/L, there was no clear relationship between the 2 variables at 6 (n = 49; Fisher exact test, P = .0650; exact trend, P = .0575) or 24 months (n = 13; Fisher exact test, P = 1.000; exact trend, P = .3357). A significant relationship was observed at 12 months (n = 40; Fisher exact test, P = .0269; exact trend, P = .0383). There were 29 patients with no or mild bleeding at diagnosis who had follow-up at 12 months. Six (21%) reported no sites of bleeding between 6 and 12 months, 14 (48%) reported 1 site of bleeding during this time, 4 (14%) reported 2 sites of bleeding, and 5 (17%) reported ≥3 sites of bleeding. In addition, there were 11 patients with moderate or severe hemorrhage at diagnosis; between 6 and 12 months 1 patient (9%) reported no bleeding sites, 1 (9%) reported 1 site of bleeding, 5 (45%) reported 2 sites of bleeding, and 4 (36%) reported ≥3 sites of bleeding.
Management
Red blood cell transfusions were infrequent (10 total occurring in 7 children, 4 reported at the 6-month visit, 2 at the 12-month visit, and 4 at 24 months). Six of these 10 transfusions were associated with bleeding reported at >2 sites. Of the 4 transfusions that had bleeding at <2 sites: 3 reported epistaxis and 1 had no bleeding reported. Platelet transfusions were uncommonly used (21 total, 12 reported at the 6-month visit, 4 at the 12-month visit, and 5 at 24 months). Twelve of these (57%) had bleeding reported at >2 sites. There were 177 reported hospitalizations, 94 patients (11%) at 6 months, 45 patients (6%) at 12 months, and 38 patients (10%) at 24 months. During the 24-month follow-up period, 10 patients underwent splenectomy, 5 (n = 854) being reported at 6 months and 5 (n = 716) at 12 months.
The most common platelet-enhancing therapies administered were similar among institutions and between 28 days and 6 months and included: steroids (17%) followed by IVIG (14%); between 6 and 12 months, steroids (13%) and IVIG (11%); and between 12 and 24 months, IVIG (11%) and steroids (10.5%).
Table 2 describes treatment based on number of bleeding sites in children with a platelet count <20 × 109/L. There was a significant trend for increased treatment with increasing number of bleeding sites at 6 and 12 months. This trend was not seen at 24 months.
Patients with platelet count <20 × 109/L who received drug therapy (including platelet transfusions)
| No. of bleeding sites reported since last research visit . | Drug treatment reported . | ||
|---|---|---|---|
| Between 28 d and 6 mo (%), n = 61* . | Between 6 and 12 mo (%), n = 50† . | Between 12 and 24 mo (%), n = 17‡ . | |
| None | 0/1 (0) | 3/12 (25) | 0/3 (0) |
| 1 | 19/25 (76) | 11/18 (61) | 5/6 (83) |
| ≥2 | 33/35 (94) | 15/20 (75) | 6/8 (75) |
| No. of bleeding sites reported since last research visit . | Drug treatment reported . | ||
|---|---|---|---|
| Between 28 d and 6 mo (%), n = 61* . | Between 6 and 12 mo (%), n = 50† . | Between 12 and 24 mo (%), n = 17‡ . | |
| None | 0/1 (0) | 3/12 (25) | 0/3 (0) |
| 1 | 19/25 (76) | 11/18 (61) | 5/6 (83) |
| ≥2 | 33/35 (94) | 15/20 (75) | 6/8 (75) |
Fisher exact test, P = .0148; exact trend, P = .0094.
Fisher exact test, P = .0250; exact trend, P = .0101.
Fisher exact test, P = .0543; exact trend, P = .0982.
Discussion
We report here long-term prospective data regarding remission rates and bleeding severity in a large cohort of children with ITP. We determined that the majority of children eventually undergo remission despite a prolonged history of ITP. In addition, we demonstrated that the prevalence of severe thrombocytopenia, defined here as a platelet count <20 × 109/L, is low in children with persistent or chronic ITP and that major hemorrhage is infrequent during any phase of the disease. Furthermore, drug therapy is usually reserved for children with more severe bleeding.
The results of this investigation parallel findings from earlier studies. Although approximately one-third of children with ITP had persistent thrombocytopenia at 6 months, 16% of them achieved durable disease remission by 12 months, a figure slightly less than the 25% rate of platelet count <150 × 109/L identified in ICIS Registry I and other natural history studies.1,5,12,13 This finding may in part be ascribed to the strict definition of remission we used in this study. In addition, we excluded the 10 children who underwent splenectomy in order to capture only spontaneous remissions even though it is possible some or many of these might have achieved remission without splenectomy.
