Key Points
IL-7 does not disrupt viral latency in highly pure resting latently infected CD4+ T cells from HIV-infected subjects receiving ART.
IL-7 therapy leads to a 70% increase in the absolute number of circulating CD4+ T cells harboring integrated HIV DNA 4 weeks posttherapy.
Abstract
HIV persists in latently infected memory CD4+ T cells during antiretroviral therapy (ART). When administered to HIV-infected subjects receiving suppressive ART, interleukin-7 (IL-7) increases the number of CD4+ T cells by promoting their survival and proliferation. However, little is known about the impact of IL-7 on HIV persistence during ART. By isolating large numbers of CD4+ T cells from HIV-infected subjects, we demonstrate that IL-7 enhances viral production in productively infected cells but does not disrupt viral latency in latently infected cells. When administered to virally suppressed subjects, IL-7 led to the rapid proliferation of memory CD4+ T cells, which resulted in a 70% increase in the absolute number of circulating CD4+ T cells harboring integrated HIV DNA 4 weeks after therapy. The genetic diversity of the viral reservoir increased transiently in the majority of the subjects studied before returning to baseline values. Altogether, our results indicate that IL-7 promotes the mechanisms of HIV persistence during ART by enhancing residual levels of viral production and inducing proliferation of latently infected cells, and suggest that IL-7 does not represent a suitable candidate therapeutic strategy for HIV eradication. This trial was registered at www.clinicaltrials.gov as #NCT00099671 (AIDS Clinical Trials Group protocol 5214).
Introduction
Antiretroviral therapy (ART) has extended the life expectancy of HIV-infected individuals but has been unsuccessful in eradicating the virus. HIV persists through multiple mechanisms in HIV-infected subjects who have been virally suppressed with ART for prolonged periods of time.1,2 In addition to residual levels of viral replication that may continuously replenish the HIV reservoir,3 the persistence of a small pool of latently infected memory CD4+ T cells constitutes a major barrier to HIV eradication.4-6 Latently infected CD4+ T cells, like the bulk of memory CD4+ T cells, are maintained by T-cell survival and homeostatic proliferation in response to the γc cytokine IL-7.7 Indeed, elevated plasma interleukin-7 levels (IL-7) levels during suppressive ART are associated with increased proliferation of CD4+ T cells,8 conservation of viral sequences over time and stability in the frequency of cells harboring HIV DNA.7
The administration of IL-7 to virally suppressed subjects restores a T-cell homeostatic equilibrium not always achieved by the control of viral load alone under ART.9,10 IL-7 therapy leads to a significant increase in the absolute number of CD4+ T cells as a result of increased T-cell cycling and survival and, to a lesser degree, increased thymic output.11
If the immunologic benefits of IL-7 are well documented, little is known about the impact of IL-7 therapy on the maintenance and/or clearance of the HIV reservoir. IL-7 was originally proposed as a potential eradication agent, as it was shown to induce reactivation of viral production in latently infected cells in severe combined immunodeficiency-hu mice and in long-term culture of peripheral blood mononuclear cells (PBMCs) obtained from HIV-infected subjects receiving ART.12,13 However, in a recent study using an in vitro model of HIV latency, Bosque et al demonstrated that IL-7 fails to reactivate HIV production in latently infected cells,14 in accordance with the observations of Vassena et al indicating that IL-7 does not induce HIV replication in CD4+ T cells isolated from infected subjects.15 The apparent contradictive results of these studies may result from the diversity of the approaches used to characterize the impact of IL-7 on HIV latency. In the present study, we used authentic latently infected CD4+ T cells from virally suppressed subjects receiving ART to assess the impact of IL-7 on viral latency and measured the effects of IL-7 administration on the size and genetic diversity of the HIV reservoir in vivo.
Methods
Patient population
Eighteen HIV-seropositive patients on stable suppressive ART and 7 chronically infected subjects with no history of ART enrolled in this study. All subjects signed informed consent approved by the Oregon Health and Science University and the Martin Memorial Health Systems (Stuart, FL) review boards or the Royal Victoria Hospital and the Centre de Recherche du Centre Hospitalier de l'Universite de Montreal (Montreal, QC, Canada) review boards. All patients underwent leukapheresis to collect large numbers of PBMCs.
In addition, we studied PBMC samples collected from 10 subjects enrolled in the ACTG5214 study. The study was approved by the institutional review boards of all participating sites and written informed consent was obtained from all participants. Seven of the participants received a single dose of subcutaneous recombinant human IL-7 on day 0 (3-60 µg/kg) and 3 control subjects received placebo. Details of the clinical trial (AIDS Clinical Trials Group protocol 5214, NCT00099671) have been previously published.9 These studies were conducted in accordance with the Declaration of Helsinki.
Isolation of CD4 T cells
Total CD4+ T cells were isolated from PBMCs of successfully treated and viremic subjects using magnetic bead–based negative selection (Stemcell Technologies). To further isolate resting memory CD4+ T cells, total CD4+ T cells were stained with the following antibodies: CD3–Pacific Blue, CD4–Alexa 700, CD45RA-allophycocyanin (APC)-Cy7, HLA-DR–APC, CD69-APC, CD25-APC (all from BD Biosciences). Dead cells were excluded with the LIVE/DEAD Aqua marker (Invitrogen). Live resting memory CD4+ T cells (CD3+CD4+CD45RA−HLA-DR−CD69−CD25−) were sorted on a BD FACSAria to very high purity (>99%).
Measurement of HIV production
Isolated CD4+ T cells (5 × 106 cells per well) or highly purified resting memory CD4+ T cells (1 × 106 cells per well) were cultured in the presence of antiretrovirals (ARVs) (100nM efavirenz, 180nM zidovudine, 200nM raltegravir). Cells were stimulated with 10 ng/mL IL-7 (R&D Systems) or with Dynabeads Human T-expander CD3/CD28 (Invitrogen) at a concentration of 1 bead per cell. Medium was harvested every 3 days and used for quantification of viral particles and replaced with fresh medium containing ARVs and IL-7 when appropriate. Freshly collected cell-culture supernatants were centrifuged for 1 hour at 25 000 g to pellet HIV particles. Viral RNAs were extracted using the Qiamp viral RNA kit (Qiagen) and quantified using an ultrasensitive seminested real-time reverse transcription–polymerase chain reaction (RT-PCR) with a detection limit of a single copy of HIV RNA. Extracted viral RNA was reverse transcribed and subjected to 16 cycles of amplification with the following primers: forward: 5′-ATG CCA CGT AAG CGA AAC TCT GGG TCT CTC TDG TTA GAC-3′; reverse: 5′-CCA TCT CTC TCC TTC TAG C-3′. Preamplified products were diluted and subjected to a nested real-time PCR for 40 cycles on the Rotor-Gene Q by using the following primers and probes: forward: 5′-ATG CCA CGT AAG CGA AAC T-3′; reverse: 5′-CTG AGG GAT CTC TAG TTA CC-3′; probe: 5′-LC-640-CAC TCA AGG CAA GCT TTA TTG AGG C-BBQ-3′. In all experiments, serial dilutions of HIV particles (LAI strain) in culture medium were processed in parallel of experimental samples.
Integrated HIV DNA quantification
CD4+ T cells were isolated by negative magnetic bead selection and cell lysates were directly used in a nested Alu PCR to quantify both integrated HIV DNA and CD3 gene copy numbers, as previously described.7
Flow cytometry
The expression of proliferation and activation markers in cultured T cells as well as in PBMCs from subjects who received IL-7 were measured by flow cytometry by using the following 11-color antibody panel: CD3-A700 (BD Biosciences), CD4-Qdot605 (Invitrogen), CD8–Pacific Blue (BD Biosciences), CD45RA-APCH7 (BD Biosciences), CCR7-phycoerythrin-Cy7 (BD Biosciences), CD27-Qdot655 (Invitrogen), HLA-DR–PerCP (BD Biosciences), PD-1–APC (eBioscience), CD127-phycoerythrin (BD Biosciences), Ki67–fluorescein isothiocyanate (BD Biosciences). Dead cells were excluded with the LIVE/DEAD Aqua marker (Invitrogen). Cells were acquired on an LSRII flow cytometer using the FACSDiva software (Becton Dickinson) and analyzed using FlowJo (TreeStar Inc).
HIV diversity
The diversity of HIV DNA populations in subjects receiving IL-7 was assessed by sequencing the V1-V3 loops of gp120, as described previously.16 To minimize possible virus population sampling bias, a minimum of 20 independent PCR products obtained from each sample were used for cloning, which allows a similar measure of population diversity to the single genome sequencing assay.17 Pairwise distances among sequences were calculated after alignment by using Geneious (Biomatters Ltd). Average genetic distances were calculated as the mean of pairwise distances in a given population. Phylogenetic trees were built using Geneious. Distance-matrix–based trees were estimated with the neighbor-joining method using the Kimura 2-parameter model of nucleotide substitution excluding positions where gaps were present in any sequence.
Statistical analysis
We performed 2-tailed Mann-Whitney U and Wilcoxon paired tests with Prism 4.0 software. The nonparametric Kruskall-Wallis test was used to compare genetic diversities of proviral populations.
Results
IL-7 shows minimal effect on viral production in CD4+ T cells from virally suppressed subjects
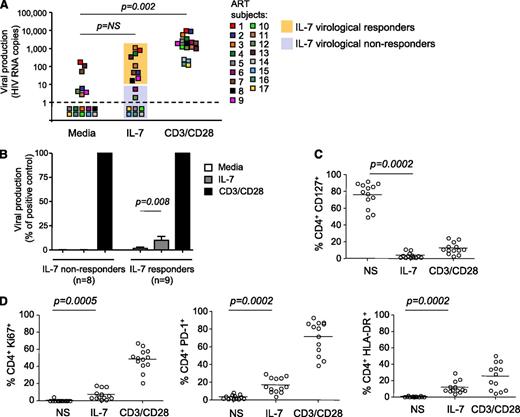
To determine whether IL-7 induced viral production in CD4+ T cells isolated from virally suppressed subjects, we used a novel assay that allows us to directly measure viral release from primary CD4+ T cells after stimulation. Large numbers of highly purified CD4+ T cells obtained from 17 subjects on suppressive ART (Table 1) and cultured in the presence of ARVs to prevent new rounds of infection were stimulated with IL-7 or with anti-CD3/CD28 antibodies as a positive control. Viral release was measured in the supernatant by ultrasensitive RT-PCR with a detection limit of a single copy of HIV RNA (Figure 1A). A subset of subjects showed low but reproducible levels of viral production (>10 HIV RNA copies per milliliter) following IL-7 stimulation (IL-7 virological responders, n = 9/17, 53%). These levels of viral production, although significant (P = .008), represented only 10% of the production induced by T-cell receptor (TCR) stimulation (Figure 1B). CD4+ T cells from the other subset of subjects (IL-7 virological nonresponders, n = 8/17, 47%) did not produce HIV in response to IL-7, although viral production was readily detected following TCR stimulation of these samples (Figure 1A-B).
Profiles of HIV-infected subjects
| Subject . | Plasma viral load, copies per mL* . | CD4 T cells, per μL . | CD8 T cells, per μL . | ART at the time of study† . | Duration of therapy, year . |
|---|---|---|---|---|---|
| ART subjects | |||||
| 1 | <50 | 235 | 399 | 3TC, D4T, NVP | 7 |
| 2 | <50 | 490 | 369 | FTC, RAL, TDF | 3 |
| 3 | <50 | 1116 | 737 | 3TC, ABC, EFV | 6 |
| 4 | <50 | 434 | 583 | 3TC, ABC, EFV | 3 |
| 5 | <50 | 396 | 645 | 3TC, ABC, AZT | 4 |
| 6 | <50 | 375 | 515 | EFV, FTC, TDF | 7 |
| 7 | <50 | 1065 | 1468 | EFV, FTC, TDF | 8 |
| 8 | <50 | 1177 | 935 | 7 | |
| 9 | <50 | 367 | 652 | 3TC, ABC, LPV/RTV | 5 |
| 10 | <50 | 558 | 972 | 3TC, ABC, LPV/RTV | 9 |
| 11 | <50 | 794 | 608 | 3TC, AZT, EFV | 7 |
| 12 | <50 | 422 | 1269 | 3TC, ABC, LPV/RTV, TDF | 4 |
| 13 | <50 | 959 | 822 | 3TC, ABC, ATV | 6 |
| 14 | <50 | 1183 | 912 | 3TC, ABC, ATV | 3 |
| 15 | <50 | 436 | 1125 | 3TC, ABC, LPV/RTV, TDF | 12 |
| 16 | <50 | 1220 | 1206 | FTC, LPV/RTV, TDF | 6 |
| 17 | <50 | 890 | 673 | 3TC, AZT, NVP | 4 |
| 18 | <50 | 291 | 491 | 3TC, ABC, ATV/RTV | 3 |
| Untreated subjects | |||||
| 1 | 17 000 | 321 | 450 | No | NA |
| 2 | 24 000 | 415 | 821 | No | NA |
| 3 | 25 138 | 364 | 544 | No | NA |
| 4 | 32 594 | 368 | 491 | No | NA |
| 5 | 95 543 | 566 | 1330 | No | NA |
| 6 | 268 417 | 880 | 1250 | No | NA |
| 7 | 19 041 | 657 | 544 | No | NA |
| Subject . | Plasma viral load, copies per mL* . | CD4 T cells, per μL . | CD8 T cells, per μL . | ART at the time of study† . | Duration of therapy, year . |
|---|---|---|---|---|---|
| ART subjects | |||||
| 1 | <50 | 235 | 399 | 3TC, D4T, NVP | 7 |
| 2 | <50 | 490 | 369 | FTC, RAL, TDF | 3 |
| 3 | <50 | 1116 | 737 | 3TC, ABC, EFV | 6 |
| 4 | <50 | 434 | 583 | 3TC, ABC, EFV | 3 |
| 5 | <50 | 396 | 645 | 3TC, ABC, AZT | 4 |
| 6 | <50 | 375 | 515 | EFV, FTC, TDF | 7 |
| 7 | <50 | 1065 | 1468 | EFV, FTC, TDF | 8 |
| 8 | <50 | 1177 | 935 | 7 | |
| 9 | <50 | 367 | 652 | 3TC, ABC, LPV/RTV | 5 |
| 10 | <50 | 558 | 972 | 3TC, ABC, LPV/RTV | 9 |
| 11 | <50 | 794 | 608 | 3TC, AZT, EFV | 7 |
| 12 | <50 | 422 | 1269 | 3TC, ABC, LPV/RTV, TDF | 4 |
| 13 | <50 | 959 | 822 | 3TC, ABC, ATV | 6 |
| 14 | <50 | 1183 | 912 | 3TC, ABC, ATV | 3 |
| 15 | <50 | 436 | 1125 | 3TC, ABC, LPV/RTV, TDF | 12 |
| 16 | <50 | 1220 | 1206 | FTC, LPV/RTV, TDF | 6 |
| 17 | <50 | 890 | 673 | 3TC, AZT, NVP | 4 |
| 18 | <50 | 291 | 491 | 3TC, ABC, ATV/RTV | 3 |
| Untreated subjects | |||||
| 1 | 17 000 | 321 | 450 | No | NA |
| 2 | 24 000 | 415 | 821 | No | NA |
| 3 | 25 138 | 364 | 544 | No | NA |
| 4 | 32 594 | 368 | 491 | No | NA |
| 5 | 95 543 | 566 | 1330 | No | NA |
| 6 | 268 417 | 880 | 1250 | No | NA |
| 7 | 19 041 | 657 | 544 | No | NA |
NA, not applicable.
Viral load was measured by the Amplicor HIV-1 monitor ultrasensitive method (Roche), with a detection limit of 50 copies per milliliter of plasma.
Antiretroviral therapy: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; FTC, Emtricitabine; LPV, lopinavir; NPV, nevirapine; RAL, raltegravir; RTV, ritonavir; TDF, tenofovir.
IL-7 shows minimal effect on viral production in CD4+T cells from virally suppressed subjects. (A) Viral production in cell-culture supernatants of CD4+ T cells isolated from subjects receiving ART and stimulated with 10 ng/mL IL-7 or with antibodies to CD3/CD28 for 6 days in the presence of ARVs. Viral release was measured by ultrasentitive RT-PCR in the supernatant. (B) Viral production in CD4+ T cells from IL-7 nonresponders and IL-7 responders in media alone or after IL-7 stimulation, relative to the positive control (CD3/CD28). (C) Expression of the IL-7 receptor (CD127) in CD4+ T cells after stimulation with IL-7 or with antibodies to CD3/CD28 was measured by flow cytometry. (D) Effect of IL-7 on the expression of activation and proliferation markers. CD4+ T cells from 13 virally suppressed subjects were stimulated with 10 ng/mL IL-7 or with antibodies to CD3/CD28 for 6 days. The levels of expression of Ki67, HLA-DR, and PD-1 on live CD4+ T cells were measured by flow cytometry.
IL-7 shows minimal effect on viral production in CD4+T cells from virally suppressed subjects. (A) Viral production in cell-culture supernatants of CD4+ T cells isolated from subjects receiving ART and stimulated with 10 ng/mL IL-7 or with antibodies to CD3/CD28 for 6 days in the presence of ARVs. Viral release was measured by ultrasentitive RT-PCR in the supernatant. (B) Viral production in CD4+ T cells from IL-7 nonresponders and IL-7 responders in media alone or after IL-7 stimulation, relative to the positive control (CD3/CD28). (C) Expression of the IL-7 receptor (CD127) in CD4+ T cells after stimulation with IL-7 or with antibodies to CD3/CD28 was measured by flow cytometry. (D) Effect of IL-7 on the expression of activation and proliferation markers. CD4+ T cells from 13 virally suppressed subjects were stimulated with 10 ng/mL IL-7 or with antibodies to CD3/CD28 for 6 days. The levels of expression of Ki67, HLA-DR, and PD-1 on live CD4+ T cells were measured by flow cytometry.
To exclude the possibility that the low to undetectable levels of viral production in CD4+ T cells from virally suppressed subjects following IL-7 stimulation resulted from the inability of these cells to respond to the cytokine, we measured the level of expression of the α chain of the IL-7 receptor (CD127) as well as the effect of IL-7 on cell viability and on the expression of proliferation and activation markers. The memory subsets that encompass the HIV reservoir7 expressed high levels of the IL-7 receptor (85%, 76%, and 84% in central, transitional, and effector memory CD4+ T cells, respectively, supplemental Figure 1A, available on the Blood website). CD4+ T cells from all subjects were responsive to IL-7, as demonstrated by dramatic downregulation of the IL-7 receptor (76%-4% CD127+CD4+ T cells, P = .0002, Figure 1C). In addition, IL-7 promoted survival of CD4+ T cells isolated from all virally suppressed subjects tested (P = .02, supplemental Figure 1B) and led to significant increases in the frequency of cells expressing Ki67 (P = .0005), HLA-DR (P = .0002), and PD-1 (P = .0002) (Figure 1D). Altogether, these results demonstrated that IL-7 normally activates CD4+ T cells from virally suppressed subjects, and indicated that the absence of viral production upon IL-7 stimulation in a subset of the samples tested could not be attributed to the lack of IL-7 receptor expression or to an intrinsic defect in the IL-7 signaling pathway in these cells.
IL-7 enhances viral production in productively infected cells but does not disrupt HIV latency
CD4+ T cells isolated from IL-7 responders were more likely to spontaneously produce low levels of virus (67% of IL-7 responders, 12% of IL-7 nonresponders, P = .02), a hallmark of untreated, viremic subjects. This suggested that IL-7 could enhance minimal preexisting levels of viral production, thereby accounting for the low levels of IL-7–induced viral production observed in a subset of virally suppressed subjects. In line with this hypothesis, productively infected CD4+ T cells isolated from viremic subjects consistently showed enhanced levels of viral production upon IL-7 stimulation (P = .02, Figure 2A). Of note, the levels of viral production induced by IL-7 were strongly correlated with the baseline levels of viral production (P < .0001, Figure 2B), whereas such a correlation was not observed after TCR stimulation of CD4+ T cells from the same donors (P > .05, data not shown). Taken together, these results suggested that the modest levels of viral production observed in some virally suppressed subjects upon IL-7 stimulation could be attributed to a low number of productively infected CD4+ T cells that persist in a fraction of subjects receiving suppressive ART.3,18
IL-7 enhances viral production in productively infected cells but does not disrupt HIV latency. (A) Quantification of viral production in cell-culture supernatants of CD4+ T cells isolated from 7 viremic untreated HIV-infected subjects. Similar methods than in Figure 1A were used. (B) Correlation between the levels of spontaneous viral production and viral production induced by IL-7 in CD4+ T cells isolated from 7 viremic subjects. (C) Viral production in total CD4+ T cells and highly pure resting memory CD4+ T cells following stimulation with IL-7 and CD3/CD28 in 4 virally suppressed subjects.
IL-7 enhances viral production in productively infected cells but does not disrupt HIV latency. (A) Quantification of viral production in cell-culture supernatants of CD4+ T cells isolated from 7 viremic untreated HIV-infected subjects. Similar methods than in Figure 1A were used. (B) Correlation between the levels of spontaneous viral production and viral production induced by IL-7 in CD4+ T cells isolated from 7 viremic subjects. (C) Viral production in total CD4+ T cells and highly pure resting memory CD4+ T cells following stimulation with IL-7 and CD3/CD28 in 4 virally suppressed subjects.
To assess the impact of IL-7 on resting latently infected cells, we sorted resting memory CD4+ T cells (CD45RA−HLA-DR−CD69−CD25−) from 4 subjects who showed enhanced viral production upon IL-7 exposure and assessed the ability of IL-7 to induce viral production in this highly pure population (>98%) of resting CD4+ T cells (Figure 2C). The depletion of activated CD4+ T cells completely abrogated the spontaneous release of viral particles. Although IL-7 induced viral production in total CD4+ T cells from these 4 subjects (mean value = 1685 HIV RNA copies per 106 cells), it had very limited or no effect in latently infected resting memory CD4+ T cells (mean value = 4 HIV RNA copies per 106 cells). Of note, stimulation of these cells through the TCR led to high levels of viral release, indicating that these cells harbored inducible virus that could not be reactivated with IL-7. These results clearly demonstrated that IL-7 is very inefficient at inducing HIV production in highly pure latently infected CD4+ T cells isolated from subjects receiving suppressive ART.
IL-7 therapy increases the size of the HIV reservoir and maintains the genetic diversity of viral quasispecies in vivo
We evaluated the impact of IL-7 administration on the HIV reservoir in 7 subjects who had received a single injection of IL-7 and in 3 placebo controls (Table 2). Blood samples were collected before IL-7 administration (day 0) as well as 4 and 28 days after injection. IL-7 administration induced a 3- to 10-fold increase in the frequency of Ki67+ CD4+ T cells at day 4, which returned to baseline levels after 28 days (Figure 3A, supplemental Figure 2A-B). Similarly, the frequencies of CD4+ T cells expressing HLA-DR and PD-1 significantly increased at day 4 and normalized at day 28. These observations confirmed that IL-7 upregulates expression of HLA-DR, PD-1, and Ki67, confirming our in vitro assay (Figure 1D), and indicated that IL-7 triggers the activation and proliferation of CD4+ T cells in virally suppressed subjects.
Characteristics of study participants (ACTG5214)
| Participant ID . | IL-7 dose, μg/kg . | Day . | Plasma viral load, copies per mL . | CD4 T cells, per μL . | CD8 T cells, per μL . | Integrated HIV DNA, copies per 106 CD4+ T cells . | Integrated HIV DNA, copies in 1 mL of blood . |
|---|---|---|---|---|---|---|---|
| 91356 | 3 | 0 | <50 | 970 | 1461 | <3 | NA |
| 3 | 4 | <50 | 910 | 1049 | <3 | NA | |
| 3 | 28 | <50 | 804 | 1096 | <3 | NA | |
| 91360 | 10 | 0 | <50 | 451 | 1576 | 477 | 215 |
| 10 | 4 | <50 | 856 | 1843 | 793 | 679 | |
| 10 | 28 | <50 | 484 | 1461 | 591 | 286 | |
| 91390 | 60 | 0 | <50 | 575 | 425 | 192 | 110 |
| 60 | 4 | <50 | 708 | 566 | 180 | 127 | |
| 60 | 28 | <50 | 1225 | 995 | 179 | 219 | |
| 251343 | 3 | 0 | 365 | 1165 | 1057 | 76 | 89 |
| 3 | 4 | <50 | 886 | 803 | 68 | 60 | |
| 3 | 28 | <50 | 987 | 917 | 85 | 84 | |
| 251657 | 3 | 0 | <50 | 564 | 1199 | 226 | 127 |
| 3 | 4 | <50 | 749 | 1622 | 362 | 271 | |
| 3 | 28 | <50 | 610 | 1325 | 431 | 263 | |
| 272430 | Placebo | 0 | <50 | 889 | 489 | 871 | 774 |
| Placebo | 4 | <50 | 750 | 403 | 908 | 681 | |
| Placebo | 28 | <50 | 756 | 378 | 656 | 496 | |
| 381546 | 30 | 0 | <50 | 240 | 590 | 1333 | 320 |
| 30 | 4 | 79 | NA | NA | 1521 | NA | |
| 30 | 28 | <50 | 453 | 1122 | 1522 | 689 | |
| 381649 | Placebo | 0 | 298 | 569 | 584 | 349 | 199 |
| Placebo | 28 | 963 | 747 | 921 | 295 | 220 | |
| 381666 | 30 | 0 | <50 | 212 | 1023 | 1726 | 366 |
| 30 | 4 | 83 | 298 | 1342 | 1614 | 481 | |
| 30 | 28 | <50 | 446 | 1841 | 1416 | 632 | |
| 381691 | Placebo | 0 | <50 | 823 | 1546 | 613 | 504 |
| Placebo | 4 | <50 | 545 | 1155 | 545 | 297 | |
| Placebo | 28 | <50 | 722 | 1804 | 404 | 292 |
| Participant ID . | IL-7 dose, μg/kg . | Day . | Plasma viral load, copies per mL . | CD4 T cells, per μL . | CD8 T cells, per μL . | Integrated HIV DNA, copies per 106 CD4+ T cells . | Integrated HIV DNA, copies in 1 mL of blood . |
|---|---|---|---|---|---|---|---|
| 91356 | 3 | 0 | <50 | 970 | 1461 | <3 | NA |
| 3 | 4 | <50 | 910 | 1049 | <3 | NA | |
| 3 | 28 | <50 | 804 | 1096 | <3 | NA | |
| 91360 | 10 | 0 | <50 | 451 | 1576 | 477 | 215 |
| 10 | 4 | <50 | 856 | 1843 | 793 | 679 | |
| 10 | 28 | <50 | 484 | 1461 | 591 | 286 | |
| 91390 | 60 | 0 | <50 | 575 | 425 | 192 | 110 |
| 60 | 4 | <50 | 708 | 566 | 180 | 127 | |
| 60 | 28 | <50 | 1225 | 995 | 179 | 219 | |
| 251343 | 3 | 0 | 365 | 1165 | 1057 | 76 | 89 |
| 3 | 4 | <50 | 886 | 803 | 68 | 60 | |
| 3 | 28 | <50 | 987 | 917 | 85 | 84 | |
| 251657 | 3 | 0 | <50 | 564 | 1199 | 226 | 127 |
| 3 | 4 | <50 | 749 | 1622 | 362 | 271 | |
| 3 | 28 | <50 | 610 | 1325 | 431 | 263 | |
| 272430 | Placebo | 0 | <50 | 889 | 489 | 871 | 774 |
| Placebo | 4 | <50 | 750 | 403 | 908 | 681 | |
| Placebo | 28 | <50 | 756 | 378 | 656 | 496 | |
| 381546 | 30 | 0 | <50 | 240 | 590 | 1333 | 320 |
| 30 | 4 | 79 | NA | NA | 1521 | NA | |
| 30 | 28 | <50 | 453 | 1122 | 1522 | 689 | |
| 381649 | Placebo | 0 | 298 | 569 | 584 | 349 | 199 |
| Placebo | 28 | 963 | 747 | 921 | 295 | 220 | |
| 381666 | 30 | 0 | <50 | 212 | 1023 | 1726 | 366 |
| 30 | 4 | 83 | 298 | 1342 | 1614 | 481 | |
| 30 | 28 | <50 | 446 | 1841 | 1416 | 632 | |
| 381691 | Placebo | 0 | <50 | 823 | 1546 | 613 | 504 |
| Placebo | 4 | <50 | 545 | 1155 | 545 | 297 | |
| Placebo | 28 | <50 | 722 | 1804 | 404 | 292 |
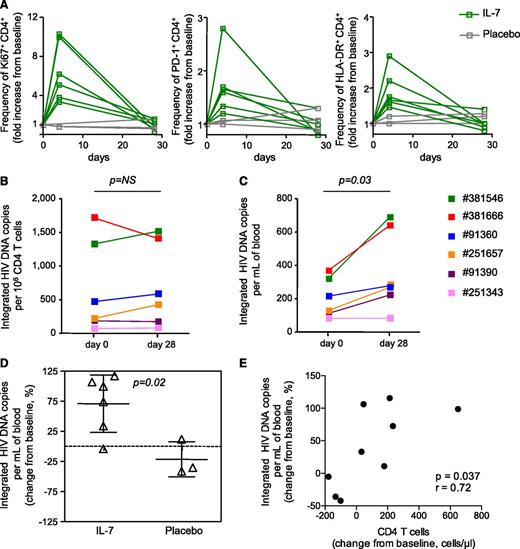
IL-7 administration increases the absolute number of CD4+T cells harboring integrated HIV DNA. (A) Fold changes in the frequencies of CD4+ T cells expressing Ki67, PD-1, and HLA-DR, 4 and 28 days following IL-7 administration as measured by flow cytometry. (B) Frequency of CD4+ T cells harboring integrated HIV DNA at baseline and 28 days after IL-7 administration. (C) Number of CD4+ T cells harboring integrated HIV DNA per milliliter of blood at baseline and 28 days after IL-7 administration. (D) Change from baseline in the absolute number of integrated HIV DNA copies per milliliter of blood in subjects who received IL-7 and in placebo controls. (E) Correlation between the change in CD4+ T-cell numbers and the increase in the frequency of cells harboring HIV integrated DNA per milliliter of blood.
IL-7 administration increases the absolute number of CD4+T cells harboring integrated HIV DNA. (A) Fold changes in the frequencies of CD4+ T cells expressing Ki67, PD-1, and HLA-DR, 4 and 28 days following IL-7 administration as measured by flow cytometry. (B) Frequency of CD4+ T cells harboring integrated HIV DNA at baseline and 28 days after IL-7 administration. (C) Number of CD4+ T cells harboring integrated HIV DNA per milliliter of blood at baseline and 28 days after IL-7 administration. (D) Change from baseline in the absolute number of integrated HIV DNA copies per milliliter of blood in subjects who received IL-7 and in placebo controls. (E) Correlation between the change in CD4+ T-cell numbers and the increase in the frequency of cells harboring HIV integrated DNA per milliliter of blood.
We then investigated the effect of IL-7 therapy on the size of the HIV reservoir by measuring the frequency of CD4+ T cells harboring integrated HIV DNA before and after in vivo administration of the cytokine (Figure 3B). IL-7 therapy did not affect the frequency of CD4+ T cells harboring integrated HIV DNA, suggesting that all CD4+ T cells, including those harboring HIV-integrated DNA, proliferated following administration of the cytokine. As CD4+ T cells expand in response to IL-7, we determined the effect of this intervention on the absolute number of CD4+ T cells harboring integrated HIV DNA. IL-7 therapy led to a significant increase in the number of CD4+ T cells harboring integrated HIV DNA per milliliter of blood (P = .03, Figure 3C). On average, virally suppressed subjects receiving IL-7 showed a 70% increase in the absolute number of CD4+ T cells harboring integrated HIV DNA and this increase was significant when compared with changes in the placebo group (P = .02, Figure 3D). The increase in the number of cells harboring integrated HIV DNA per milliliter of blood was correlated with the increase in CD4 T-cell number (P = .037, Figure 3E).
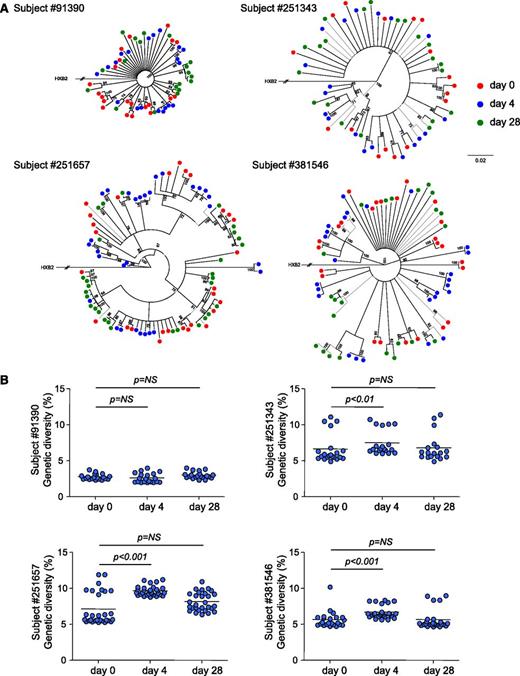
To obtain further insights on the consequences of IL-7 therapy on HIV persistence, we measured the genetic diversity of the viral reservoir before and after IL-7 administration in 4 subjects. Phylogenetic analysis demonstrated that sequences from each subject formed tight and distinct clusters (supplemental Figure 3), consistent with the absence of contamination during amplification. The analysis of sequences obtained at days 0, 4, and 28 revealed the lack of temporal clustering, with viral sequences intermingling between the 3 time points studied (Figure 4A). Three of the 4 subjects displayed a significant increase in the genetic diversity of proviral populations 4 days after IL-7 therapy (Figure 4B). However, 28 days after administration of the cytokine, the genetic diversity of proviral populations returned to baseline values, suggesting that IL-7 therapy did not drive preferential expansion of particular quasispecies but rather induced proliferation of all reservoir cells. Taken together, our results indicated that IL-7 therapy increases the absolute number of CD4+ T cells harboring HIV-integrated DNA without affecting the diversity of the HIV reservoir, suggesting that IL-7 administration induces cell division of latently infected cells without viral reactivation, thereby confirming our in vitro experiments.
IL-7 administration transiently increases the genetic diversity of the HIV reservoir. (A) Neighbor-joining trees derived from HIV sequences obtained from 4 virally suppressed subjects who received IL-7. CD4+ T cells from samples collected at baseline (green dots) and after 4 (blue dots) and 28 days (red dots) following IL-7 therapy were sorted, and a minimum of 20 clones were sequenced in each case. The numbers near nodes indicate the percentage of bootstrap replicates. The scale refers to the distance between sequences. (B) HIV genetic diversities at baseline and 4 and 28 days after IL-7 administration. Each dot represents the mean genetic distance between one given clone and the entire population. The horizontal bar represents the mean and is reflecting the genetic diversity of the viral population at the different time points.
IL-7 administration transiently increases the genetic diversity of the HIV reservoir. (A) Neighbor-joining trees derived from HIV sequences obtained from 4 virally suppressed subjects who received IL-7. CD4+ T cells from samples collected at baseline (green dots) and after 4 (blue dots) and 28 days (red dots) following IL-7 therapy were sorted, and a minimum of 20 clones were sequenced in each case. The numbers near nodes indicate the percentage of bootstrap replicates. The scale refers to the distance between sequences. (B) HIV genetic diversities at baseline and 4 and 28 days after IL-7 administration. Each dot represents the mean genetic distance between one given clone and the entire population. The horizontal bar represents the mean and is reflecting the genetic diversity of the viral population at the different time points.
Discussion
IL-7 plays a crucial role in T-cell homeostasis19-21 and has been shown to efficiently induce proliferation of CD4+ T cells in lymphopenic conditions such as HIV infection.9,10 Recently, IL-7 therapy has been demonstrated to favorably impact T-cell functions by promoting their proliferation and expansion in subjects receiving suppressive ART.11 However, the effects of IL-7 administration on the mechanisms of HIV persistence and on the size of the latent reservoir are still unclear.
In this study, we used large numbers of CD4+ T cells obtained from virally suppressed subjects to demonstrate that IL-7 is a poor inducer of HIV production in latently infected cells. By comparing CD4+ T cells from chronically infected subjects and highly pure resting memory CD4+ T cells from successfully treated subjects, we observed that IL-7 enhances viral production in productively infected cells but has no effect in latently infected cells. The low levels of viral production observed in a fraction of samples obtained from virally suppressed subjects following IL-7 stimulation may result from the enhancement of viral production in a few productively infected cells that can be detected even after prolonged ART.3 Indeed, virally suppressed subjects who showed viral production in response to IL-7 were more likely to spontaneously produce low levels of viral particles, a phenomenon that was abrogated when cells expressing an activated phenotype were depleted. This suggests that the residual levels of viral production observed in a fraction of virally suppressed subjects originate from a small pool of activated cells that are responsive to IL-7. These results are in accordance with a recent study conducted on samples obtained from subjects enrolled in the ACTG5214 trial aimed at identifying the sources of HIV detected during transient viremic episodes following IL-7 administration. HIV sequences detected during viral “blips” were closely related to those present before and after cytokine administration.22 This suggests that the low-level viremia induced by IL-7 likely reflects predominantly transient induction of virus from a preexisting pool of productively infected cells rather than activation of silent quasispecies. This observation suggests that IL-7 therapy may enhance low levels of viral production in anatomical sites such as the gut in which productively infected cells can be detected during ART,18,23 thereby promoting residual inflammation and HIV persistence.
In sharp contrast with productively infected cells in which viral production was enhanced by IL-7, resting memory CD4+ T cells did not produce viral particles in response to the cytokine. This observation could not be attributed to the unresponsiveness of these cells to IL-7 as demonstrated by the increase in the expression of activation markers such as Ki67 and PD-1 following IL-7 stimulation, as previously reported.7,24 Our results demonstrate that this response to IL-7 is not accompanied by production of HIV particles, indicating that the activation of the Janus kinase/signal transducer and activator of transcription signaling pathway induced by IL-7 (signal transducer and activator of transcription 5) is not sufficient to revert viral latency in authentic latently infected CD4+ T cells. In accordance with a recent study using an in vitro model of HIV latency in central memory CD4+ T cells,14 our data demonstrate that IL-7 induces survival and proliferation of latently infected CD4+ T cells isolated from virally suppressed subjects in the absence of viral reactivation.
More importantly, our in vitro data were confirmed in vivo in virally suppressed subjects who received IL-7. We first confirmed that IL-7 administration led to a rapid and dramatic increase in the frequency of cycling T cells.9,10 Because the frequency of cells harboring integrated HIV DNA within the CD4 compartment remained stable after IL-7 administration, it is likely that all CD4+ T cells, whether latently infected or not, proliferated similarly in response to the cytokine. This resulted in a significant increase in the absolute number of infected cells per milliliter of blood 4 weeks after IL-7 administration, indicating that IL-7 therapy expands the pool of infected CD4+ T cells during ART.
In both studies where IL-7 was administered to virally suppressed subjects, high doses of the cytokine (10 μg/kg or higher) frequently induced transient episodes of viremia.9,10 Of note, 3 of 7 subjects included in the present study received low-dose IL-7 (3 μg/mL). Although our results and those from a recent study22 strongly suggest that IL-7 is inducing a transient viral burst primarily by amplifying virus present before IL-7 therapy, rather than inducing production from a previously silent reservoir, a study examining a larger number of subjects receiving high doses of IL-7 is warranted.
One limitation to our study is that viral DNA, even in its integrated form, does not necessarily reflect replication competent virus. The limited number of cells available from these subjects did not allow us to measure the number of infectious units per million cells by the classical coculture assay.5 Nevertheless, a model in which IL-7 induces proliferation of latently infected CD4+ T cells harboring replication competent virus in the absence of viral reactivation is supported by our current and previous in vitro observations7 as well as by the recent study of Bosque et al.14 In addition, the maintenance in the viral diversity of proviral quasispecies that accompanied the increase in the absolute number of infected cells also reinforces a model in which infected CD4+ T cells proliferate in response to the cytokine.
Of note, a transient and significant increase in the diversity of proviral quasispecies was observed at day 4 in 3 of the 4 study subjects. This may result from the rapid and transient redistribution of infected cells between blood and tissues after IL-7 administration,25 as injection of IL-7 in the rhesus macaque model has been shown to provoke rapid and massive T-cell homing before T cells migrate back into the blood stream.26
In summary, our study provides in vitro and in vivo evidences supporting a role for IL-7 in HIV persistence during ART by enhancing residual levels of viral production and by inducing proliferation of latently infected CD4+ T cells. Our results indicate that in spite of its ability to induce substantial functional and quantitative changes in T cells during ART, IL-7 therapy alone will not achieve HIV eradication. Combining IL-7 therapy with novel therapeutic strategies aimed at interfering with the long-term mechanisms of viral persistence may achieve restoration of T-cell function together with a reduction in the size of the latent reservoir during ART.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients for their participation in this study. The authors also thank Mohamed-Rachid Boulassel, Rebeka Bordi, Brenda Jacobs, and Kathyrin Penniman for recruitment and clinical assistance with patients, Stephanie Santos and Nicola Faraci for technical assistance with blood samples, and Yu Shi and Kim Kusser for flow cytometric cell sorting. The authors thank the members of the Cleveland Immunopathogenesis Consortium for advice and helpful discussions.
R.F. is supported by the American Foundation for AIDS Research (amfAR, fellowship number 108264). The work of I.S. is supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases. This work was supported in part by the amfAR (107175-44-RGRL), the Fonds de la recherche en Santé du Québec (Réseau Syndrome d'immunodéficience acquise et maladies infectieuses), the Canadian Institutes of Health Research (HOP 103230), and the National Institutes of Health (1U19AI096109 to the Delaney AIDS Research Enterprise to find a cure). J.-P.R. holds the Louis Lowenstein Chair in Hematology & Oncology.
Authorship
Contribution: C.V. designed and performed experiments, analyzed data, designed figures, and wrote the manuscript; R.F., S.D., and M.B.L. performed experiments and analyzed data; I.S., M.M.L., M.R., and J.-P.R. provided samples from research subjects and helped with writing the manuscript; R.-P.S. interpreted data and wrote the manuscript; and N.C. designed the study, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicolas Chomont, Vaccine and Gene Therapy Institute Florida, 9801 SW Discovery Way, Port St. Lucie, FL 34987; e-mail: nchomont@vgtifl.org.