Key Points
Human arterial ring assay is an innovative system for the three-dimensional study of tumor angiogenesis.
This assay can be exploited for antiangiogenic drug screening and gene function analysis on human vessels.
Abstract
The intrinsic complexity of the process of vessel formation limits the efficacy of cellular assays for elucidation of its molecular and pharmacologic mechanisms. We developed an ex vivo three-dimensional (3D) assay of sprouting angiogenesis with arterial explants from human umbilical cords. In this assay, human arterial rings were embedded in basement membrane extract gel, leading to a network of capillarylike structures upon vascular endothelial growth factor (VEGF) A stimulation. The angiogenic outgrowth consisted of endothelial cells, which actively internalized acetylated–low-density lipoprotein, surrounded by pericytes. Computer-assisted quantification of this vascular network demonstrated considerable sensitivity of this assay to several angiogenic inhibitors, including kinase inhibitors and monoclonal antibodies. We also performed targeted gene knockdown on this model by directly infecting explanted umbilical arteries with lentiviruses carrying short-hairpin RNA. Downregulation of VEGFR2 resulted in a significant reduction of the sprouting capability, demonstrating the relevance of human vascular explants for functional genomics studies. Furthermore, a modification of this assay led to development of a 3D model of tumor-driven angiogenesis, in which angiogenic outgrowth was sustained by spheroids of prostate cancer cells in absence of exogenous growth factors. The human arterial ring assay bridges the gap between in vitro endothelial cell and animal model, and is a powerful system for identification of genes and drugs that regulate human angiogenesis.
Introduction
Significant progress in angiogenesis research has been made after observations that a neoplastic mass does not grow above a few millimeters in diameter without recruiting new vessels.1,2 Numerous cellular and animal models have been developed to design robust and exhaustive assays able to mechanistically decipher the angiogenic cascade and to validate new antiangiogenic drugs. Endothelial cell (EC) assays can mimic individual steps of the angiogenic cascade, such as cell migration, proliferation, and capillarylike formation.3-5 However, different cell types—such as fibroblasts, pericytes, and smooth muscle cells—play a crucial role during new vessel formation by releasing soluble factors and establishing homotypic and heterotypic interactions. In addition, collective cell migration and extracellular matrix invasion are critical steps in the angiogenic process but are difficult to investigate with cultured cells.6 Characterization of angiogenesis has therefore been established using mainly animal models. However, although powerful insights into the molecular and cellular mechanisms of angiogenesis have been elucidated by means of transgenic and knockout mouse models,2,6 the complexity of animal models often limits detailed mechanistic interpretation of experimental findings. Moreover, the contribution of inflammatory cellular infiltrate and the discrepancy between developmental and adult angiogenesis complicate the understanding of in vivo models.7
A functional solution to bridge the gap between endothelial cultured cells and in vivo animal models is the use of ex vivo assays, in which explants of vascular tissues are embedded in extracellular matrix gels and produce new vascular sprouts that differentiate in capillarylike structures. Rat and mouse aortic ring (mAR) assays are frequently used to evaluate the efficacy of pro- and antiangiogenic molecules and the effect they have on different genetically modified mouse lines. Adaptations of this assay allow direct monitoring of the angiogenic process and the study of endothelial tip cell dynamics.8-11 However, rat and mAR models display certain disadvantages: a high age- and strain-dependent variability of assays,12 as well as variations owing to the region of the explanted aorta13 ; autonomous angiogenic outgrowth, limiting studies on proangiogenic factors14 ; the necessity to sacrifice animals; and the failure to detect species-specific antiangiogenic compounds in preclinical screening assays.15
In this study, we developed an ex vivo three-dimensional (3D) assay of sprouting angiogenesis with arterial explants from human umbilical cords. This assay can be exploited for antiangiogenic drug screening and, when coupled with lentiviral-mediated gene expression, for direct gene function analysis on developing human vessels. Moreover, we provide evidence that tumor cells are able to autonomously sustain the development of vascular structures.
Methods
Human AR angiogenesis assay
Umbilical cords from healthy women were provided by the Sant’Anna and Mauriziano Hospitals, Torino, Italy, under the approval and the institutional guidelines of the University of Torino Ethical Committee and in compliance with the international laws and policies. Human umbilical arteries were explanted from umbilical cords (supplemental Video 1), fibro-adipose tissue was dissected away (supplemental Video 2), and they were sectioned in 1- to 2-mm-long arterial rings (ARs) (supplemental Video 3). ARs were placed on 48-well culture dishes precoated with 100 µL of basement membrane extract (BME) (8 mg/mL, regular BME Matrigel, BD Biosciences) and then sealed in position with an overlay of 100 µL of BME and covered with 500 µL of endothelial cell basal medium (EBM-2; Clonetics) 5% fetal calf serum (FCS) plus heparin with addition of specific growth factors—vascular endothelial growth factor (VEGF)-A165, epidermal growth factor (EGF), insulinlike growth factor-1 (IGF-1), or basic fibroblast growth factor (FGF2) (all from R&D)—or complete EGM-2 (Clonetics). Medium was changed every 2 to 3 days. Tubular structures were examined with an inverted bright-field microscope (Leica Microsystem, Heerbrugg, Switzerland) and photographed. Lengths of the capillarylike structures were quantified with the image analysis software WinRhizo Pro (Regent Instruments Inc.).
Whole-mount immunofluorescence staining
Human ARs (hARs) were cultured in BME gel drop on glass-bottom dishes (WillCo Wells). They were then equilibrated in phosphate-buffered saline (PBS) and fixed with zinc-buffered formalin overnight. After sample permeabilization with 0.2% Triton-X100 for 2 hours and saturation with 10% donkey serum for 1 hour, primary antibodies—rabbit anti-NG2 (1:100, Chemicon), goat anti-VEcadherin (1:100, Santa Cruz Biotechnology), and rabbit anti-VEGFR2 (1:100, Cell Signaling) mAb—were diluted in PBS 2.5% donkey serum and incubated overnight at 4°C in a humidified chamber. hARs were washed in PBS, incubated for 1 hour at room temperature with secondary antibodies—Alexa-488 conjugated donkey α-rabbit, Alexa-555 conjugated donkey α-goat, and Alexa-555 conjugated donkey α-rabbit (1:250, Invitrogen)—or Alexa-488 conjugated phalloidin (Invitrogen) and then observed using a confocal laser-scanning microscope.