Over a quarter of children with ITP at 12 months subsequently underwent remission by 24 months from diagnosis. Although we cannot comment on the durability of remissions in this patient group, it does support the observation that some children will achieve remission even years following their diagnosis. These findings are consistent with the published 0.21 and 0.33 cumulative probabilities of remission at 12 and 24 months in children with chronic ITP.2 They are also reflective of recently published data from a prospective Nordic cohort showing that children with persistent ITP at 6 months had remission rates of 15% by 12 months and 36% at 2 years.14 Overall, applying a platelet count of 100 × 109/L to define remission did not significantly impact remission rates (Figure 3) and supports there being a group of patients that achieve a platelet count ≥100 × 109/L and maintain that value without declining again. Interestingly, durable remission rates were higher using the higher threshold of 150 × 109/L between 28 days and 6 months compared with the threshold of 100 × 109/L. This corresponds to a large percentage of patients having a platelet count between 100 and 150 × 109/L (Figure 2) at 28 days. This lower threshold may therefore not be adequate early in the course of the disease. Further work is necessary to validate these findings and determine whether the threshold based on adult data are applicable in pediatric patients.
These results highlight that even in ITP patients with a platelet count <20 × 109/L, life-threatening bleeding was rarely observed and approximately half of children with such severe thrombocytopenia had either no bleeding or hemorrhage at only 1 site even 24 months from diagnosis. Quite striking was that there were no reported episodes of intracranial hemorrhage, and few bleeding events were significant enough to require red blood cell transfusions, a criterion used by Bolton-Maggs and Moon to define severe hemorrhage.10,11 Children with a platelet count <20 × 109/L were more likely to experience a greater number of bleeding sites than children with higher platelet counts. Interestingly, however, the number of children reporting only 1 site of bleeding, usually skin, was fairly uniform between the 2 groups. This provides further evidence for the fact that severe thrombocytopenia may be permissive but is not sufficient for major hemorrhage to occur. This may also in part indicate the frequency of “normal” bleeding in children, regardless of the platelet count, such as bruising with activity or seasonal epistaxis. However, further site-specific data to characterize the nature of the reported bleeding was not collected in this study.
Ideally, from a clinical perspective, perhaps individual patients could be risk-stratified for future bleeding complications based on the degree of hemorrhage at presentation. We therefore compared severity of bleeding at diagnosis with hemorrhage documented at 6, 12, and 24 months. However, we were unable to identify a strong relationship partially due to infrequency of moderate or severe bleeding at diagnosis. Additionally, some patients with moderate or severe bleeding at diagnosis may have been more likely to receive therapy that would decrease risk of major bleeding or development of chronic ITP later on such as treatment with IVIG at diagnosis.15
In this study, drug therapy was infrequently used in the absence of bleeding, irrespective of platelet count but was more likely to be administered to children with multiple bleeding sites. These data suggest a trend toward treatment being reserved for patients with more severe bleeding manifestations rather than because of a specific platelet count, consistent with recently published guidelines.16,17 This trend was not present at 24 months, however, perhaps because of small numbers of patients or because of a greater emphasis on other factors such as health-related quality of life at that point in time long after diagnosis. We did not collect specific data on treatments beyond corticosteroids, anti-D immunoglobulin, IVIG, splenectomy, local measures, and transfusions. Thus, it is possible that some study subjects received rituximab or additional immunosuppressive therapy not captured by the registry data. As the first report of children with ITP treated with rituximab was published in 2005,18 some patients enrolled later in the registry might have received this agent. Conversely, some children received treatment despite having no reported bleeding sites, perhaps due to factors such as proximity to the medical center, parental anxiety, child activity level, and desire to participate in sports.19 Because data were not collected on quality of life, it is difficult to comment on the rationale for such treatment in this group of patients.
