Key Points
PF4 mediates VSMC injury responses.
PF4-driven effects are, in part, KLF4 dependent.
Abstract
Activated platelets release many inflammatory molecules with important roles in accelerating vascular inflammation. Much is known about platelet and platelet-derived mediator interactions with endothelial cells and leukocytes, but few studies have examined the effects of platelets on components of the vascular wall. Vascular smooth muscle cells (VSMCs) undergo phenotypic changes in response to injury including the production of inflammatory molecules, cell proliferation, cell migration, and a decline in the expression of differentiation markers. In this study, we demonstrate that the platelet-derived chemokine platelet factor 4 (PF4/CXCL4) stimulates VSMC injury responses both in vitro and in vivo in a mouse carotid ligation model. PF4 drives a VSMC inflammatory phenotype including a decline in differentiation markers, increased cytokine production, and cell proliferation. We also demonstrate that PF4 effects are mediated, in part, through increased expression of the transcription factor Krüppel-like factor 4. Our data indicate an important mechanistic role for platelets and PF4 in VSMC injury responses both in vitro and in vivo.
Introduction
Vascular inflammation and smooth muscle cell injury initiate and accelerate a variety of cardiovascular diseases, including atherosclerosis, accelerated graft arteriosclerosis (AGA), and ischemia-reperfusion injury. Platelets have an important role in vascular inflammation and vessel wall remodeling, but the key platelet mediators that drive vascular inflammation are not well defined.1,2 During thrombosis, platelets adhere to the exposed extracellular matrix of the vessel wall and secrete inflammatory molecules. Platelets not only adhere to a damaged vessel wall but also adhere to an intact inflamed endothelium without forming an obstructive thrombus, such as at sites of atherosclerotic lesion development and transplant endothelium.3-6 Platelet-derived inflammatory mediators include adhesion molecules (integrins, P-selectin), secreted small molecules (ADP, thromboxane, serotonin), chemokines, and cytokines. Major platelet-derived chemokines and cytokines include platelet factor 4 (PF4/CXCL4), proplatelet basic protein and its breakdown products β-thromboglobulin/NAP-2/CXCL7, RANTES/CCL5, interleukin (IL)-1α, IL-1β, IL-8, and transforming growth factor-beta.7
PF4 was the first described CXC class chemokine and is an abundant platelet protein.8,9 PF4 is best known for its pathogenic role in heparin-induced thrombocytopenia.10 PF4 is not a major thrombotic molecule, as PF4−/− mice have no difference in tail bleeding time, but their time to thrombus formation is prolonged.11 Other studies suggest that PF4 may have diverse roles in angiogenesis, thrombosis, megakaryopoiesis, and atherosclerosis.9,11-13 PF4 has been noted penetrating deep into vascular smooth muscle layers after vessel injury and in the ApoE−/− mouse model of atherosclerosis.13,14 These studies demonstrate a potentially important, but undefined, role for PF4 in vascular wall injury responses.
Vascular smooth muscle cells (VSMCs) contribute substantially to the proinflammatory environment associated with postinjury vessel remodeling. Unlike most other muscle types, VSMCs retain the capacity for phenotype changes. Differentiated or mature VSMCs do not proliferate or produce inflammatory mediators, but in response to injury, VSMCs change their gene expression profile to a less differentiated state, often referred to as a synthetic, dedifferentiated, or inflammatory phenotype, and begin to proliferate and migrate. They also begin to produce inflammatory mediators including IL-6, which directly stimulates VSMC migration and proliferation.15-18 Numerous inflammatory molecules such as platelet-derived growth factors, IL-1β, endothelial growth factors, and fibroblast growth factor have been implicated as contributing to the transition to an inflammatory phenotype. Many of these are, in large part, platelet derived. We have now found that PF4 also has a major role in driving VSMC phenotypic changes.
Inflammatory mediators ultimately activate transcription factors to initiate gene expression pattern changes associated with VSMC phenotypes. Together, myocardin and serum response factor promote gene expression typical of a differentiated phenotype.19,20 Other transcription factors, such as Krüppel-like factor 4 (KLF4), regulate gene expression leading to a synthetic phenotype.21 In response to injury or inflammation, KLF4 blunts myocardin expression and decreases serum response factor binding, reducing the expression of genes associated with a differentiated phenotype, and increasing the expression of genes associated with an inflammatory phenotype.21,22 KLF4 expression is rapidly increased after VSMC injury, and conditional deletion of KLF4 in VSMCs leads to less of a decline in VSMC differentiation markers and decreased VSMC proliferation.22 Our results indicate that PF4 is an upstream mediator of KLF4 expression.
Other studies have suggested that platelets and PF4 may exert in vitro effects on VSMCs,14,23,24 but its in vivo relevance and downstream mechanisms have not been shown. We now demonstrate that PF4 accelerates VSMC inflammatory responses to injury, in part by stimulating the expression of the transcription factor KLF4.
Methods
Animal studies
Male mice on a C57Bl6/J background greater than 10 generations were used throughout. PF4−/− mice were provided by M. Anna Kowalska, The Children’s Hospital of Pennsylvania.4 Complete carotid ligation was performed on mice anesthetized using 2.0% isoflurane, placed on a heated surgical board and given flunixin (2.5 mg/kg SQ). A midline cervical incision was made and the left common carotid isolated and ligated. For platelet depletion, rat anti-mouse GPIbα (CD42b) or control IgG was intraperitoneally injected into mice 1 day after the ligation following the instructions of the manufacturer, Emfret Analytics (Eibelstadt, Germany). This greatly reduces platelet numbers for about 3 to 4 days.25 All animal studies were approved by the University of Rochester Animal Care and Use Committee.
For bone marrow transplant, mice were irradiated and injected intravenously with 2 × 106 bone marrow cells isolated from either wild-type (WT) or IL-6−/− mice (Jax labs). At 3 weeks later, confirmation of hematopoietic genotype was determined by polymerase chain reaction (PCR) genotyping of peripheral white blood cells.
Histochemistry and immunohistochemistry
Ligated carotid arteries were removed, fixed in methanol/acetic acid, and paraffin embedded. Hematoxylin and eosin–stained slides were prepared using standard methods. Tissues were sectioned at 5 µm, and slides were deparaffinized and rehydrated to phosphate-buffered saline with endogenous peroxidase activity blocked with 30-minute incubation in 3% H2O2 in phosphate-buffered saline. PF4 antibody and secondary antibody were also both from PeproTech, with primary antibody diluted 1:1000.
Vessel wall area was determined using Image Pro software, taking vessel area measurements at 3 different points 200 µm apart moving away from the ligature. The average surface area was then determined by the mean of the 3 areas in an individual ligated vessel. This was normalized to the vessel area of the internal control nonligated right carotid artery to account for any differences in the size of the mouse or variables not related to the injury itself.
Cell culture and in vitro assays
Human coronary artery smooth muscle cells (HCASMCs) were purchased from Invitrogen (Carlsbad, CA) and kept in Medium 231 plus smooth muscle cell growth supplements (Invitrogen). At 1 week before the experiments, cell culture media were changed to differentiation media (Medium 231 plus smooth muscle cell differentiation supplements, Invitrogen). Differentiated HCASMCs were treated with endotoxin-free human PF4 (Haematologic Technologies, Essex Junction, VT). To neutralize PF4 effects, cells were pretreated with 50 U/mL of heparin (Sigma-Aldrich, St. Louis, MO) for 30 minutes and then incubated with heparin and PF4 together.
Cell proliferation was assessed by the 2,3-bis (2-methoxy-2-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (R&D Systems, Minneapolis, MN). A total of 20 000 cells were seeded in each well of a 12-well plate, and 24 hours later, XTT reagent was added to the plate and the cell number determined by the absorbance at 460 nm.
Cell migration was determined by a transwell assay. Briefly, 3 × 105 differentiated cells were seeded in the upper chamber of transwell (24-mm, 8-µm pore, Corning, Lowell, MA). PF4 was added in the lower chamber at the concentration of 1 µg/mL in Medium 231. At 18 hours later, cells were fixed with 4% paraformaldehyde, and the upper side of the membrane was wiped clean by a cotton swab. Cells underneath the membrane were visualized by Crystal Violet staining (Sigma-Aldrich). Cell numbers were counted under the microscope.
Glutathione S-transferase (GST) and glutathione S-transferase–receptor-associated protein (GST-RAP) expression plasmids were provided by Chieko Mineo, University of Texas Southwestern Medical Center. Anti-LRP1 antibody and control IgG were purchased from Molecular Innovations. Cells were treated with RAP or anti-LRP1 for 30 minutes before addition of PF4.
Enzyme-linked immunosorbent assay (ELISA)
ELISA kits were purchased from R&D Systems. Mouse plasma and cell culture supernatant were subjected to ELISA following the instructions of the manufacturer.
Real-time quantitative PCR
Total RNA was extracted from mouse carotid arteries or HCASMCs using RNAeasy Kits from Qiagen (Valencia, CA) and was converted to complementary DNA (cDNA) using the High Capacity RNA-to-cDNA kit from Applied Biosystems (Foster City, CA), according to the instructions of the manufacturer. cDNA was subjected to quantitative PCR using the SYBR Green Supermix (Bio-Rad, Hercules, CA). Predesigned primers were purchased from Qiagen. At least 3 internal controls (β2 microglobulin, ribosomal protein L4, and TATA box-binding protein) were used for quantification.
Silencing RNA knockdown and adenovirus infection
KLF4-specific small-interfering RNA (siRNA) and scrambled siRNA were purchased from Applied Biosystems and were transfected into HCASMCs with a Nucleofector device and a VSMC kit (Lonza, Walkersville, MD). For every million cells, 50 pmol of siRNA was used. KLF4 and a control LacZ adenovirus were amplified and purified by the Gene Transfer Vector Core at The University of Iowa (Iowa City, Iowa).20 HCASMCs were incubated with adenovirus at 37°C overnight. KLF4 expression was confirmed by western blotting.
Western blotting
HCASMC lysates were collected and loaded on a 12% polyacrylamide gel. After the electrophoresis, proteins were transferred to nitrocellulose and were immunoblotted using mouse monoclonal antibody against KLF4 (Abnova, Walnut, CA) or actin (recognizes all isoforms; BD Biosciences, San Diego, CA). Bound primary antibodies were detected by horseradish peroxidase–conjugated anti-mouse IgG (Sigma-Aldrich).
Statistics
Unless otherwise indicated, all statistics were performed using the paired Student t test.
Results
Platelets promote early inflammatory responses to vascular injury
Platelets are important mediators of inflammation associated with chronic inflammatory diseases such as atherosclerosis and arthritis.1,2,26 A role for specific platelet-derived mediators in VSMC injury responses that initiate chronic vascular pathologic conditions has not been addressed. To begin to explore this question, we used a complete carotid ligation model of VSMC injury with C57Bl6/J (B6) mice.27 In this well-established model, the left carotid artery of the mice is completely ligated, occluding blood flow and leading to VSMC injury responses.22,28 Starting 1 day after carotid ligation, the mice were treated with control IgG or platelet-depleting antibody every 4 days to maintain reduced platelet counts.3,25 Plasma was collected on multiple days, and plasma PF4 was measured to determine whether platelets are activated during the VSMC injury response. Plasma PF4 levels were increased in control IgG–treated mice (Figure 1A), indicating ongoing platelet activation. As expected, plasma PF4 levels were greatly reduced in platelet-depleted mice, confirming that platelets are the primary source of PF4 in this model system (Figure 1A). Because other studies have demonstrated that IL-6 is critical to VSMC synthesis and remodeling responses, we measured plasma IL-6 to determine whether platelet activation is associated with vascular injury responses.17,18,29-31 IL-6 levels were increased in control IgG–treated mice in a pattern very similar to plasma PF4 (Figure 1B), but platelet-depleted mice had no change in IL-6 levels. These data indicate that after carotid ligation, plasma IL-6 levels are increased in a platelet-dependent manner.
Platelets increase carotid ligation–induced VSMC inflammation in mice. (A) Platelets are activated after carotid ligation. The left carotid artery was permanently ligated, and the mice were treated with platelet-depleting antibody or control IgG. Plasma PF4 was measured by ELISA (n = 5 ±SD; *P < .01 vs 0). (B) Platelets promote inflammation in carotid ligation model. Plasma IL-6 was measured by ELISA in control and platelet-depleted mice after carotid ligation (n = 5 ± standard deviation [SD]; *P < .01 vs 0).
Platelets increase carotid ligation–induced VSMC inflammation in mice. (A) Platelets are activated after carotid ligation. The left carotid artery was permanently ligated, and the mice were treated with platelet-depleting antibody or control IgG. Plasma PF4 was measured by ELISA (n = 5 ±SD; *P < .01 vs 0). (B) Platelets promote inflammation in carotid ligation model. Plasma IL-6 was measured by ELISA in control and platelet-depleted mice after carotid ligation (n = 5 ± standard deviation [SD]; *P < .01 vs 0).
PF4 augments VSMC injury responses to carotid ligation
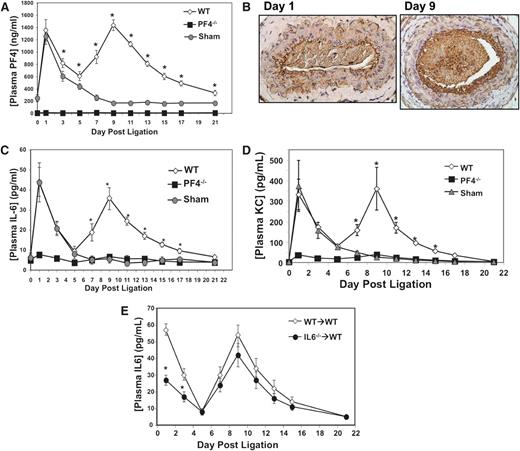
PF4 is not only a plasma marker of platelet activation but also induces strong inflammatory responses from many cells and tissues.4,8,12 Therefore, we performed carotid ligations on WT and PF4−/− mice, and a group of sham surgeries (WT mice with surgery, but no ligature placed around the carotid). Both WT and sham mice had greatly increased plasma PF4 levels within 24 hours of surgery (Figure 2A), indicating that an early phase of surgery related platelet activation. Plasma PF4 levels then declined, but in WT mice with carotid ligation, there was a second wave of platelet activation as indicated by a large increase in plasma PF4 levels between days 5 and 15 after ligation, peaking at ∼day 9 (Figure 2A). The second peak in plasma PF4 on day 9 coincides with vascular remodeling changes, including large “activated”-appearing VSMC and a more cellular vascular lumen compared with day 1 (supplemental Figure 1).
PF4 drives VSMC proinflammatory response in vivo. (A) The left common carotid artery of WT and PF4−/− mice were ligated, or a sham surgery performed, and plasma PF4 was measured (n = 5 ± SD; *P < .01 vs sham). (B) PF4 is present in the vessel wall on day 9. Immunohistochemistry studies of ligated carotid arteries on days 1 and 9 (representative images). (C) Plasma IL-6 and (D) plasma KC are increased after ligation in WT mice, but not PF4−/− mice, in a similar pattern to PF4 (n = 5 ± SD; *P < .01 vs sham). (E) Ligation-induced IL-6 is primarily vessel wall derived. WT mice were given WT or IL-6−/− bone marrow, and carotid ligations were performed. Postsurgical IL-6 is similar in WT and IL-6−/− bone marrow mice (n = 6 ± SD; *P < .05 vs WT).
PF4 drives VSMC proinflammatory response in vivo. (A) The left common carotid artery of WT and PF4−/− mice were ligated, or a sham surgery performed, and plasma PF4 was measured (n = 5 ± SD; *P < .01 vs sham). (B) PF4 is present in the vessel wall on day 9. Immunohistochemistry studies of ligated carotid arteries on days 1 and 9 (representative images). (C) Plasma IL-6 and (D) plasma KC are increased after ligation in WT mice, but not PF4−/− mice, in a similar pattern to PF4 (n = 5 ± SD; *P < .01 vs sham). (E) Ligation-induced IL-6 is primarily vessel wall derived. WT mice were given WT or IL-6−/− bone marrow, and carotid ligations were performed. Postsurgical IL-6 is similar in WT and IL-6−/− bone marrow mice (n = 6 ± SD; *P < .05 vs WT).
To determine whether plasma PF4 is representative of increased PF4 levels within the injured vessel, we isolated ligated left carotid arteries on days 1 and 9 postligation and performed anti-PF4 immunohistochemistry studies. One day postligation, light PF4-positive areas were primarily lining the vessel surface and within the lumen, but there was very limited PF4 staining in the wall itself (Figure 2B, left; control nonligated right carotid arteries, supplemental Figure 2). On day 9, the ligated left carotid artery had a large increase in PF4-positive staining in the lumen, indicating increased platelet numbers, and densely positive areas of PF4 staining in the carotid intima (Figure 2B, right). These data demonstrate that PF4 is present in the vessel wall postinjury and that direct PF4 interactions with VSMC occur.
To begin to determine the physiological role of PF4 in vascular injury, plasma IL-6 and KC (mouse IL-8 homolog) were quantified in sham, WT, and PF4−/− mice on multiple days. In both WT and sham mice, IL-6 and KC levels increased in a pattern mirroring PF4 concentrations (Figure 2C-D). Strikingly, PF4−/− mice had no change in either plasma IL-6 or KC levels at any point after ligation (Figure 2C-D). To determine the primary source of PF4-dependent cytokine responses, leukocytes, or VSMCs, we performed bone marrow transplantation of WT mice with either WT or IL-6−/− marrow. After marrow recovery, carotid ligations were performed and plasma IL-6 measured at multiple time points. IL-6−/− bone marrow mice had significantly reduced plasma IL-6 levels compared with WT bone marrow mice 1 day after ligation (Figure 2E). However, after day 5 plasma IL-6 levels were not significantly different at any point between IL-6−/− and WT bone marrow mice (Figure 2E). These data indicate that hematopoietic cells are a major source of the early postsurgical IL-6, but the vessel then becomes the primary source of IL-6. In addition, Il6 messenger RNA (mRNA) expression in the ligated left carotid arteries relative to the control right carotid arteries was greatly increased in WT, but not PF4−/−, mice (supplemental Figure 3). Together, these data indicate that PF4 has a central role in VSMC cytokine production in response to injury.
PF4 mediates induction of a VSMC inflammatory phenotype
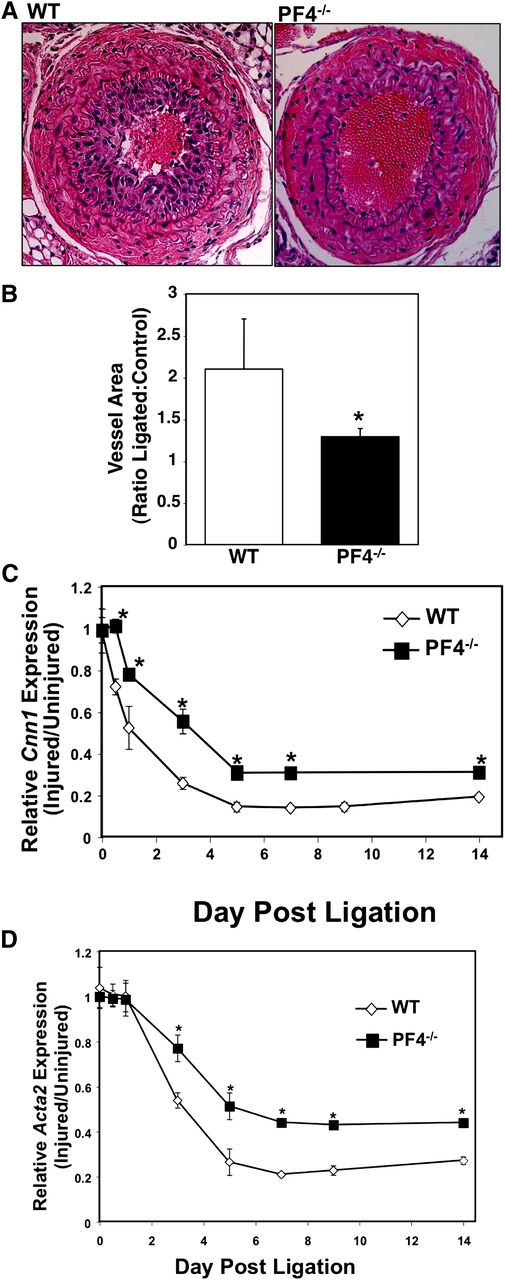
B6 mice have limited neointimal formation postligation compared with other strains, but they do have VSMC hyperplasia and vessel wall thickening.32 To determine whether PF4 stimulates VSMC proliferation and hyperplasia in addition to inflammatory mediator production, the ligated and control carotid arteries were isolated on day 21, and the ligated vessel area relative to the control nonligated right carotid arteries was determined at multiple levels away from the ligature. WT mice had greatly increased ligated vessel area compared with PF4−/− mice (Figure 3A-B), demonstrating that PF4 has a role in driving vascular wall remodeling. The VSMC inflammatory phenotype is also associated with decreased expression of differentiation markers such as calponin-1 and α actin (gene names Cnn1 and Acta2, respectively). Cnn1 and Acta2 mRNA expression in WT and PF4−/− mouse carotid arteries were determined by quantitative reverse-transcription PCR (qRT-PCR) on multiple days after ligation. The WT mice had a more rapid decline in the expression of both differentiation markers and an overall decrease in expression postinjury compared with PF4−/− mice (Figure 3C-D). These data demonstrate that PF4 is a central mediator of the VSMC inflammatory phenotype.
Mice lacking PF4 have an attenuated VSMC inflammatory phenotype switch. (A) Representative image and (B) quantification of vessel wall area. Ligated carotid arteries were isolated 21 days after ligation, and the vessel wall area was measured at multiple levels of 200 µm apart and expressed as a ratio compared with internal control nonligated right carotid (n = 5 ± SD; *P < .01 vs WT). (C-D) PF4−/− mice have less of a decline in VSMC differentiation markers in vivo. Carotids were isolated multiple days after ligation, and mRNA expression was quantified by qRT-PCR. (C) Cnn1 expression and (D) acta2 (n = 5 ± SD; *P < .01 vs WT).
Mice lacking PF4 have an attenuated VSMC inflammatory phenotype switch. (A) Representative image and (B) quantification of vessel wall area. Ligated carotid arteries were isolated 21 days after ligation, and the vessel wall area was measured at multiple levels of 200 µm apart and expressed as a ratio compared with internal control nonligated right carotid (n = 5 ± SD; *P < .01 vs WT). (C-D) PF4−/− mice have less of a decline in VSMC differentiation markers in vivo. Carotids were isolated multiple days after ligation, and mRNA expression was quantified by qRT-PCR. (C) Cnn1 expression and (D) acta2 (n = 5 ± SD; *P < .01 vs WT).
PF4 directly stimulates VSMC inflammatory responses. To confirm this, HCASMCs were cultured with multiple physiologically relevant concentrations of human PF4, and secreted IL-6 levels were measured 24 and 48 hours later. To rule out PF4 contaminant effects, HCASMCs were pretreated with heparin to neutralize PF4.33 PF4 dose-dependently increased IL-6 production (Figure 4A). To test whether PF4 has similar effects at very high concentrations, we treated HCASMCs with up to 5 µg/mL of PF4, and IL-6 production remained the same at all concentrations greater than 1 µg/mL (supplemental Figure 4). A PF4-induced IL-6 time course was also determined, and PF4 increased IL-6 levels the most between 12 and 20 hours after PF4 treatment, an effect that was inhibited by heparin before treatment (supplemental Figure 5). Similarly, IL-8 levels were measured in the supernatant of control or PF4 (1-µg/mL)–treated HCASMCs and were found to also increase in response to PF4 (supplemental Figure 6). These data demonstrate that PF4 directly induces VSMC cytokine production.
PF4 directly stimulates VSMC cytokine production. (A) Dose response. VSMCs were treated with PF4, and IL-6 levels were measured by ELISA (n = 4 ± SD; *P < .01 vs 0). (B-C) LRP1 inhibition attenuates PF4-mediated cytokine production. HCASMCs were treated with (B) control GST or GST-RAP (15 µg/mL), or (C) control IgG or LRP1-blocking antibody (25 µg/mL), before overnight incubation with PF4 (1 µg/mL). IL-6 and IL-8 in the supernatant were measured by ELISA (n = 3 ± SD; *P < .01).
PF4 directly stimulates VSMC cytokine production. (A) Dose response. VSMCs were treated with PF4, and IL-6 levels were measured by ELISA (n = 4 ± SD; *P < .01 vs 0). (B-C) LRP1 inhibition attenuates PF4-mediated cytokine production. HCASMCs were treated with (B) control GST or GST-RAP (15 µg/mL), or (C) control IgG or LRP1-blocking antibody (25 µg/mL), before overnight incubation with PF4 (1 µg/mL). IL-6 and IL-8 in the supernatant were measured by ELISA (n = 3 ± SD; *P < .01).
PF4 is reported to signal through CXCR3 and low-density lipoprotein receptor-related protein-1 (LRP1) in other cell types.9,13 CXCR3-blocking antibody had no effect on PF4-induced HCASMC cytokine production (not shown). To determine whether PF4-induced cytokine production is LRP signaling dependent, we treated HCASMCs with nothing, control GST, or with a recombinant LRP-blocking protein, GST-RAP, before PF4 treatment. LRP blocking inhibited PF4-induced IL-6 and IL-8 secretions (Figure 4B). RAP inhibits LRP receptors other than LRP1, so to better demonstrate the specificity of LRP1, we pretreated HCASMCs with LRP1-blocking antibody or control IgG before addition of PF4. The LRP1-blocking antibody also inhibited PF4-mediated HCASMC cytokine production (Figure 4C), demonstrating that PF4 signals through LRP1 in VSMCs.
To further demonstrate that PF4 drives a VSMC inflammatory phenotype, we measured the expression of CNN-1 and ACTA-2 in response to PF4. After 24 hours of PF4 (1-µg/mL) treatment, HCASMCs had decreased expression of both CNN-1 and ACTA-2 (Figure 5A), as determined by qRT-PCR. Compared with control buffer–treated cells, PF4 increased HCASMC migration in a transwell chamber assay similar to a moderate dose of tumor necrosis factor-alpha (100 ng/mL) used as a positive control (Figure 5B). PF4-mediated migration was blocked by heparin (Figure 5B). The number of HCASMCs was also increased by PF4 treatment (24 hours) compared with control-treated cells (Figure 5C). These data indicate that PF4 directly promotes an inflammatory/synthetic VSMC phenotype.
PF4 promotes a VSMC synthetic phenotype. (A) VSMCs were treated with PF4 (1 µg/mL), and the expression of CNN1 and ACTA2 was determined by qRT-PCR (n = 4 ± SD; *P < .01 vs control). (B) Transwell chamber cell migration. VSMC migration after 18 hours to the basal aspect of a transwell chamber was determined in response to buffer or PF4 (1 µg/mL). Heparin was used as a negative control and tumor necrosis factor-alpha (100 ng/mL) as a positive control (n = 3 ± SD; *P < .01 vs control). (C) Cell proliferation. A total of 20 000 cells were plated, and the number of cells was counted 24 hours later using XTT staining (n = 3 ± SD; *P < .01 vs control).
PF4 promotes a VSMC synthetic phenotype. (A) VSMCs were treated with PF4 (1 µg/mL), and the expression of CNN1 and ACTA2 was determined by qRT-PCR (n = 4 ± SD; *P < .01 vs control). (B) Transwell chamber cell migration. VSMC migration after 18 hours to the basal aspect of a transwell chamber was determined in response to buffer or PF4 (1 µg/mL). Heparin was used as a negative control and tumor necrosis factor-alpha (100 ng/mL) as a positive control (n = 3 ± SD; *P < .01 vs control). (C) Cell proliferation. A total of 20 000 cells were plated, and the number of cells was counted 24 hours later using XTT staining (n = 3 ± SD; *P < .01 vs control).
PF4 VSMC proinflammatory response is, in part, KLF4 dependent
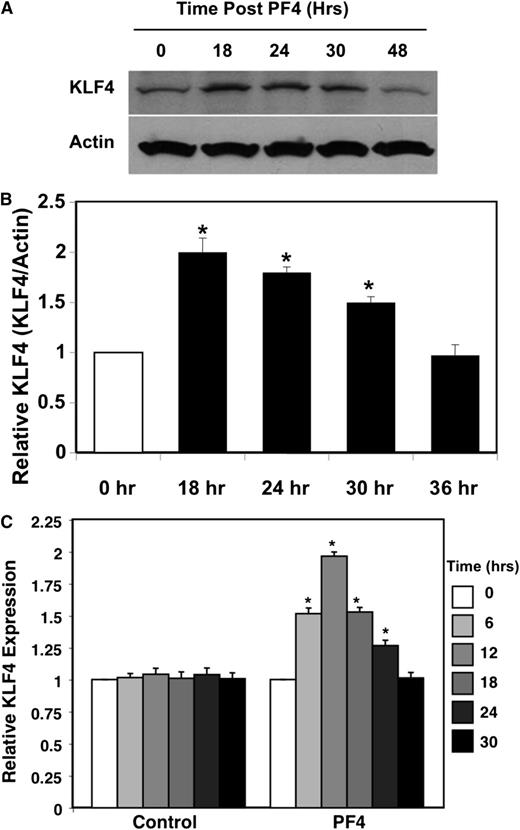
KLF4 is a transcription factor that directs cell fate and differentiation of many cell types, including VSMCs.21,22,34 Relatively small changes in KLF family member expression can result in large changes in physiological outcomes.22,35 To begin to determine whether PF4-mediated VSMC inflammatory phenotype may, in part, be KLF4 dependent, we treated HCASMCs with PF4 (1 µg/mL) and measured KLF4 by both western blot and qRT-PCR. PF4 increased KLF4 protein expression between 18 and 30 hours after treatment (Figure 6A-B). Similar results were noted with KLF4 mRNA expression; PF4 increased KLF4 within 6 hours of treatment, peaking at 12 hours, and then declined to baseline by 30 hours (Figure 6C).
PF4 increases KLF4. (A) Protein expression. HCASMCs were treated with PF4, and KLF4 expression was determined by western blot (representative). (B) Quantification of band density relative to actin control (n = 3 ± SD; *P < .01 vs 0). (C) PF4 increases HCASM cell KLF4 mRNA expression. VSMCs were treated with PF4, and KLF4 mRNA was quantified by qRT-PCR (n = 3 ± SD; *P < .01 vs 0).
PF4 increases KLF4. (A) Protein expression. HCASMCs were treated with PF4, and KLF4 expression was determined by western blot (representative). (B) Quantification of band density relative to actin control (n = 3 ± SD; *P < .01 vs 0). (C) PF4 increases HCASM cell KLF4 mRNA expression. VSMCs were treated with PF4, and KLF4 mRNA was quantified by qRT-PCR (n = 3 ± SD; *P < .01 vs 0).
PF4-induced cytokine production is, in part, KLF4 dependent. We overexpressed KLF4 in HCASMCs using a KLF4-expressing adenovirus (5 and 10 multiplicity of infection [MOI]), with a LacZ adenovirus vector (10 MOI) as a negative control (Figure 7A). IL-6 levels were measured in the cell supernatant following overnight incubation. The control LacZ adenovirus mildly increased IL-6 levels, and these levels were further increased by the addition of PF4 (1 µg/mL) (Figure 7B). KLF4 overexpression greatly increased IL-6 production at both 5 and 10 MOI compared with control (Figure 7B) but had no effect on IL-8 (supplemental Figure 7). As further evidence for KLF4 dependence of PF4-mediated IL-6 production, HCASMCs were treated with KLF4-specific or scramble control siRNA. Cells were then treated with PF4 or a control buffer. Cells treated with KLF4-specific siRNA had significantly attenuated IL-6 production and KLF4 expression after PF4 treatment compared with control siRNA–treated cells (Figure 7C-D). KLF4 siRNA significantly reduced IL-8 production, but to a much lesser extent than IL-6 (supplemental Figure 8). These data indicate that PF4-induced VSMC IL-6 is, in large part, KLF4 dependent.
PF4 drives VSMC proinflammatory response in a KLF4-dependent manner. (A) KLF4 overexpression. HCASMCs were infected with a KLF4-expressing adenovirus (5 and 10 MOI), a control LacZ adenovirus (10 MOI), or were null infected. Controls were also treated with buffer or PF4 (1 µg/mL). Representative KLF4 expression western blot. (B) Overexpression of KLF4 increases IL-6 production (n = 3 ± SD; *P < .01). (C) Inhibition of KLF4 expression decreases IL-6. HCASMCs were treated with scramble control siRNA or KLF4-specific siRNA before PF4. IL-6 was measured by ELISA (n = 3 ± SD; *P < .01). (D) Western blot confirmation of KLF4 knockdown by siRNA. (E) PF4 increases Klf4 expression in vivo. Ligated left carotid Klf4 expression relative to internal control nonligated right carotid was determined by qRT-PCR in WT and PF4−/− mice (n = 5 ± SD; *P < .05, **P < .01 vs PF4−/−).
PF4 drives VSMC proinflammatory response in a KLF4-dependent manner. (A) KLF4 overexpression. HCASMCs were infected with a KLF4-expressing adenovirus (5 and 10 MOI), a control LacZ adenovirus (10 MOI), or were null infected. Controls were also treated with buffer or PF4 (1 µg/mL). Representative KLF4 expression western blot. (B) Overexpression of KLF4 increases IL-6 production (n = 3 ± SD; *P < .01). (C) Inhibition of KLF4 expression decreases IL-6. HCASMCs were treated with scramble control siRNA or KLF4-specific siRNA before PF4. IL-6 was measured by ELISA (n = 3 ± SD; *P < .01). (D) Western blot confirmation of KLF4 knockdown by siRNA. (E) PF4 increases Klf4 expression in vivo. Ligated left carotid Klf4 expression relative to internal control nonligated right carotid was determined by qRT-PCR in WT and PF4−/− mice (n = 5 ± SD; *P < .05, **P < .01 vs PF4−/−).
KLF4 has been shown to be increased in VSMC injury responses in vivo, and mice lacking VSMC KLF4 have less of a decline in differentiation marker expression after injury.21,22,36 We performed carotid ligation on WT and PF4−/− mice, and at multiple time points during a 2-week postinjury period, Klf4 mRNA expression in the ligated vessel relative to the internal control nonligated vessel was determined. Similar to plasma PF4 and IL-6, Klf4 expression varied in WT mice, with the largest increases noted on days 0.5 and 7, immediately before plasma IL-6 levels increased (Figure 7E). Strikingly, Klf4 expression was unchanged in PF4−/− mice (Figure 7E). These data demonstrate that PF4 has a major role in driving Klf4 expression in vitro and in vivo in response to injury.
Discussion
Our work demonstrates that PF4 is a potent platelet-derived mediator of VSMC inflammatory phenotypes and injury responses.1,2 PF4 has a central role in stimulating VSMC cytokine production and the loss of VSMC differentiation both in vitro and in vivo. Furthermore, we show that the platelet-driven VSMC alterations are, in large part, mediated by a PF4-induced increase in the expression of the transcription factor KLF4. Platelets are increasingly associated with inflammation and tissue damage, making identification of the primary platelet-derived mediators and the mechanisms involved very important. Platelet inhibitors such as aspirin and clopidogrel are commonly used after cardiovascular events, but their use is associated with increased bleeding risks. By identifying specific platelet-derived mediators that lead to increased vascular injury, the rational design of compounds targeting these immune molecules may be possible, while minimizing untoward bleeding risk.
PF4 is the major mediator of platelet-driven VSMC injury responses, but our work does not rule out other platelet mediators that may have additive effects. Because vascular injury can take on many forms, including mechanical (surgery), trauma, transplantation, or atherosclerosis, platelets may not have the same function in all types of vessel injury. For example, platelets have been shown to maintain vascular integrity in some settings37,38 ; however, similar to our study results, PF4 has been shown to accelerate atherogenesis in an ApoE−/− mouse model. It should also be noted that PF4 has weak chemotactic activity compared with other chemokines. The migration assay using transwell chambers were performed for ∼18 hours and may not be a direct result of PF4. Rather, PF4-induced IL-6 and/or IL-8 production may also contribute to the noted PF4-mediated increase in HCASMC migration.
Carotid ligation is a common means to model VSMC inflammatory phenotypes. Our results demonstrate that sham mice have substantial platelet activation and cytokine production very early, which is part of the surgical injury response. In PF4−/− mice, even this early surgery-related cytokine response is almost completely abrogated, indicating that platelets and PF4 have a major role in general acute inflammatory responses. It is also interesting to note that plasma PF4, IL-6, and KC all have biphasic response patterns after ligation. Histologic study indicates that the second increase starting at ∼day 5 after ligation represents when there is ongoing vessel wall remodeling, likely leading to increased platelet activation and PF4 release. This observation may have important implications when considering the vessel remodeling that occurs in pathologic conditions such as deep vein thrombosis or after a vascular stent. It is also important to consider that the degree of carotid ligation–associated neointimal formation is strain dependent.39 Because the PF4−/− mice are on a B6 background, our studies were entirely performed using this strain. It may be interesting in the future to backcross PF4−/− mice to other strains to better examine other genetic variables. Our data demonstrate a potentially important physiological role for PF4 in VSMC injury that may apply to diseases such as atherosclerosis, chronic transplant vasculopathy, or vascular grafts. A future goal will be to explore our current discoveries in animal models of these diseases and in human participants.
KLF4 is a transcription factor with key regulatory roles in cell differentiation and growth and is associated with injury and inflammatory responses in numerous mature cell types.33,34,40,41 Gene expression responses and phenotypic outcomes mediated by KLF4 are dependent on the cell type and the cellular environment, contributing to the variety of responses in which KLF4 has been associated. Relatively small changes in KLF4 expression, like most KLF family members, can result in large changes in functional outcomes,22,42-44 and KLF4 functions are sometimes seemingly contradictory. For example, KLF4 has been implicated as a requirement for IL-17 production from Th17 T-cells,45 but KLF4 has also been implicated as a negative regulator of T-cell proliferation.40 Furthermore, much data indicate that KLF4 is necessary for monocyte differentiation and induction of a proinflammatory phenotype.42,46 However, other studies have found that KLF4 may function in macrophage differentiation by promoting an anti-inflammatory M2 phenotype.35 This apparent conflict in the role for KLF4 in the same cell type may be a function of environment and the inflammatory mediator milieu that drives its expression and other yet-to-be-identified interacting molecules. KLF4 expression is an important step in VSMC dedifferentiation, as conditional deletion of KLF4 from VSMCs delays the decline in the expression of differentiation markers after carotid ligation.22 However, KLF4 expression has also been associated with both decreased and increased VSMC proliferation.22,47,48 Our data indicate that PF4 increases VSMC proliferation in vivo and in vitro. Therefore, PF4 may influence VSMC proliferation by transcription factor–signaling networks in combination with KLF4.
Mouse models of atherosclerosis indicate that PF4 is proatherogenic, and PF4 has been found to penetrate deep into the vessel wall beyond the endothelial cell layer.11-13 PF4 has been found to permeate deep into the vessel wall after endothelial injury, emphasizing the potential extravascular importance of PF4 in vascular biology studies.14 Platelets also have key roles in other vascular inflammatory diseases associated with VSMC phenotypic changes. Platelet activation and deposition in transplant tissue is predictive of subsequent graft failure,3,49 and a major cause of chronic allograft failure is AGA, typified by VSMC proliferation and proinflammatory cytokine responses. Very early in the course of transplant rejection, platelets are activated by inflamed endothelial cells within the allograft vasculature and accelerate leukocyte recruitment and graft rejection.25 On the basis of our study, the role of platelets in AGA may be just as important as their role in acute rejection and include stimulating VSMC proinflammatory phenotypes that contribute to AGA.
Platelets have important roles in vascular injury and remodeling. Results of our study indicate that these platelet-accelerated events may, in large part, be driven by PF4.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
C.N.M. is supported by R01HL093179 and R01HL094547 from the National Institutes of Health.
Authorship
Contribution: G.S. designed and performed research, analyzed data, and wrote the paper; D.J.F., X.L., D.M., and K.K. performed research; V.A.K. contributed reagents and analyzed data; J.M.M. designed research, contributed vital new reagents, and analyzed data; S.T. performed research; and C.N.M. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Craig Morrell, University of Rochester School of Medicine and Dentistry, 601 Elmwood Ave, Rochester, NY 14642; e-mail: craig_morrell@urmc.rochester.edu.

![Figure 1. Platelets increase carotid ligation–induced VSMC inflammation in mice. (A) Platelets are activated after carotid ligation. The left carotid artery was permanently ligated, and the mice were treated with platelet-depleting antibody or control IgG. Plasma PF4 was measured by ELISA (n = 5 ±SD; *P < .01 vs 0). (B) Platelets promote inflammation in carotid ligation model. Plasma IL-6 was measured by ELISA in control and platelet-depleted mice after carotid ligation (n = 5 ± standard deviation [SD]; *P < .01 vs 0).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/21/10.1182_blood-2012-09-454710/4/m_4417f1.jpeg?Expires=1765888156&Signature=1tmv~T8lF5xJAJBlZfRpdJ2JX9rJWWhD2BRuZ5aQX3jiLdblg6Y6Fwn7kxKs2SKKFtPBi-ecr0PFOum~cJMcFbw1hW0r73asDzPiPMpDrt8WYjZKONQINFq9fkyoo8v-mERHnP82IxafoAOQleAxlXqpC~FmEN~jgFnmnm2O6nUezqO1P46GaynMAiKsBQFLQT~ncJ~bb~FBP-skDaFRkipyQMk7CNTTeulxOrpTWcppznBOkcTx5nZneUR-KH3xAsl0bLv2~MZj4ZRRvOFSoxP4wdoyPRAU2TpZymp~vNqzdSoM60FtAD9j7ApQXuO7yD7XtAu~72WcJBwH3iiLyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)