Ac-LDL uptake
Incorporation analysis of Alexa-488–conjugated acetylated–low-density lipoprotein (Ac-LDL) (Invitrogen) was performed to assess the uptake ability of angiogenic outgrowth, as previously described.16 hARs were incubated with 15 µg/mL of Alexa-488 Ac-LDL or Alexa-488 10 000 kDa Dextran (Invitrogen) in EGM-2 for 4 hours at 37°C and washed 3 times in PBS. For live nuclear cell detection, hARs were incubated with cell-permeant nucleic acid stain (Syto63, Invitrogen). Ac-LDL uptake was observed in live hARs using a confocal laser-scanning microscope.
Computer assisted image analysis
Brightfield photomicrographs were modified by Image Pro Plus, as shown in supplemental Figure 3A. Kernel well.7 × 7 (strength: 7 pixels) filter makes binarylike modification of the images without user-defined threshold selection. After Kernel modification, capillarylike structures were selected by WinRhizo Pro (Regent Instruments Inc.), image analysis software studied to recognize elongated particles (“threshold” = 120; “image smoothing” = medium; “length/width ratio” <4; “background” = black). Angiogenic sprouting capability was quantified as total sprouts length (sum of every sprout from each hAR), normalized with the control. This image analysis process was a completely automatic quantification of angiogenic sprouting (supplemental Figure 3A). For branching index analysis, we manually counted all branching points and divided them by WinRhizo-quantified sprouts length.
Whole-mount confocal imaging and digital elaboration
Immunostained or green fluorescent protein (GFP)-transduced hARs were analyzed using a confocal laser-scanning microscope (TCS SP2 with DM IRE2; Leica Microsystem) equipped with 5×/0.15 HCX PL Fluotar, 10×/0.30 HCX PL Fluotar, and 20×/0.70 HCX PL APO objective. Confocal images are the maximum projections of a partial z-section.
For 3D rendering, after digital background subtraction and blind deconvolution using ImageJ plugins, high-resolution confocal image stacks of whole-mount hARs stained with anti-VEcadherin Ab were reconstructed by isosurface rendering using Imaris software (version 6.3.0, Bitplane, AG). Isosurface rendering is a computer-generated representation of a specified range of fluorescence intensities in a dataset that allows the creation of an artificial solid object of a specific area.
Angiogenic drug treatment experiments
Sunitinib and combretastatin were purchased from Axon Medchem. SU5416 (VEGFR2 kinase inhibitor III) and PI-103 were purchased from Calbiochem. Avastin (Bavacizumab) was purchased from Roche. Polyclonal rabbit α-human VEGF-A165 Ab was purchased from Thermo Scientific. All drugs were solubilized in dimethyl sulfoxide (DMSO). DMSO alone or control IgG treatment did not modulate angiogenesis. Dose-response inhibitor curve fittings for measurement of IC50 were performed with GraphPad Prism 5 and showed R2 values >0.7.
Mouse aortic ring angiogenesis assay
Animal housing and procedures involving mice were completed in accordance with the Italian Ministry of Health (D.L. 162/1979) and the ethical guidelines for animal care of the European Community Council (directive 86/609/ECC). Experiments performed were approved by the local ethical committee (Ethical Committee for Animal experimentation of the Fondazione Piemontese per la Ricerca sul Cancro). mAR assays were performed as previously described.9 In brief, thoracic aortas were removed from 8- to 12-week-old wild-type C57/BL6 mice (Charles River) and fibroadipose tissue was dissected away. Aortas were sectioned into 1-mm-long aortic rings and incubated for 2 days in serum-free medium with antibiotics. Forty-eight–well culture dishes were coated with 100 µL of BME (8 mg/mL, regular BME Matrigel, BD Biosciences) and allowed to solidify. A single mAR per well was sealed in place with an overlay of 100 µL of BME and covered with 500 µL of EGM-2 (Clonetics). Medium was changed every 2 to 3 days. Tubular structures were examined with a bright-field contrast microscope (Leica Microsystem) and photographed. Lengths of the capillarylike structures were quantified with the imaging software WinRhizo Pro (Regent Instruments Inc.).
Stable lentiviral-mediated AR transduction
mARs were incubated for 48 hours in 50 µL of M199 (Sigma, St. Louis, MO) plus 8 μg/mL of Polybrene (Sigma) and 5 × 105 purified and concentrated virions. hARs were incubated for 48 hours in 100 µL of EGM-2 (Clonetics) plus 8 μg/mL of Polybrene (Sigma) and 2 × 106 purified and concentrated virions.
Tumor human angiogenesis assay
hARs were prepared as described before. The day before gel embedding, to induce formation of autonomous 3D spheroidal aggregates of cells (STC), 6 × 104 LNCaP cells or DsRed-transduced LNCaP cells were seeded into nonadherent, round-bottomed, 96-well plates and cultured overnight at 37°C in DMEM (Cambrex) containing 1% methylcellulose (Sigma) plus 10% FCS. The following day, 2 LNCaP STCs were washed once in PBS and twice in methylcellulose-free medium and embedded into BME gels in the proximity of hARs. Explants and cell spheroids in 20 µL of BME were then sealed in place with an overlay of BME gel and covered with 10% FCS EBM-2 (Clonetics) or EGM-2 (Clonetics), with a change of medium every 2 to 3 days. Tubular structures were examined with a bright-field contrast microscope (Leica Microsystem) and photographed. Lengths of the capillarylike structures were quantified with the imaging software WinRhizo Pro (Regent Instruments Inc.).
Further details of our materials and methods, including cell cultures, immunohistochemistry, lentiviral preparation, purification and concentration, sprouted cells extraction, cytofluorimetric analysis, quantitative reverse transcriptase–polymerase chain reaction, and statistical analysis are available online as supplemental information.
Results
Establishment of the hAR angiogenesis assay
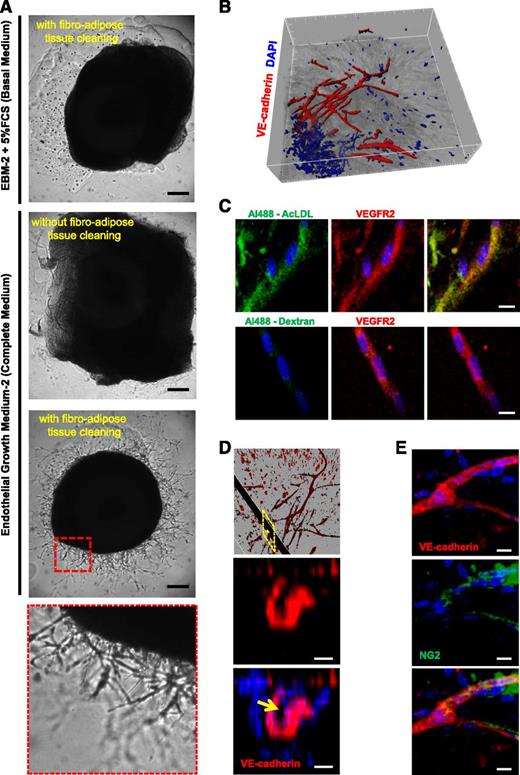
To develop 3D hAR cultures, we explanted arteries from human umbilical cords (supplemental Video 1) and carefully dissected them by removing the periarterial fibroadipose tissue (Figure 1A and supplemental Video 2). We transversely cut the artery to generate rings (supplemental Video 3), which were then embedded in BME gel (Figure 1A). These AR cultures were maintained in culture medium (EGM-2) and outgrowth began from around day 8 to day 12. Sprouting cells formed capillarylike structures, which continued to grow and branch until day 30. Microscopic analysis of these multicellular structures showed their mean diameter was 8.3 ± 2.2 μm (supplemental Figure 1A), resembling the mean diameter of human capillaries.17 Unlike mouse and rat ARs, which autonomously produce capillarylike structures,14,18 hARs only sprouted in the presence of angiogenic growth factor (GF) (Figure 1A). However, no EC outgrowth was observed without the prior removal of periarterial fibroadipose tissue (Figure 1A).
Characterization of 3D hAR cultures. (A) hARs sprout in the presence of EGM-2 but only after fibroadipose tissue cleaning. Representative micrographs of hARs with or without fibroadipose tissue cleaning, cultured with 5% FCS EBM-2 (Basal medium) or with EGM-2 (complete medium). The bottom micrograph is a magnification of the red dotted line square. Scale bar represents 200 μm. (B) 3D isosurface rendering of hAR sprouting of whole-mount immunofluorescence staining for VE-cadherin (red) and nuclei (blue) acquired through confocal microscopy. Tickmarks on axis represent 50 μm. (C) Confocal images of hAR sprouts treated with Ac-LDL or Dextran in green and stained with anti-VEGFR2 Ab in red and with DAPI in blue. Scale bar represents 10 μm. (D) Sprout lumen is shown by 3D rendering of magnification of (B) and z-stack section of yellow dotted line rectangle of hAR sprouting outgrowth stained for VE-cadherin (red) and nuclei (blue). The yellow arrow indicates the capillary lumen. Scale bar represents 5 μm. (E) Pericytes coverage. Immunostaining of whole-mount hARs with anti-VEcadherin (red), NG-2 (green), and nuclear staining with DAPI (blue). Scale bar represents 10 μm.
Characterization of 3D hAR cultures. (A) hARs sprout in the presence of EGM-2 but only after fibroadipose tissue cleaning. Representative micrographs of hARs with or without fibroadipose tissue cleaning, cultured with 5% FCS EBM-2 (Basal medium) or with EGM-2 (complete medium). The bottom micrograph is a magnification of the red dotted line square. Scale bar represents 200 μm. (B) 3D isosurface rendering of hAR sprouting of whole-mount immunofluorescence staining for VE-cadherin (red) and nuclei (blue) acquired through confocal microscopy. Tickmarks on axis represent 50 μm. (C) Confocal images of hAR sprouts treated with Ac-LDL or Dextran in green and stained with anti-VEGFR2 Ab in red and with DAPI in blue. Scale bar represents 10 μm. (D) Sprout lumen is shown by 3D rendering of magnification of (B) and z-stack section of yellow dotted line rectangle of hAR sprouting outgrowth stained for VE-cadherin (red) and nuclei (blue). The yellow arrow indicates the capillary lumen. Scale bar represents 5 μm. (E) Pericytes coverage. Immunostaining of whole-mount hARs with anti-VEcadherin (red), NG-2 (green), and nuclear staining with DAPI (blue). Scale bar represents 10 μm.
To characterize these capillarylike structures, we performed whole-mount staining with an anti-VE-cadherin Ab, a marker of mature EC. Confocal microscopy analysis showed that the majority of outgrowing cells were VE-cadherin–positive, whereas 3D rendering showed the complexity of the vascular network (Figure 1B and supplemental Video 4). The endothelial functionality of sprouting cells was evaluated using fluorescent Ac-LDL.16 Outgrowing EC, detected by the anti-VEGFR2 antibody, rapidly internalized Ac-LDL, showing positivity to Ac-LDL 4 hours after treatment (Figure 1C) and remaining fluorescent for 7 days (supplemental Figure 1B)
To further verify that outgrowing structures have capillaries features, as previously demonstrated in rat AR assays,19,20 we analyzed sprouting cells by confocal microscopy and immunohistochemistry and observed the presence of lumen with a mean diameter of 4.4 ± 1.2 μm in a restricted number of outgrowing structures (Figure 1D and supplemental Figure 1C). Moreover, in some regions of the tubular structures, NG2-positive cells were clearly visible (Figure 1E). NG2 is a well-characterized marker of mural cells,21 suggesting that mural cells surround the capillarylike structures.
Immunohistochemical analysis unveiled 2 putative sources of EC that contributed to vascular outgrowth. Arterial lumen–derived CD31-, CD34-, and VE-cadherin–positive cells invaded the tunica intima and migrated outward toward the BME gel. Furthermore, we detected VEGFR2/TIE2-positive cells in the external layer of hARs (supplemental Figure 2A-B). After 15 days of stimulation, multicellular structures were detected, sprouting from this external layer and contributing to the vascular network (supplemental Figure 2A-B).
Sensitivity to angiogenic factors
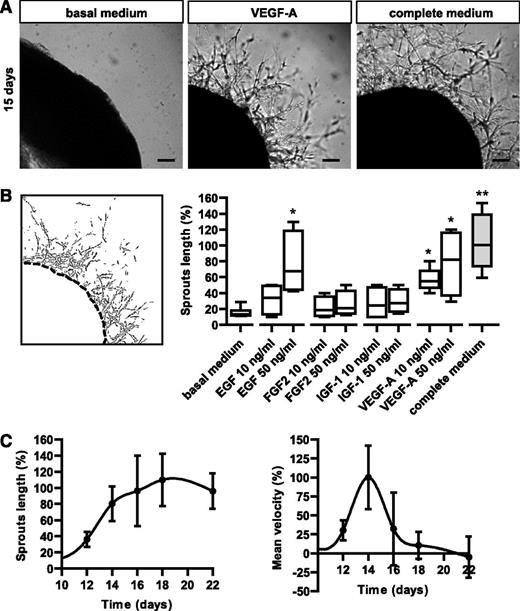
One of the major limitations of 3D sprouting assays has been the challenge of outgrowth quantification. Here we developed a computer-assisted image analysis method that automatically measured the length and area of angiogenic outgrowths (supplemental Figure 3A). Using this quantification method, we measured the hAR response to different angiogenic GFs. hARs were cultured in basal medium with different growth factors. Although VEGF-A and EGF significantly stimulated hAR outgrowth, other GFs such as basic FGF2 and IGF-1 were not able to significantly induce endothelial sprouting (Figure 2A-B). The complete culture medium (EGM-2) containing all these GFs was slightly more efficient in inducing sprouting than VEGF or EGF alone at 50 ng/mL (Figure 2A-B).
Modulation of angiogenic sprouts by growth factors. (A) Representative micrographs of hARs treated with basal medium (EBM-2), basal medium plus VEGF-A (10 ng/mL), or complete medium (EGM-2) for 15 days. Scale bar represents 200 μm. (B) The left panel shows representative output data of computer-assisted image analysis, as shown in supplemental Figure 3A. The right panel shows quantification of the angiogenic outgrowth at 15 days of hAR culture. 5% FCS EBM-2 plus heparin (basal medium) with the addition of indicated concentrations of specific GFs or complete EGM-2 (complete medium). Values shown are box-and-whisker plots, normalized to the median of complete medium hARs, of 3 independent experiments, each from 6 different umbilical cords (*P < .05 vs basal medium hARs; **P < .01). (C) Time course of angiogenic outgrowth lengths (normalized to day 16) and velocity (normalized to day 14) of hARs cultured with complete medium (EGM-2). Values shown are mean ± SD of 3 independent experiments, each from 6 different umbilical cords. Graphs represent the computer-assisted quantification shown in supplemental Figure 2A.
Modulation of angiogenic sprouts by growth factors. (A) Representative micrographs of hARs treated with basal medium (EBM-2), basal medium plus VEGF-A (10 ng/mL), or complete medium (EGM-2) for 15 days. Scale bar represents 200 μm. (B) The left panel shows representative output data of computer-assisted image analysis, as shown in supplemental Figure 3A. The right panel shows quantification of the angiogenic outgrowth at 15 days of hAR culture. 5% FCS EBM-2 plus heparin (basal medium) with the addition of indicated concentrations of specific GFs or complete EGM-2 (complete medium). Values shown are box-and-whisker plots, normalized to the median of complete medium hARs, of 3 independent experiments, each from 6 different umbilical cords (*P < .05 vs basal medium hARs; **P < .01). (C) Time course of angiogenic outgrowth lengths (normalized to day 16) and velocity (normalized to day 14) of hARs cultured with complete medium (EGM-2). Values shown are mean ± SD of 3 independent experiments, each from 6 different umbilical cords. Graphs represent the computer-assisted quantification shown in supplemental Figure 2A.
Moreover, we followed outgrowth dynamics in time-course experiments and observed a sigmoidal growth curve, characterized by sprout degeneration after 20 days of culture (Figure 2C). In the velocity-time graph, the maximum growth velocity was achieved at 14 days after BME embedding (Figure 2D).
Different from murine aortas, which are explanted and processed immediately, umbilical cords can be preserved at 4°C for days after clamping. However, a reduction in sprouting efficiency (40%) was observed when arteries were processed from umbilical cords >2 days after clamping (supplemental Figure 4A-B).
Antiangiogenic drug validation
To evaluate the quality and reproducibility of the hAR assay, we calculated the Z′-factor, a statistical value designed to reflect the dynamic range of the assay as well as the variation associated with signal measurements (supplemental Figure 5A).22 Z′-test on EGM-2 (positive control) and EBM-2 (negative control) cultured hARs unveiled that the Z′-factor for the hAR assay was 0.526 (supplemental Figure 5B), whereas the Z′-factor for the mAR assay—a widely used ex vivo assay in angiogenesis studies8-11 —was 0.353 (supplemental Figure 5C). Therefore, under similar experimental conditions, hAR is a more effective assay than mAR (supplemental Figure 5B-C). Furthermore, we performed a prospective statistical power analysis to calculate the minimum sample size required to obtain at least 50% of the sprouting length with a confidence level of 0.05. We calculated that 6 hARs were required to obtain a statistical power of 0.80, which is the standard for adequacy. This sample size was therefore used in all of our experiments.
To explore the suitability of the hAR assay for testing antiangiogenic drugs, we treated hARs with different classes of drugs undergoing preclinical trials or those approved for cancer treatment: (1) Small ATP-binding inhibitors: Sunitinib (VEGFRs and PDGFRs inhibitor, approved for clinical treatment of GIST [gastrointestinal stromal tumor] and kidney cancer), SU5416 (Semaxanib, reversible but long-term VEGFR2 kinase activity inhibitor23,24 ), and PI-103 (a dual phosphatidylinositol 3-kinase α (PI3Kα)/mTOR ATP-binding inhibitor25 ); (2) blocking antibodies: Avastin (moAb against VEGF, approved for clinical treatment of several solid tumors) and a polyclonal VEGF-blocking Ab; and (3) vascular-disruption drug: Combretastatin (vascular disrupting-agent, microtubulin-destabilizing drug, in phase II clinical trials in solid tumors26 ).
All of these antiangiogenic drugs inhibited hAR outgrowth in a concentration-dependent manner and, remarkably, their IC50 (Sunitinib 0.4 nM, SU5416 98 nM, PI-103 0.9 μM, Avastin 1.3 μg/mL and Combretastatin 1.9 nM) was lower compared with cellular studies (Figure 3A and supplemental Figure 6A-B).27-30 Elevated sensitivity to antiangiogenic drugs of the hAR assay was confirmed by comparative experiments using the murine model, in which the antiangiogenic effects of Sunitinib and PI-103 (supplemental Figure 6C), in terms of IC50, were 1.8 nM and 2.1 μM, respectively (Figure 3B and supplemental Figure 6D). Therefore, the hAR model proved to be twice more sensitive to the tested angiogenic inhibitors than the mAR model (Figure 3B).
Validation of antiangiogenic drugs with hAR assays. (A) hARs treated with antiangiogenic drugs. Quantification of angiogenic outgrowth at 15 days of culture in EGM-2. hARs treated with indicated concentrations of Sunitinib, SU5416, PI-103, Avastin, and anti-VEGF Ab. For Combretastatin, a vascular-disrupting agent, the treatment started at day 19 (shown in supplemental Figure 6B). Values shown are box-and-whisker plots, normalized to the median of DMSO or control IgG-treated hARs, of 3 independent experiments, each from 6 different umbilical cords (*P < .05 vs DMSO or control [ctr] IgG hARs; **P < .01; ***P < .001). (B) Evaluation of IC50 of antiangiogenic drugs on human and mAR assays. Nonlinear regression R2 > 0.7.
Validation of antiangiogenic drugs with hAR assays. (A) hARs treated with antiangiogenic drugs. Quantification of angiogenic outgrowth at 15 days of culture in EGM-2. hARs treated with indicated concentrations of Sunitinib, SU5416, PI-103, Avastin, and anti-VEGF Ab. For Combretastatin, a vascular-disrupting agent, the treatment started at day 19 (shown in supplemental Figure 6B). Values shown are box-and-whisker plots, normalized to the median of DMSO or control IgG-treated hARs, of 3 independent experiments, each from 6 different umbilical cords (*P < .05 vs DMSO or control [ctr] IgG hARs; **P < .01; ***P < .001). (B) Evaluation of IC50 of antiangiogenic drugs on human and mAR assays. Nonlinear regression R2 > 0.7.
Ex vivo human gene knockdown
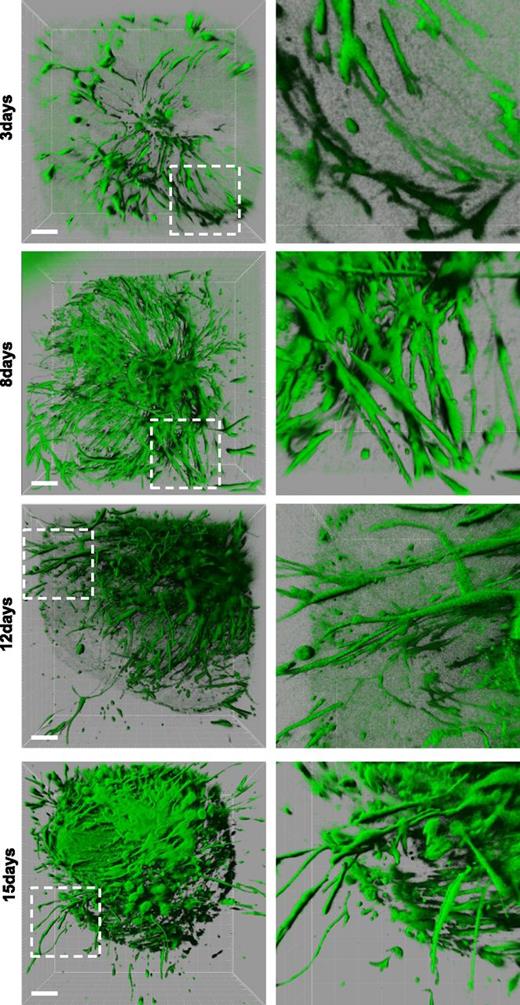
Gene-silencing technology has considerably improved medical and biological research but has rarely been applied to human tissues. We performed a gene target knockdown on hARs by directly infecting explanted umbilical arteries with lentiviruses carrying short hairpin RNA (shRNA). To set up the ex vivo lentiviral transduction and evaluate infection efficiency, we infected hARs with a GFP-producing lentivirus. Confocal microscopy analysis showed that most of the cells constituting the vascular outgrowths were fluorescent, indicating that quiescent arterial ECs were efficiently infected by lentiviruses (Figure 4). The most efficient infection was achieved using 2 × 106 viral particles per hAR for 48 hours (supplemental Figure 7A). Interestingly, the time-course 3D imaging showed that angiogenic outgrowth originated both from lumens and external layers of hAR (Figure 4), as suggested by Immunohistochemical analysis (supplemental Figure 2B-C).
Morphogenesis of GFP lentiviral-transduced hARs. hARs stably transduced with lentiviruses carrying pLKO.1-GFP before gel embedding. Three-dimensional rendering reconstruction of high-resolution confocal stacks of representative hARs at indicated days of culture. Magnification is shown in the right column. Scale bar represents 200 µm.
Morphogenesis of GFP lentiviral-transduced hARs. hARs stably transduced with lentiviruses carrying pLKO.1-GFP before gel embedding. Three-dimensional rendering reconstruction of high-resolution confocal stacks of representative hARs at indicated days of culture. Magnification is shown in the right column. Scale bar represents 200 µm.
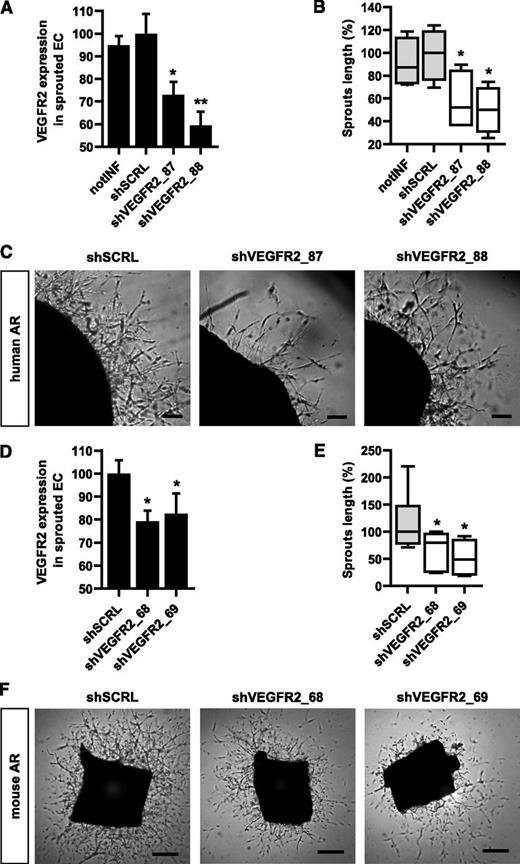
To evaluate the effects of gene silencing in hARs, we targeted VEGFR2, it being the crucial gene for modulating angiogenic processes. We selected the shRNA-targeting VEGFR2 by screening EC from umbilical cord veins (supplemental Figure 8A-B). hARs were infected with the 2 most efficient VEGFR2-silencing shRNAs (shVEGFR2_87 and shVEGFR2_88) or with scramble shRNA (shSCRL) as a control, and VEGFR2 expression was evaluated on EC extracted from the gel (Figure 5A and supplemental Figure 9A-B). Both shVEGFR2_87 and shVEGFR2_88 were effective in reducing VEGFR2 levels compared with scramble shRNA (Figure 5A and supplemental Figure 9A-B). hARs infected with shVEGFR2_87 and shVEGFR2_88 demonstrated significantly reduced growth of capillarylike structures compared with shSCRL (Figure 5B-C).
Lentiviral-mediated stable knockdown of VEGFR2 in hAR and mAR models. (A) Graph reports VEGFR2 expression in hAR-sprouted cells extracted from BME. hARs are transduced with lentiviruses carrying VEGFR2-targeted shRNA (shVEGFR2_87 and shVEGFR2_88) and scramble shRNA (shSRCL), or not infected (notINF). VEGFR2 mean fluorescence signals of VEGFR2-positive cells are shown and expressed as relative percentage of VEGFR2 levels of shSCRL hARs (*P < .05 vs shSCRL hARs; **P < .01). (B-C) Quantifications and representative micrographs of hARs transduced with lentiviruses carrying VEGFR2-targeted shRNA (shVEGFR2_87 and shVEGFR2_88) and scramble shRNA (shSRCL) or not infected (notINF). hARs were observed after 15 days of EGM-2 culture and are representative of 3 experiments, each from 6 umbilical cords. Values shown are box-and-whisker plots of sprout lengths normalized to the median of shSCRL hARs. Scale bar represents 200 µm (*P < .05 vs shSCRL hARs). (D) Graph showing VEGFR2 expression in mAR-sprouted cells extracted from BME. VEGFR2 mean fluorescence signals of VEGFR2-positive cells are shown and expressed as relative percentage of VEGFR2 levels of shSCRL mARs (*P < .05 vs shSCRL mARs). (E-F) Quantifications and representative micrograph of mARs transduced with lentiviruses carrying VEGFR2-targeted shRNA (shVEGFR2_69 and shVEGFR2_70) and scramble shRNA (shSCRL). mARs were observed after 5 days of EGM-2 culture and are representative of 3 experiments, each from 5 different mice. Values shown are box-and-whiskers plots of sprout lengths, normalized to the median of shSCRL mARs. Scale bar = 200 µm (*P < .05 vs shSCRL mARs).
Lentiviral-mediated stable knockdown of VEGFR2 in hAR and mAR models. (A) Graph reports VEGFR2 expression in hAR-sprouted cells extracted from BME. hARs are transduced with lentiviruses carrying VEGFR2-targeted shRNA (shVEGFR2_87 and shVEGFR2_88) and scramble shRNA (shSRCL), or not infected (notINF). VEGFR2 mean fluorescence signals of VEGFR2-positive cells are shown and expressed as relative percentage of VEGFR2 levels of shSCRL hARs (*P < .05 vs shSCRL hARs; **P < .01). (B-C) Quantifications and representative micrographs of hARs transduced with lentiviruses carrying VEGFR2-targeted shRNA (shVEGFR2_87 and shVEGFR2_88) and scramble shRNA (shSRCL) or not infected (notINF). hARs were observed after 15 days of EGM-2 culture and are representative of 3 experiments, each from 6 umbilical cords. Values shown are box-and-whisker plots of sprout lengths normalized to the median of shSCRL hARs. Scale bar represents 200 µm (*P < .05 vs shSCRL hARs). (D) Graph showing VEGFR2 expression in mAR-sprouted cells extracted from BME. VEGFR2 mean fluorescence signals of VEGFR2-positive cells are shown and expressed as relative percentage of VEGFR2 levels of shSCRL mARs (*P < .05 vs shSCRL mARs). (E-F) Quantifications and representative micrograph of mARs transduced with lentiviruses carrying VEGFR2-targeted shRNA (shVEGFR2_69 and shVEGFR2_70) and scramble shRNA (shSCRL). mARs were observed after 5 days of EGM-2 culture and are representative of 3 experiments, each from 5 different mice. Values shown are box-and-whiskers plots of sprout lengths, normalized to the median of shSCRL mARs. Scale bar = 200 µm (*P < .05 vs shSCRL mARs).
The VEGFR2 knockdown results in hARs were then compared with the mAR model. VEGFR2 knockdown efficiency was evaluated (Figure 5D) after transduction with the 2 most effective shRNAs for silencing mouse VEGFR2 (supplemental Figure 8C-D) in mARs. Gene silencing of VEGFR2 in mARs resulted in a significant reduction of sprouting capability (Figure 5E-F), similar to that obtained in hARs.