We recognize that this analysis has limitations. Rates of thrombocytopenia at each chosen time point are likely influenced by follow-up patterns. Patients with a normal platelet count and/or no bleeding symptoms are less likely to continue to seek follow-up. Yet, this would be expected to bias the data toward an increase in the percentage of children at each time point with severe thrombocytopenia. The converse would be true if children who were lost to follow-up had severe bleeding events that were not recorded. Recent data from the UK registry suggest that children with clinically significant bleeding may not be necessarily captured by large registry-based studies.20 Moreover, due to a lack of a validated retrospective bleeding severity instrument, data regarding hemorrhage events and their severity were not specifically captured beyond 28 days following diagnosis.7 In the present analysis, we therefore applied the number of bleeding sites as a surrogate for bleeding severity. We felt this to be the preferred approach as the majority of children with bleeding at only 1 site experienced skin manifestations only, there were no reported episodes of intracerebral hemorrhage, and red blood cell transfusions were infrequent. It is possible, however, that some children are misclassified based on this strategy, for example the 3 episodes of epistaxis that were associated with a packed red blood cell transfusion. A limitation of this registry is the timing of bleeding events reported between study visits at 6, 12, and 24 months following diagnosis may not be related to the platelet count at the follow-up visit which could have been performed many months following the event. For example, a patient could have a bleeding event at 3 months yet the platelet count at that time might not be known or not next recorded until 6 months following diagnosis. Additionally, specific timing of drug therapy for bleeding events and the resultant platelet count was not known.
The ICIS Registry II data presented here provide follow-up data on an extremely large cohort of children with newly diagnosed ITP and confirm that ITP is a benign and self-limited condition for the majority of affected children. Even in the setting of prolonged and severe thrombocytopenia, major hemorrhage was uncommon and fatal hemorrhage was not encountered. These observations support the emerging trend toward reserving drug therapy for ITP patients with major bleeding manifestations or troublesome impairment of quality of life.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.E.N. analyzed data and wrote the manuscript; P.I., T.K., and G.R.B. designed and performed the research; G.R.B., P.H.B.B.-M., E.N., C.M.B., and V.S.B. performed the research; S.K.V. performed statistical analysis; L.A. analyzed and organized data; and all authors were involved in manuscript preparation.
Conflict-of-interest disclosure: ICIS receives unrestricted research support from CSL Behring, Amgen, and Glaxo Smith Kline. E.N. receives research funding from Glaxo Smith Kline. T.K. receives research funding from CSL Behring, Amgen, and Glaxo Smith Kline. P.H.B.B.-M. receives financial support for Glaxo Smith Kline, and the UK ITP support association. The remaining authors declare no competing financial interests.
A complete list of the members of the Intercontinental Cooperative ITP Study Group (ICIS) appears in “Appendix.”
Correspondence: Cindy E. Neunert, Georgia Regents University, 1120 15th St, BG-2011 Augusta, GA 30912; e-mail: cneunert@gru.edu.
Appendix: study group members
The investigators in ICIS ITP Registry II are: Argentina: Hugo Donato, Regina Kohan, Maria Cristina Rapetti. Australia: Peter Downie, Ping Han, Maria Kirby, Michael Marks, Benjamin Saxon, Heather Tapp. Belarus: Tatjana Uglova. Bulgaria: Ilia Kalev, Vesselina Kenderova. Canada: Victor Blanchette, David Eisenstat, Sara Israels, Robert Klaassen, Patricia McCusker, Mariana Silva, Kent Stobart, John Wu, Rochelle Yanofsky. Croatia: Srdjana Culic. Egypt: Mohsen Elalfy. Germany: Alexander Claviez, U. Göbel, Gisela Janssen, Hermann Müller, Charlotte Niemeyer, Ursula Schulte-Overberg-Schmidt, S. Weinspach. Greece/Hellas: Helen Platokouki. Israel: Ariel Koren, Hannah Tamary. Italy: Carlo Baronci, Momcilo Jankovic. Japan: Junichi Kitazawa, Hirishi Miyata, Hideo Mugishima, Hisaya Nakadate, Hiroyuki Shichino. Korea: Heung Sik Kim. Lebanon: Roula Farah. Poland: Grazyna Wrobel. Russia: Victor Petrov, Xenia Pshenichnaya. South Africa: Linda Wainwright. Switzerland: Regula Angst, Doris Auf der Maur, Pierino Avoledo, Walter Baer, Daniela Berger, Pierluigi Brazzola, Matthias Cremer, Nadège Huegli, Thomas Kühne, Hulya Ozsahin, Sylvia Schärer. Turkey: Yesim Aydinok. United Kingdom: Irene Roberts, Anne-Marie O’Hea. USA: Ashraf Abdelmonem, Carolyn Bennett, George Buchanan, Timothy Griffin, Michael R. Jeng, Vikramjit Kanvar, Greg Kirkpatrick, Sharon Lockhart, Eric Lowe, Lisa A. Michaels, Linda A. Stout, Jakica Tancabelic, Sherri Zimmerman. Yugoslavia: Lidija Dokmanovic.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal