Key Points
The novel FVIII variant (FVIII-RH) has enhanced stability and procoagulant activity in both in vitro and in vivo models.
FVIII-RH is efficacious and safe; thus, it is an attractive molecule for protein replacement and as a transgene in gene-therapy strategies.
Abstract
Recombinant canine B-domain deleted (BDD) factor VIII (FVIII) is predominantly expressed as a single-chain protein and exhibits greater stability after activation compared with human FVIII-BDD. We generated a novel BDD-FVIII variant (FVIII-RH) with an amino acid change at the furin cleavage site within the B domain (position R1645H) that mimics the canine sequence (HHQR vs human RHQR). Compared with human FVIII-BDD, expression of FVIII-RH protein revealed a 2.5-fold increase in the single-chain form. Notably, FVIII-RH exhibited a twofold increase in biological activity compared with FVIII-BDD, likely due to its slower dissociation of the A2-domain upon thrombin activation. Injection of FVIII-RH protein in hemophilia A (HA) mice resulted in more efficacious hemostasis following vascular injury in both the macro- and microcirculation. These findings were successfully translated to adeno-associated viral (AAV)-based liver gene transfer in HA mice. Expression of circulating FVIII-RH was approximately twofold higher compared with AAV-FVIII-BDD–injected mice. Moreover, FVIII-RH exhibits superior procoagulant effects compared with FVIII-BDD following a series of hemostatic challenges. Notably, the immunogenicity of FVIII-RH did not differ from FVIII-BDD. Thus, FVIII-RH is an attractive bioengineered molecule for improving efficacy without increased immunogenicity and may be suitable for both protein- and gene-based strategies for HA.
Introduction
Hemophilia is an X-linked bleeding disease caused by deficiency in coagulation factor VIII (FVIII) or factor IX. Hemophilia A (HA) affects 1:5000 males born worldwide and represents 80% of the cases of hemophilia. Current management of hemophilia is based on protein replacement, but due to its high cost, only ∼20% of patients receive regular treatment. Gene replacement, gene repair, or genome editing are promising strategies for the curative treatment of hemophilia.1 Recent success with the use of adeno-associated viral (AAV) vectors for liver expression of clotting factor IX (hemophilia B) raises the possibility that a similar approach for HA could be envisioned.2 Preclinical studies in mouse models followed by canine models of severe HA using liver-directed AAV vectors showed that the therapeutic dose likely will be higher than those determined for hemophilia B.3-5 Thus, there is a fundamental interest in optimizing the expression of the transgene that could reduce the therapeutic vector dose.
FVIII is processed from a single-chain polypeptide with a domain structure of A1-A2-B-A3-C1-C2 in which the large B domain (40% of the protein) is not essential for hemostatic function. This formed the basis for the development of a shorter version of FVIII with the removal of most of the B domain, with only 14 residual amino acids (B-domain deleted, BDD [FVIII-BDD]).6,7 Recombinant FVIII-BDD has been efficacious and safe in the clinical management of HA patients over the last several decades.8 Recently, we generated a canine version of the BDD-FVIII protein.9 We uncovered that, in comparison with human FVIII, the canine protein exhibits enhanced biological activity. Canine BDD-FVIII protein was secreted predominantly in a single-chain form, whereas the human BDD-FVIII is predominantly secreted as a heterodimer form. The activated form of canine FVIII was more stable than the activated human FVIII.9 The underlying mechanisms for these differences are not known. We hypothesized that canine BDD-FVIII remained as a single-chain protein due to differences in the amino acid recognition sequence for the intracellular protease-paired basic amino acid cleaving enzyme (PACE)/furin. The consensus cleavage sequence is characterized by arginine-x-x-arginine (RxxR), and alignments of several mammals showed that canine FVIII had a unique sequence with histidine (HxxR), whereas RxxR was the common sequence in other species, including human, porcine, and murine FVIII.10-13 Here we demonstrate that in a series of biochemical studies and in vivo mouse models of HA that a single amino acid change at position R1645 to H (FVIII-RH) of human FVIII-BDD generated an enhanced functional FVIII variant without any modification in immunogenicity, as compared with the human FVIII-BDD.
Materials and methods
Production of recombinant FVIII-BDD forms
Human FVIII-BDD complementary DNA (cDNA) was used as a template for the generation of FVIII-RH using a QuickChange II-XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). Recombinant proteins were produced in baby hamster kidney cells and purified, as previously described in Sabatino et al.9 Final concentrations of FVIII protein forms were determined by absorbance at 280 nm using an extinction coefficient (E280, 1%) of 1.60 and a molecular weight of 160 000. Protein purity was assessed by loading 3 μg of FVIII protein on reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by staining with Coomassie blue. FVIII proteins, processed by thrombin (10 nM) for 30 minutes, were run on SDS-PAGE. The ratio between FVIII single-chain (SC) protein to heavy chain (HC, A1 and A2; 90 kDa)/light chain (LC, A3-C1-C3; 73 kDa) was calculated by optical densitometry using the TotalLab Quant software (Newcastle upon Tyne, United Kingdom). Carbohydrate analysis was carried out using recombinant N-glycosidase F (New England Biolabs, Ipswich, MA) or neuraminidase (QA-Bio, Palm Desert, CA), as reported before in Arruda et al.14 FVIII activity was determined by 1- or 2-stage activated partial thromboplastin time (aPTT) assays, as previously described in Sabatino et al.9
Decay of activated FVIII activity
We determined the decay of activated forms of FVIII-BDD and FVIII-RH using a modified 2-stage aPTT. Briefly, 20 nM purified FVIII-BDD or FVIII-RH was activated in the presence of 40 nM α-thrombin (Haematologic Technologies, Essex Junction, VT). The reaction was quenched by 60 nM hirudin (Sigma-Aldrich, St. Louis, MO) and serially diluted. Activity was assayed by aPTT at several time points thereafter and quantitated based on known concentrations of BDD-FVIII (Refacto; Pfizer, Cambridge, MA). Observed decay rates were calculated by fitting to a single exponential decay.
Recombinant AAV vectors production
AAV serotype 8 vectors were produced by a triple transfection protocol, as previously described in Sabatino et al.4 Three vector lots of each FVIII transgene were produced and quantified by silver staining in the same gel to optimize the determination of the amounts of physical particles delivered by each vector.
Animal models and vector injection
The Institutional Animal Care and Use Committee approved all animal experiments. Adult HA mice on a C57BL6/129Sv background or HA mice were crossed with CD4-knockout mice to obtain HA/CD4null mice. Transgenic HA mice with platelet-specific expression of human BDD-FVIII-BDD were previously reported in Greene et al.15 Animals received AAV vector by tail vein injection diluted in saline solution.
FVIII antigen and activity levels in mice
Levels of circulating FVIII antigen were determined from citrated plasma using matched-pair antibodies to FVIII (Affinity Biologicals, Ancaster, ON, Canada) using serial dilutions of BDD-FVIII (Refacto; Pfizer, New York, NY). Coagulant activity of FVIII-BDD and FVIII-RH in citrated mouse plasma was determined by Coatest SP4 FVIII (Chromogenix, Milan, Italy).
Antibodies to FVIII and AAV capsid
Detection of mouse FVIII-specific IgG antibodies to human FVIII was performed using full-length FVIII (Recombinate; Baxter, Deerfield, IL) at a concentration of 20 U/mL to capture the total IgG antibodies in mouse serum using enzyme-linked immunosorbent assay. Murine IgG (Sigma-Aldrich) was used as a standard by coating serial dilutions, and IgG was detected with 1:2000 antimurine IgG (H+L) conjugated to horseradish peroxidase (Millipore, Billerica, MA). Inhibitory antibody titers to FVIII were detected by Bethesda assays using pooled normal human plasma to determine the residual activity of the protein in human FVIII-deficient plasma, as described previously in Sabatino et al.4 Detection of antibodies to AAV capsid was carried out as reported in Haurigot et al,16 with minor modifications.
Tail clipping assay
Mice were prewarmed at 37°C, the tail was transected at a 3-mm diameter, and blood was collected for 10 minutes. Total blood loss (μL) was determined by converting the hemoglobin content using standard curves established with known amounts of mouse whole blood.17
Carotid artery injury model
Ferric chloride (FeCl3)–induced injury was carried out as described in Ivanciu et al17 and Schlachterman et al.18 We used 2 concentrations of FeCl3 (7.5% or 15%) for 2 minutes at the adventitial surface of the vessel to induce injury, and the vessel was then washed with saline. In the case of injury after tail vein infusion of FVIII protein, the time from infusion to injury was 15 minutes.
Cremaster arteriole injury model
Laser-induced arteriole injury of the cremaster muscle was performed as reported in Ivanciu et al17 and Schlachterman et al.18 We determined platelet and fibrin accumulation at the injury site following injection of Alexa555-labeled rat anti-CD1 and Alexa647-labeled antifibrin, respectively. Brightfield and fluorescence images were collected over 3 to 4 minutes and analyzed, as previously described in Ivanciu et al.17
Copy number and messenger RNA analysis
Livers from mice receiving AAV were collected for genomic DNA and RNA isolation. cDNA was synthesized using the SuperScript First-Strand Synthesis System (Invitrogen, Grand Island, NY). Real-time quantitative polymerase chain reaction (PCR) was used to detect either gene copy number or messenger RNA transcript levels specific for human FVIII using TaqMan or SYBR Green (Applied Biosystems, Carlsbad, CA), and it was standardized to murine 18S ribosomal RNA where applicable: human FVIII forward, 5′-GGGAAGTTGGAGACACACTGTTG-3′; human FVIII reverse: 5′- TGGCCATCTTCTACAGTCACTG-3′; human FVIII probe, fluorescein aminohexylamide–labeled: 5′-CGGAATCACTGATGTCCGTCCTTTGTATTC-3′; murine 18S forward: 5′-CGCTTCCTTACCTGGTTGAT- 3′; and murine 18S reverse: 5′-GAGCGACCAAAGGAACCATA-3′. The amount of FVIII was standardized to serial dilutions of linearized pED expression plasmid containing BDD-human FVIII cDNA.
Statistical analysis
All data are presented as mean ± SEM. Comparison of data among experimental groups was analyzed by unpaired Student t test or analysis of variance.
Results
Biochemical characterization of FVIII R1645H (FVIII-RH) variant
We generated a human FVIII derivative with the canine (H1645) sequence recognized by PACE/furin (Figure 1A-B). We found that FVIII-RH yields 0.27 mg/L, which is 2.5 times higher than the 0.10 mg/L for FVIII-BDD. The latter is within the typical yields we reported for FVIII-BDD (0.16 mg/L) using an identical expression system in baby hamster kidney cells.9 Recombinant human FVIII-BDD and FVIII-RH proteins migrate in a similar fashion on SDS-PAGE under reducing conditions (Figure 1A), and the percentage of single-chain form increased by 2.5-fold for FVIII-RH compared with FVIII-BDD (52% vs 20% single-chain form, respectively) by densitometric analysis. The slower migration pattern of the heavy chain was noted for FVIII-RH compared with FVIII-BDD (Figure 1A). We sought to determine whether carbohydrate posttranslational modifications differ between FVIII forms. Removal of N-linked glycans or sialic acid residues resulted in similar migration of the heavy chain from both FVIII forms (data not shown). Thus, it remains possible that the O-linked glycan pattern may be different between FVIII-RH and FVIII-BDD. Both FVIII variants were correctly processed by thrombin to yield the expected heterotrimer forms (Figure 1A). We next monitored the decay of FVIII variants upon activation with thrombin using clotting assays. The data showed that the half-life of activated FVIII-RH was ∼ threefold longer (3.9 minutes vs 1.4 minutes) than that of activated FVIII-BDD (Figure 1C). These findings reflect a slow dissociation of the A2 domain from the A1/A3-C1-C2 heterodimer from FVIII-RH compared with FVIII-BDD. Moreover, the superior activity of FVIII-RH was also determined by a 2-stage aPTT assay (Figure 1D). In these experiments, a dilution of FVIII-containing plasma samples is preincubated with thrombin for 30 seconds before the measurement of FVIII activity, using a clotting assay.19 The data showed a rapid onset of activated FVIII-RH generation compared with FVIII-BDD (Figure 1D); however, the increased activity of FVIII cannot be detected using the 1-stage aPTT assay.
Biochemical characterization of the recombinant FVIII-R1645H (RH) variant. (A) SDS-PAGE results of 3 μg of FVIII-RH and FVIII-BDD staining with Coomassie blue before (−) and after (+) incubation with thrombin are shown. (B) The alignment of partial sequences of FVIII B domain from distinct species is shown. (C) The graph shows the decay of FVIII-RH and FVIII-BDD following thrombin activation. Purified proteins (20 nM) were activated in the presence of α-thrombin, and the residual activity of FVIII was determined by a 2-stage aPTT assay. (D) FVIII clotting activity was determined by 1-stage (without thrombin) or 2-stage (with thrombin) aPTT assay; data represent triplicated experiments.
Biochemical characterization of the recombinant FVIII-R1645H (RH) variant. (A) SDS-PAGE results of 3 μg of FVIII-RH and FVIII-BDD staining with Coomassie blue before (−) and after (+) incubation with thrombin are shown. (B) The alignment of partial sequences of FVIII B domain from distinct species is shown. (C) The graph shows the decay of FVIII-RH and FVIII-BDD following thrombin activation. Purified proteins (20 nM) were activated in the presence of α-thrombin, and the residual activity of FVIII was determined by a 2-stage aPTT assay. (D) FVIII clotting activity was determined by 1-stage (without thrombin) or 2-stage (with thrombin) aPTT assay; data represent triplicated experiments.
FVIII-RH exhibits enhanced prohemostatic effects in murine models of HA upon vascular injury
To evaluate the efficacy of FVIII-RH compared with FVIII-BDD in vivo, we used a series of vascular injury models for both microcirculation and macrocirculation. To define the role of FVIII-RH (Figure 2, black lines) or FVIII-BDD (gray lines) in microcirculation, we injected HA mice with 2 doses of the FVIII protein variants. We monitored platelet accumulation and fibrin deposition over time. In this model, a laser-induced inside-out injury in the arteriole of the cremaster muscle failed to sustain thrombus formation in untreated HA mice.18 The data shown in Figure 2 demonstrate that both parameters of thrombus formation for FVIII-RH were superior to those of FVIII-BDD at the low dose of protein injection. Similar findings were also observed in the high-dose cohort; fibrin formation was higher following FVIII-RH protein infusion compared with FVIII-BDD. The kinetics of platelet accumulation was faster for FVIII-RH in both dose cohorts compared with FVIII-BDD (Figure 2A). The data represent a total of 10 to 15 injuries (n = 3 mice per FVIII variant) for the low-dose cohort, and 25 injuries (n = 5 mice per FVIII variant) for the high-dose cohort. Thus, FVIII-RH exhibits enhanced hemostatic effects in microcirculation.
Platelet and fibrin accumulation following laser-induced arteriole injury in HA mice upon infusion of FVIII variant proteins. The graphs show the time course of real-time thrombus formation in mice injected with recombinant protein FVIII-BDD (gray) or FVIII-RH (black) with a total of 10 to 15 injuries (n = 3 mice per FVIII variant) for the low-dose cohort and 25 injuries (n = 5 mice per FVIII variant) for the high-dose cohort. Median fluorescence intensity (MFI) for platelet (A) and fibrin (B) are plotted vs time.
Platelet and fibrin accumulation following laser-induced arteriole injury in HA mice upon infusion of FVIII variant proteins. The graphs show the time course of real-time thrombus formation in mice injected with recombinant protein FVIII-BDD (gray) or FVIII-RH (black) with a total of 10 to 15 injuries (n = 3 mice per FVIII variant) for the low-dose cohort and 25 injuries (n = 5 mice per FVIII variant) for the high-dose cohort. Median fluorescence intensity (MFI) for platelet (A) and fibrin (B) are plotted vs time.
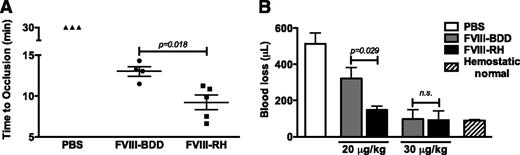
We next tested the procoagulant effects of FVIII variants with a FeCl3–induced injury in the carotid artery model. In the untreated HA mice, no clot is formed for the duration of the experiment (30 minutes) using a FeCl3 concentration of 7.5%. Injection of the proteins in HA mice showed that the time to full occlusion for FVIII-RH (n = 5 mice) is shortened compared with FVIII-BDD (n = 4 mice) (P = .018) (Figure 3A). In all protein-injected animals, the thrombi formed were stable throughout the duration of the experiment. Last, we monitored blood loss following tail clipping (at a 3-mm diameter) in HA mice (n = 3 per group per dose) over a 10-minute period after injection of protein. The data presented in Figure 3B demonstrate that FVIII-RH resulted in a reduction of blood loss compared with FVIII-BDD (P = .023) at low doses (20 μg/kg), but not at higher doses (Figure 3B). These findings were not due to differences in the protein pharmacokinetics, because there were no distinctions in recovery (15-minute time point) and the calculated half-life of both FVIII-RH and FVIII-BDD (data not shown). Taken together, these findings suggest that FVIII-RH–induced hemostasis in macrocirculation-injury models is superior to FVIII-BDD protein, notably at the lowest dose tested.
Hemostatic effect of recombinant FVIII variant protein in HA mice. (A) The graph shows the time of full occlusion of the carotid artery upon 7.5% FeCl3–induced injury. HA mice (C57BL/6) received 20 μg/kg of FVIII-RH (n = 5), FVIII-BDD (n = 4), or phosphate-buffered saline (PBS) (n = 3). (B) Blood loss following tail clipping is shown in the graph. HA mice (C57BL6/129Sv) received tail vein injection of FVIII-RH or FVIII-BDD at doses indicated or PBS (n = 5 mice per group). Hemostatically normal mice (n = 3, C57BL/6) were used as normal controls. Total blood loss (μL) was measured after tail transection at a 3-mm diameter, and blood was collected for 10 minutes. n.s., not significant.
Hemostatic effect of recombinant FVIII variant protein in HA mice. (A) The graph shows the time of full occlusion of the carotid artery upon 7.5% FeCl3–induced injury. HA mice (C57BL/6) received 20 μg/kg of FVIII-RH (n = 5), FVIII-BDD (n = 4), or phosphate-buffered saline (PBS) (n = 3). (B) Blood loss following tail clipping is shown in the graph. HA mice (C57BL6/129Sv) received tail vein injection of FVIII-RH or FVIII-BDD at doses indicated or PBS (n = 5 mice per group). Hemostatically normal mice (n = 3, C57BL/6) were used as normal controls. Total blood loss (μL) was measured after tail transection at a 3-mm diameter, and blood was collected for 10 minutes. n.s., not significant.
FVIII-RH is associated with increased circulating expression levels in a dose-dependent manner following AAV liver gene therapy
Due to the promising data obtained with the FVIII-RH protein, we sought to determine whether these findings could be translated into a model of continuous endogenous expression of these variants. Transgene expression was under the control of a hepatocyte-specific promoter (Figure 4). HA/CD4-deficient mice received AAV-FVIII-BDD or AAV-FVIII-RH at doses ranging from 8 × 1012 vector genomes (vg)/kg to 4 × 1013 vg/kg. In all 3 cohorts, the expression levels of FVIII-RH were ∼ twofold higher than FVIII-BDD (Figure 5A). FVIII clotting activity determined by chromogenic assay showed a good correlation with FVIII antigen levels (Figure 5B).
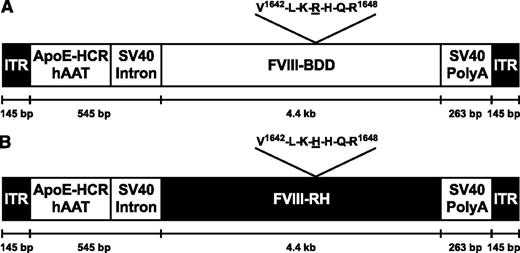
AAV vectors for the expression of FVIII forms. The schematic shows the F8 gene (encoding FVIII) expression plasmids used for the preparation of recombinant AAV vectors flanked by inverted terminal repeats (ITR). The human α1-antitrypsin (hAAT) promoter is linked to the hepatic locus control region (HCR) of a single copy of the apolipoprotein E (ApoE) enhancer. SV40 intronic and polyadenylation (poly A) sequences are from simian virus 40. Differences in the sequences used for the generation of FVIII-RH and FVIII-BDD are indicated. bp, base pairs.
AAV vectors for the expression of FVIII forms. The schematic shows the F8 gene (encoding FVIII) expression plasmids used for the preparation of recombinant AAV vectors flanked by inverted terminal repeats (ITR). The human α1-antitrypsin (hAAT) promoter is linked to the hepatic locus control region (HCR) of a single copy of the apolipoprotein E (ApoE) enhancer. SV40 intronic and polyadenylation (poly A) sequences are from simian virus 40. Differences in the sequences used for the generation of FVIII-RH and FVIII-BDD are indicated. bp, base pairs.
Expression of FVIII-RH and FVIII-BDD following AAV-mediated liver gene transfer. The graphs show circulating FVIII antigen (A) and FVIII activity by chromogenic assay (B) in HA/CD4-deficient mice 8 weeks after injection with AAV-FVIII-RH or AAV-FVIII-BDD at the doses indicated (n = 5 to 13 mice per group per dose). Liver sections of HA/CD4-deficient mice injected with AAV-FVIII-BDD (total n = 15) or AAV-FVIII-RH (total n = 14) harvested 14 to17 weeks after vector injection for the determination of (C) AAV DNA content and (D) FVIII-RNA are shown. P values determined by Student t test are shown.
Expression of FVIII-RH and FVIII-BDD following AAV-mediated liver gene transfer. The graphs show circulating FVIII antigen (A) and FVIII activity by chromogenic assay (B) in HA/CD4-deficient mice 8 weeks after injection with AAV-FVIII-RH or AAV-FVIII-BDD at the doses indicated (n = 5 to 13 mice per group per dose). Liver sections of HA/CD4-deficient mice injected with AAV-FVIII-BDD (total n = 15) or AAV-FVIII-RH (total n = 14) harvested 14 to17 weeks after vector injection for the determination of (C) AAV DNA content and (D) FVIII-RNA are shown. P values determined by Student t test are shown.
To further determine the underlying mechanism of the superior expression levels of FVIII-RH, we determined the levels of vector genomes by quantitative PCR and of FVIII RNA by reverse transcriptase-PCR in both groups of mice at weeks 14 to 17 after vector injection. Vector DNA content in the liver showed no statistical difference between gene copy numbers for FVIII-RH and FVIII-BDD (Figure 5C), as expected. There was variation in the RNA levels between groups of mice expressing FVIII-RH or FVIII-BDD, but these differences did not reach statistical significance. The correlation between FVIII expression levels with FVIII-RNA showed that increased circulating FVIII-RH levels are unlikely due to RNA content (Figure 5D). Together these data suggest that the high levels of FVIII-RH may occur, at least in part, at the posttranscriptional level and are most likely secreted more efficiently than the FVIII-BDD (Table 1).
Summary of gene transfer and expression results for mice receiving AAV8-FVIII-BDD or AAV8-FVIII-RH
| Dose . | Gene copy number (copies per cell) . | RNA transcripts (FVIII/18S RNA) . | Circulating FVIII (ng/mL) . | |||
|---|---|---|---|---|---|---|
| AAV8-BDD . | AAV8-RH . | AAV8-BDD . | AAV8-RH . | AAV8-BDD . | AAV8-RH . | |
| 8 × 1012 vg/kg | 16.8 ± 4.2 | 18.7 ± 4.0 | 0.09 ± 0.03 | 0.11 ± 0.04 | 49.9 ± 9.4 | 78.9 ± 6.6 |
| 2 × 1013 vg/kg | 64.6 ± 15.6 | 35.0 ± 9.1 | 0.04 ± 0.01 | 0.09 ± 0.03 | 71.4 ± 7.5 | 123.5 ± 19.3 |
| 4 × 1013 vg/kg | 84.7 ± 24.0 | 71.4 ± 12.9 | 0.10 ± 0.06 | 0.23 ± 0.12 | 75.1 ± 9.8 | 158.1 ± 28.3 |
| Dose . | Gene copy number (copies per cell) . | RNA transcripts (FVIII/18S RNA) . | Circulating FVIII (ng/mL) . | |||
|---|---|---|---|---|---|---|
| AAV8-BDD . | AAV8-RH . | AAV8-BDD . | AAV8-RH . | AAV8-BDD . | AAV8-RH . | |
| 8 × 1012 vg/kg | 16.8 ± 4.2 | 18.7 ± 4.0 | 0.09 ± 0.03 | 0.11 ± 0.04 | 49.9 ± 9.4 | 78.9 ± 6.6 |
| 2 × 1013 vg/kg | 64.6 ± 15.6 | 35.0 ± 9.1 | 0.04 ± 0.01 | 0.09 ± 0.03 | 71.4 ± 7.5 | 123.5 ± 19.3 |
| 4 × 1013 vg/kg | 84.7 ± 24.0 | 71.4 ± 12.9 | 0.10 ± 0.06 | 0.23 ± 0.12 | 75.1 ± 9.8 | 158.1 ± 28.3 |
FVIII-RH is more effective than FVIII-BDD in inducing hemostasis in vivo upon vascular injury models
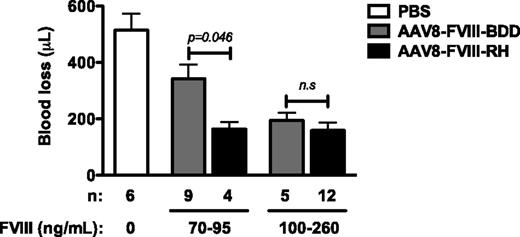
To test whether the FVIII-RH variant could correct hemostasis at comparable levels to FVIII-BDD, we grouped AAV-injected mice accordingly by FVIII expression plateau levels. We empirically normalized the FVIII levels into 2 groups (Figure 6) by antigen levels because there is a direct correlation with FVIII activity. All FVIII-expressing mice exhibited reduced blood loss compared with untreated HA mice (P < .001 for all groups) (Figure 6). There was a direct correlation between circulating FVIII levels and the volume of blood loss. At intermediate levels (70-95 ng/mL), the blood loss in the FVIII-RH mice was reduced compared with that in the FVIII-BDD mice (P < .04). However, blood loss was similar between both groups expressing FVIII at levels higher than 100 ng/mL, and these values are comparable with those of hemostatically normal mice (90-100 μL).
Blood loss following tail clipping assay in HA mice after AAV injection. HA/CD4-deficient mice received AAV vectors at a range of doses and were grouped according to the circulating FVIII-RH or FVIII-BDD levels determined at week 8 after injection. The numbers of mice in each group are indicated. Control groups included untreated HA/CD4-deficient mice or hemostatically normal C57BL/6 mice. Total blood loss (μL) was measured after tail transection at a 3-mm diameter, and blood was collected for 10 minutes. n.s., not significant.
Blood loss following tail clipping assay in HA mice after AAV injection. HA/CD4-deficient mice received AAV vectors at a range of doses and were grouped according to the circulating FVIII-RH or FVIII-BDD levels determined at week 8 after injection. The numbers of mice in each group are indicated. Control groups included untreated HA/CD4-deficient mice or hemostatically normal C57BL/6 mice. Total blood loss (μL) was measured after tail transection at a 3-mm diameter, and blood was collected for 10 minutes. n.s., not significant.
In addition, we sought to define the procoagulant effect of FVIII variants in the carotid artery model using 7.5% or 15% FeCl3 challenges. Because there is an inverse correlation between the time for occlusion and FVIII levels, the time of occlusion was not an end point in this model, as this reflects the FVIII expression levels. Here we focused on the assessment of clot stability over a period of 30 minutes as the end point. In 3 out of 7 mice expressing FVIII-BDD, the clot formed was transient, whereas all FVIII-RH animals exhibited stable clots (Table 2). We next used the 7.5% FeCl3 model to refine the differences between both forms of FVIII. The data showed transient clots formed in 3 of 10 FVIII-BDD mice, and 1 of 5 for the FVIII-RH mice, expressing less than 100 ng/mL. Moreover, since the endogenous levels following AAV delivery were relatively high, there were no differences in the fibrin deposition and platelet accumulation in the laser-induced injury model (data not shown). Again, consistent with the findings of the blood loss, there was no difference in clot stability in mice expressing >100 ng/mL of FVIII forms. Nevertheless, these data suggest that FVIII-RH induced the formation of clots with improved stability compared with FVIII-BDD.
Thrombus stability following FeCl3-mediated injury of the carotid artery in mice expressing FVIII-BDD or FVIII-RH by AAV transduction
| FeCl3 concentration . | AAV . | FVIII expression (ng/mL) . | No thrombus . | Transient occlusive thrombi . | Stable occlusive thrombi . |
|---|---|---|---|---|---|
| 15% | PBS | 0 | 2 | 0 | 0 |
| FVIII-BDD | 60-130 | 0 | 3 | 4 | |
| FVIII-RH | 60-180 | 0 | 0 | 7 | |
| 7.5% | PBS | 0 | 3 | 0 | 0 |
| FVIII-BDD | 30-90 | 0 | 3 | 6 | |
| 95-200 | 0 | 0 | 5 | ||
| FVIII-RH | 30-90 | 0 | 1 | 4 | |
| 95-250 | 0 | 0 | 8 |
| FeCl3 concentration . | AAV . | FVIII expression (ng/mL) . | No thrombus . | Transient occlusive thrombi . | Stable occlusive thrombi . |
|---|---|---|---|---|---|
| 15% | PBS | 0 | 2 | 0 | 0 |
| FVIII-BDD | 60-130 | 0 | 3 | 4 | |
| FVIII-RH | 60-180 | 0 | 0 | 7 | |
| 7.5% | PBS | 0 | 3 | 0 | 0 |
| FVIII-BDD | 30-90 | 0 | 3 | 6 | |
| 95-200 | 0 | 0 | 5 | ||
| FVIII-RH | 30-90 | 0 | 1 | 4 | |
| 95-250 | 0 | 0 | 8 |
No increased immunogenicity by the FVIII-RH variant in mice tolerant to FVIII-BDD
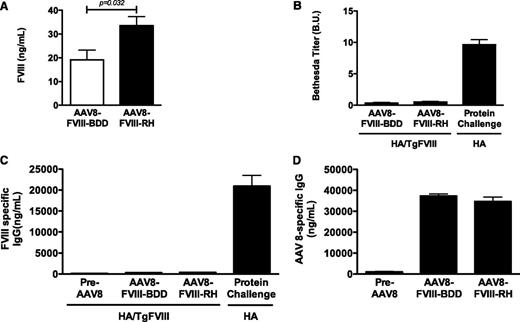
The risk of immune response to variant forms of FVIII is a major safety concern; thus, we tested whether FVIII-RH could be associated with a high risk of antibody formation.15 We used HA transgenic mice for platelet-specific expression of human FVIII-BDD with no detectable circulating FVIII. Female mice received AAV vectors encoding FVIII-RH (n = 7) or FVIII-BDD (n = 5) at doses of 8 × 1012 vg/kg. Circulating FVIII levels ranged from 10% to 33% for FVIII-BDD, and from 18% to 48% for FVIII-RH (Figure 7A), and were somewhat lower than the male HA groups. As previously reported in Davidoff et al,20 female mice expressed lower levels of the transgene by liver-directed gene transfer by AAV vectors in a serotype-independent manner. No antibody formation to human FVIII was detected by Bethesda assay (Figure 7B) or by anti-human FVIII-specific IgG during a period of 6 to 12 weeks after vector injection (Figure 7C). In contrast, naive HA mice developed antibodies following FVIII-BDD protein injection (Figure 7C) or AAV-FVIII-BDD injection (unpublished observations), as also reported by others.21 In order to confirm that the immune tolerance to FVIII forms was transgene specific, we measured anti–AAV8-specific capsid IgG levels. All animals developed a robust anti-capsid immune response (Figure 7D) at week 6 after vector injection, indicating that these animals are fully capable of generating humoral immune responses to other antigens following vector administration. Thus, the FVIII-RH variant showed no evidence of increased immunogenicity to the transgene product in mice tolerant to human FVIII-BDD.
Efficacy and immunogenicity of the expression of FVIII variants in the HA mouse model. Transgenic HA mice tolerant to human FVIII-BDD due to platelet-restricted FVIII expression received AAV8 vector encoding for FVIII-RH (n = 10) or FVIII-BDD (n = 7). (A) Circulating antigen levels of FVIII at 8 weeks after vector injection are shown in the graph. (B) The graph shows inhibitory antibody detected by Bethesda assay. Positive controls included nontolerant HA mice (n = 6) with antibody to human FVIII following repeated injections with recombinant FVIII protein. (C) The graph shows mouse anti-human FVIII antibody detected by enzyme-linked immunosorbent assay. (D) The graph shows humoral responses to AAV serotype 8 capsid at baseline and at 6 weeks after AAV injection at doses of 8 × 1012 vg/kg of AAV-FVIII-RH (n = 10) or AAV-FVIII-BDD (n = 7). P value is indicated.
Efficacy and immunogenicity of the expression of FVIII variants in the HA mouse model. Transgenic HA mice tolerant to human FVIII-BDD due to platelet-restricted FVIII expression received AAV8 vector encoding for FVIII-RH (n = 10) or FVIII-BDD (n = 7). (A) Circulating antigen levels of FVIII at 8 weeks after vector injection are shown in the graph. (B) The graph shows inhibitory antibody detected by Bethesda assay. Positive controls included nontolerant HA mice (n = 6) with antibody to human FVIII following repeated injections with recombinant FVIII protein. (C) The graph shows mouse anti-human FVIII antibody detected by enzyme-linked immunosorbent assay. (D) The graph shows humoral responses to AAV serotype 8 capsid at baseline and at 6 weeks after AAV injection at doses of 8 × 1012 vg/kg of AAV-FVIII-RH (n = 10) or AAV-FVIII-BDD (n = 7). P value is indicated.
Discussion
Recent developments in the bioengineering of coagulation factors for the treatment of hemophilia resulted in a series of novel proteins with advantageous biological functions. Molecules with prolonged half-life or high specific activity, as well as recombinant expression systems that optimize the rates of protein production, are in preclinical development or in early-phase clinical trials.22,23 The ultimate goal is to obtain a therapeutic protein with enhanced biological activity without increased immunogenicity.
Our early studies on the biochemical and in vivo characterization of canine BDD-FVIII showed the attractive features of this protein, specifically its stability and/or enhanced procoagulant activity. We sought to determine whether these could be amenable to the human FVIII-BDD.9 The canine FVIII B domain has a unique putative recognition sequence for PACE/furin11 that differs from all other species tested, and thus offers the opportunity to modify the human sequence, FVIII-RH, to determine if this single amino acid change could affect the biology of the human derivative. The introduction of H1645 increased the secretion of single-chain protein by 2.5-fold compared with FVIII-BDD (20%), which is now closer to the amount of the canine single-chain form (75%). Moreover, the A2 dissociation from the A1/A3-C1-C2 heterodimer was delayed ∼ threefold and exhibits higher protein activity in a series of coagulation studies in vitro. The latter result is derived from the data on the 2-stage aPTT, a more sensitive assay for the assessment of FVIII activity. This is consistent with data on the loss of function of FVIII in hemophilia patients with mutations associated with rapid A2 domain dissociation, which resulted in low residual FVIII activity by 2-stage compared with 1-stage aPTT.24 Overall, these findings are comparable, although not identical, to those obtained in canine studies, and thus demonstrate an unanticipated role of the PACE/furin cleavage site in affecting FVIII biological functions.9
To test the impact of the FVIII-RH protein in inducing hemostasis upon vascular injuries, we challenged HA mice in macrocirculation (tail vessels and carotid artery). In both models, FVIII-RH exhibits a more effective procoagulant effect than FVIII-BDD. Moreover, monitoring of real-time thrombus formation in microcirculation showed that FVIII-RH induces a higher content of fibrin deposition and faster platelet accumulation at the injury site compared with FVIII-BDD, as better evidenced in the low-dose cohorts. Taken together, these data led us to speculate that the superior performance of FVIII-RH is likely the result of higher protein stability (delayed A2 domain dissociation) and consequent robust thrombus formation.
Translational studies on promising gene-based strategies for hemophilia are critical to defining the efficacy of the transgene product as well as its immunogenicity.1,25 Moreover, in 2 early-phase clinical trials for hemophilia B using AAV for liver-restricted expression, there was a direct correlation between the risk of unwanted immune cellular responses to the transduced cells and the vector dose administered.1,2,26 Thus, there is a fundamental interest in optimizing the expression of the transgene that could reduce the therapeutic vector dose. Several strategies to enhance vector efficacy by modifications in the vector capsid or vector genomes, transgene optimization, and chimeric molecules are ongoing.27-30 Here we sought to test liver-based gene therapy using AAV-FVIII-RH as a strategy to enhance endogenous FVIII expression levels and biological activity. Notably, circulating levels of FVIII-RH were higher than those in HA mice injected with AAV-FVIII-BDD at all 3-dose cohorts. These findings were unexpected because we anticipated that the benefits of this variant would be related mostly to the procoagulant effects. However, the rates of increased expression levels in vivo are consistent, to a certain extent, with data obtained in vitro with the higher yields of recombinant FVIII-RH expression compared with FVIII-BDD.
Analysis of the FVIII transcripts showed comparable RNA levels, although with some variability, within the same dose cohort for both FVIII variants. Thus, it is possible that increased levels of circulating FVIII-RH are unlikely solely due to RNA content. We speculate that, at least in part, FVIII-RH is secreted more efficiently than FVIII-BDD, which supports the finding of high levels of expression of the transgene product. Similar findings have been reported for in vitro recombinant expression of porcine FVIII compared with human FVIII.29 There was a linear correlation between the antigen levels and the coagulation activity levels for both FVIII forms.
Hemostatic challenges in vivo provide further evidence of the enhanced performance of FVIII-RH, such as the reduced blood loss following tail clipping and the stability of the clot formed at the carotid artery. Because of the relatively high levels of FVIIII-RH and FVIII-BDD, there were no differences in fibrin deposition and platelet content in the thrombus formation in the microcirculation. Overall, these findings from the carotid artery and the cremaster arteriole are consistent with data observed in mice expressing FVIII-BDD ectopically in platelets, which was improved by the expression of canine FVIII,15 and in the recent data using FVIII-RH in platelets (M.P., personal oral communication, October 2012).
To address the safety concern of the immunogenicity of FVIII-RH, we used HA mice tolerant to human BDD-FVIII due to transgenic expression of FVIII in platelets but not detectable in the plasma. Following vector injection, circulating levels of FVIII-RH were twofold higher than FVIII-BDD, as observed in HA/CD4-deficient mice. No antibody to human FVIII was detected in these animals. Thus, FVIII-RH does not exhibit higher immunogenicity than FVIII-BDD. These transgenic mice mounted normal humoral responses to the AAV capsid, which suggests that the tolerance to FVIII forms were transgene specific and not a general immunosuppressive response.
Cumulative data showed that the most-common epitopes associated with inhibitor formation in HA patients on protein therapy are located at the A2 and C2 domains, and these findings are also obtained in HA mice injected with human FVIII protein.31,32 To date, there is no conclusive evidence on distinct rates of inhibitor formation comparing recombinant BDD or full-length FVIII.8 Moreover, the B-domain sequence is the most polymorphic of all the FVIII domains.10,33 Together these findings suggest that minimal modifications in B-domain sequence, as reported here, may not increase FVIII immunogenicity. Thus, it is possible that the risk of inhibitors associated with FVIII-RH may not differ from the other currently used FVIII forms.
These studies provide an attractive novel variant of FVIII derived from the sequence of the canine molecule. This allowed us to take advantage of the naturally occurring canine FVIII to improve the human protein without the need of a chimeric molecule. It is interesting to note that a single amino acid change in a relatively large protein exhibits enhanced biological function as evidenced by protein-based therapy and gene-based approach. This variant may be attractive for other expression systems for recombinant protein production or therapeutic transgene products. These findings are encouraging for future studies targeting the PACE/furin cleavage site and further enhancement of FVIII molecule.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mr. Alex Tai for his assistance in the vector production.
This work was supported by a grant from the Hemophilia Association of New York (V.R.A.) and the National Institutes of Health, Heart Lung and Blood Institute (PO1HL64190) (V.R.A., R.M.C., M.P.) and (R01-HL88010) (R.M.C.).
Authorship
Contribution: J.I.S. carried out biochemical and in vivo experiments and data interpretation, N.P.I. carried out biochemical experiments, and L.I. carried out in vivo experiments and assisted in the study design; S.Z. produced the recombinant AAV vectors; D.E.S. and R.M.C. assisted in the study design, editing, and intellectual contribution to the manuscript; M.P. provided editing and intellectual contribution to the manuscript; and V.R.A. directed experimental design, conducted data analysis and interpretation, and prepared the manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Valder R. Arruda, The Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, 5056 Colket Translational Research Center, Philadelphia, PA 19104; e-mail: arruda@email.chop.edu.