These results show that, by coupling lentiviral-mediated gene silencing with ex vivo human models, it is possible to perform functional genetic studies directly on human explants.
A 3D model of tumor-driven angiogenesis
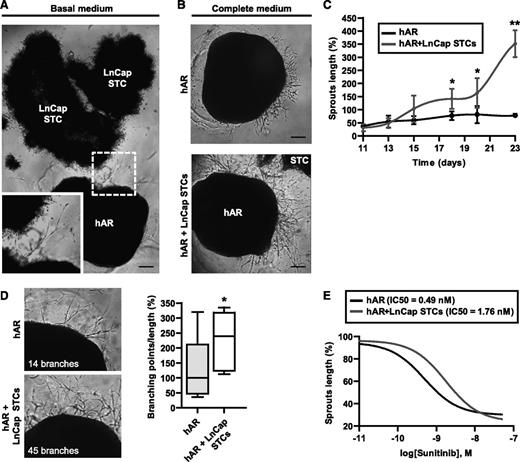
Tumor cells elicit angiogenesis through the production of proangiogenic factors, such as VEGF.31 We investigated whether tumor cells were able to sustain the formation of capillarylike structures from hARs in the absence of exogenous GFs. For these investigations, we used LNCaP cancer cells, originating from prostate carcinoma and known to produce angiogenic GFs.32 In addition, these cells showed the ability to grow as STCs in BME gel, which recapitulate several features of tumor progression.33 Angiogenic outgrowth was stimulated in hARs that were embedded in BME gel together with STC and cultured in the absence of GFs. The sprouting process started after 20 to 25 days and reached maximal outgrowth at days 30 to 35 (Figure 6A). In addition, STC synergized with exogenous angiogenic factors to support the formation of capillarylike structures (Figure 6B). Angiogenic outgrowth, stimulated by the culture medium, was strongly increased by the presence of STCs, with a considerable gain in sprout length, observable after 15 to 18 days of culture (Figure 6C). Moreover, the capillarylike structures formed in the presence of STC were more branched than in the presence of complete medium alone (Figure 6D). To exclude the contribution to angiogenic outgrowth of potentially protruding STC, we observed this process in time-lapse microscopy. Capillarylike structures were clearly derived exclusively from hAR (supplemental Figure 10A). In some experiments STC were transduced with DsRed and visualized by fluorescent microscopy, showing compact aggregate of cells that never produced protruding structures (supplemental Figure 10B). A partial directionality of the capillarylike structures toward the STC was observed, although endothelial sprouts were also present in the opposite region of the hARs (Figure 6A-B). We evaluated the sensitivity of this tumor-driven angiogenesis model by treating the cocultures with the antiangiogenic drug Sunitinib. Interestingly, Sunitinib treatment was less effective in hARs cocultured with STC compared with hARs alone (Figure 6E). The threefold difference of IC50 in response to Suntinib between hARs (0.49 nM) and STC-hARs (1.76 nM) may be explained by other angiogenic factors produced by STC.
Use of the hAR model to test the angiogenic potential of tumor cells. (A) Representative photograph of hARs cocultured with LNCaP STC for 30 days in basal medium (EBM-2 plus 10% FCS). The inset shows higher magnification of the same photograph. Scale bar represents 400 µm. (B) Representative micrographs of angiogenic outgrowth of hARs with or without LNCaP STCs for 25 days. Scale bar represents 400 µm. (C) Time course of angiogenic outgrowth length (normalized to day 25 of hARs alone) of hARs cultured with complete medium (EGM-2) with or without LNCaP STCs (shown in B). Values shown are mean ± SD of 3 independent experiments, each from 6 different umbilical cords (*P < .05 vs hARs alone; **P < .01; ***P < .001). (D) Branching density analysis in tumor hARs. Representative micrographs and quantifications of angiogenic outgrowth of hARs with or without LNCaP STCs for 25 days. Values shown are box-and-whisker plots of branching points number divided by sprout lengths and normalized to the median of hARs alone (*P < .05 vs hARs alone). (E) Dose-response normalized inhibitor curve fittings for evaluation of IC50 of Sunitinib treatment on hARs alone or with LNCaP STCs. Equation “log(inhibitor) vs normalized response” Y = 100/(1+10^((X-LogIC50))). Nonlinear regression R2 > 0.7.
Use of the hAR model to test the angiogenic potential of tumor cells. (A) Representative photograph of hARs cocultured with LNCaP STC for 30 days in basal medium (EBM-2 plus 10% FCS). The inset shows higher magnification of the same photograph. Scale bar represents 400 µm. (B) Representative micrographs of angiogenic outgrowth of hARs with or without LNCaP STCs for 25 days. Scale bar represents 400 µm. (C) Time course of angiogenic outgrowth length (normalized to day 25 of hARs alone) of hARs cultured with complete medium (EGM-2) with or without LNCaP STCs (shown in B). Values shown are mean ± SD of 3 independent experiments, each from 6 different umbilical cords (*P < .05 vs hARs alone; **P < .01; ***P < .001). (D) Branching density analysis in tumor hARs. Representative micrographs and quantifications of angiogenic outgrowth of hARs with or without LNCaP STCs for 25 days. Values shown are box-and-whisker plots of branching points number divided by sprout lengths and normalized to the median of hARs alone (*P < .05 vs hARs alone). (E) Dose-response normalized inhibitor curve fittings for evaluation of IC50 of Sunitinib treatment on hARs alone or with LNCaP STCs. Equation “log(inhibitor) vs normalized response” Y = 100/(1+10^((X-LogIC50))). Nonlinear regression R2 > 0.7.
Discussion
The intrinsic complexity of vessel sprouting limits the efficacy of cellular in vitro assays in elucidating molecular, cellular, and pharmacologic mechanisms.3-5,12-15,26 In this study, we describe a reproducible system for investigating the angiogenic cascade by controlling experimental variables and precisely quantifying angiogenic outgrowth. We developed a human angiogenesis model, in which umbilical artery rings embedded in BME produced capillarylike structures, recapitulating different steps of angiogenesis, including EC sprouting, migration, and differentiation into capillaries. Contrary to previously described similar assays (ie, those involving rat and mouse ARs, which autonomously form capillarylike structures when matrix-embedded), vessel outgrowth of hARs is completely dependent on the addition of angiogenic factors in the culture medium. Specifically, the presence of VEGF is required for endothelial sprouting from hARs, whereas VEGFR2 inhibition completely blocks this process. In contrast to FGF2, and IGF-1, which do not directly control endothelial outgrowth, a high concentration of EGF efficiently induces this process.
Three-dimensional culture models are often restricted by difficulties in achieving unbiased and reproducible quantifications. To overcome this limitation, we developed a computer-assisted image analysis strategy to quantify angiogenic outgrowth. Our experiments with antiangiogenic drugs demonstrated the power of this assay to quantitatively evaluate their inhibitory effects at different steps of the angiogenic cascade. In addition, the higher sensitivity to angiogenic inhibitors compared with other assays (eg, cultured cells or mAR) suggests the possibility of exploiting these hAR assays for screening new antiangiogenic drugs. As such, the assay is particularly applicable to biological therapeutics (such as antibodies and ligand traps) that cannot be studied in mice because of their specificity for human targets. The reproducibility and robustness of this assay make it a versatile tool for preclinical angiogenesis studies in parallel with animal trials.
A major potential application of the hAR assay is the investigation of the impact of gene loss-of-function on human angiogenesis through exploitation of lentiviral-mediated RNA interference. As proof of principle, we downregulated VEGFR2 in hAR, obtaining a significant reduction of the sprouting capability, which demonstrates the possibility of performing functional genomics studies directly on human vascular explants. Thus far, ex vivo studies of gene deficiency were limited by the lack of availability of genetically modified mice. This system, however, allows the study of all human genes potentially involved in the process of angiogenesis and can therefore be exploited as a tool to identify biomarkers and to monitor the efficacy of antiangiogenic regimens and the onset of resistance.34
Frequently, 3D models of angiogenesis tend to focus on the process itself, only including EC or cocultures with mesenchymal mural cells, without including direct interactions with a tumor component. In this study, we developed an ex vivo model where angiogenesis can be investigated under the direct influence of the tumor microenvironment. Spheroids of prostate cancer cells embedded in BME sustain the angiogenic outgrowth from hAR in the absence of exogenous growth factors. We did not investigate which STC-produced growth factors are involved in this angiogenesis process, but interestingly the STC-induced outgrowth was more resistant to VEGFR2 inhibition. This assay is one of the first examples of an autonomous system that does not require continuous exogenous stimulation. Interestingly, in this tumor hAR model, there is occasional direct contact of tumor cells with EC. The significance of these contacts and how they eventually develop is unknown and thus requires further investigation. However, the possibility of dynamic observation of tumor cells that recruit, interact, and stimulate the growth of new vessels can greatly further our understanding of tumor-driven angiogenesis.
In conclusion, hAR assay is an innovative system for the 3D study of human angiogenesis. This model allows (1) identification of new candidates that regulate sprouting angiogenesis, (2) screening for pro- or antiangiogenic drugs, (3) use of shRNA gene silencing directly on human sprouting angiogenesis, and (4) dynamic analysis of tumor microenvironmental effects on vessels.
This article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Associazione Italiana per la Ricerca sul Cancro investigator (grants IG10133 to F.B. and IG9158 to L.P.), Associazione Italiana per la Ricerca sul Cancro 5x1000 (code: 12182), Regione Piemonte: Ricerca Sanitaria Finalizzata, 2008, Converging Technologies Program, grant: “Photonic Biosensors for Early Cancer Diagnostics,” Technological Platforms for Biotechnology: grant DRUIDI, Fondazione Cassa di Risparmio Torino, Fondazione Piemontese per la Ricerca sul Cancro-ONLUS (Intramural Grant 5x1000 2008) (to L.P.), Fondo Investimenti per la Ricerca di Base RBAP11BYNP (Newton) (to F.B. and L.P.), and University of Torino-Compagnia di San Paolo: RETHE grant).
Authorship
Contribution: G.S. and L.P. conceived the idea. G.S., G.C., L.d.B., P.A.G., and R.S. performed the experiments and analyzed the data. L.P. and F.B. supervised the study. G.S and L.P. wrote the manuscript. L.d.B., P.A.G., and F.B. made suggestions and modifications. All authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luca Primo, Institute for Cancer Research and Treatment at Candiolo, Str. Prov. 142 Km. 3.95, 10060 Candiolo, Turin, Italy; e-mail: luca.primo@ircc.it.

![Figure 3. Validation of antiangiogenic drugs with hAR assays. (A) hARs treated with antiangiogenic drugs. Quantification of angiogenic outgrowth at 15 days of culture in EGM-2. hARs treated with indicated concentrations of Sunitinib, SU5416, PI-103, Avastin, and anti-VEGF Ab. For Combretastatin, a vascular-disrupting agent, the treatment started at day 19 (shown in supplemental Figure 6B). Values shown are box-and-whisker plots, normalized to the median of DMSO or control IgG-treated hARs, of 3 independent experiments, each from 6 different umbilical cords (*P < .05 vs DMSO or control [ctr] IgG hARs; **P < .01; ***P < .001). (B) Evaluation of IC50 of antiangiogenic drugs on human and mAR assays. Nonlinear regression R2 > 0.7.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/21/10.1182_blood-2012-08-452292/4/m_e129f3.jpeg?Expires=1771330242&Signature=qPo1850thBJTn0PnV30Cd7f3Xl~xwF90-b3wF4d9PwDM26091BE6aRqf2zpvuDX5B~AtaFIQxrOHlkJD4obLoQMoSo7vn70NnLGx2JA9xDIYr-lSFhwghC4U2lvFStcD5oq91lOBXfGxHRNjY-V2gtDs3aNV~wcqoikK6IltJhf~lqRPYzgMKMJcuS~72zrNqEUL1COnItekuzUTyILh0czI2hWj266dBrl5fou0fmIt8k9WNYv5OPv467umNO19Z7IyoiUgIBlp5jNumRmXZKBRS-67BIpb8yI7uvN4hCXNWX3B~l~tRr8UShOdi0218KkR48xBZGxYHBWLHQ5bEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal