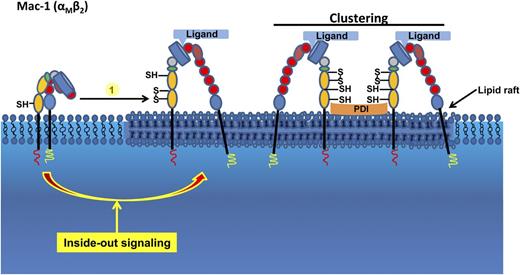
Working model of the role of PDI in the function of the β2-integrin Mac-1 on neutrophils. The neutrophil β2-integrin Mac-1 is shown in different activation states. The nonactivated state on the left side contains free thiols. Agonist-induced stimulation induces cytoplasmic events leading to inside-out signaling and the interaction of Mac-1 with its ligands. Mac-1 is modified by PDI. During neutrophil activation, sulfhydryls are generated in the αM subunit of Mac-1, leading to Mac-1 clustering. S, sulfur; SH, thiol.
Working model of the role of PDI in the function of the β2-integrin Mac-1 on neutrophils. The neutrophil β2-integrin Mac-1 is shown in different activation states. The nonactivated state on the left side contains free thiols. Agonist-induced stimulation induces cytoplasmic events leading to inside-out signaling and the interaction of Mac-1 with its ligands. Mac-1 is modified by PDI. During neutrophil activation, sulfhydryls are generated in the αM subunit of Mac-1, leading to Mac-1 clustering. S, sulfur; SH, thiol.
PDI is an enzyme that can catalyze 3 different reactions, oxidation, reduction, and isomerization of disulfide bonds including thiol-disulfide exchange.2 Although PDI has an endoplasmic reticulum retention sequence, it is also located on the cell surface.2 The oxidation of sulfhydryls to disulfides happens mainly in the endoplasmic reticulum, whereas PDI on the cell surface is thought to catalyze isomerization or reduction of disulfide bonds.2 Human PDI has a single subunit of 491 amino acids and possesses 2 regions of internal homology, each of which has an active site with the sequence of Cys-Gly-His-Cys. The peptide/protein-binding site of PDI is located in the b′ domain. An interaction of PDI with its substrate at this site appears to be required for catalytic activity.2 PDI mediates platelet aggregation and secretion, as well as activation of the αIIβ3 integrin.3 A role of PDI in platelet adhesion by β1 and β3 integrins has also been demonstrated, including adhesion by the α2β1 collagen receptor.4 A functional and physical relationship of PDI to the adhesion receptor glycoprotein Ib on platelets has also been shown.4 A recent study demonstrated that extracellular PDI can interact with endothelial cell and platelet β3 integrins during thrombus formation in vivo, thereby regulating integrin activity.5
Integrins are integral membrane proteins that mediate cell-cell adhesion and cell-matrix interactions. These molecules are essential for hematopoiesis, vascular development, immune and inflammatory responses, and hemostasis. Integrins are signaling receptors that can signal information bidirectionally across the plasma membrane. In circulating blood cells, integrins are normally in an active (resting) state with low affinity for their ligands. Upon activation, they can quickly change their conformation to an activated, high-affinity state, a process often referred to as inside-out signaling (see figure).6
In the present study, Hahm et al demonstrate a new mechanism by which the adhesiveness of the β2-integrin Mac-1 can be regulated under inflammatory conditions. The authors show that the specific deletion of PDI in myeloid cells abolishes neutrophil recruitment. By using blocking antibodies and knockout mice, they convincingly demonstrate that eliminating or blocking PDI reduces neutrophil adhesion and crawling by regulating Mac-1 adhesiveness. Although Mac-1 is also involved in neutrophil transmigration,7 the authors have not investigated this important step in this study. In addition to this, the in vivo data in the present study show that exogenous PDI can also bind to the inflamed endothelium and may regulate the activation status of endothelial cells and the expression of endothelial junctional molecules, thereby affecting neutrophil recruitment. Therefore, the role of PDI in neutrophil transmigration has to be addressed in future studies.
Other studies demonstrated that PDI interacts with αIIβ3 and αvβ3 integrins on platelets and endothelial cells, respectively.5,8 In biochemistry experiments, the authors nicely showed that PDI interacts with Mac-1 and that activated Mac-1 colocalizes with PDI in lipid rafts.1 To directly prove the interaction of PDI and Mac-1, Hahm et al1 performed surface plasmon resonance using recombinant proteins. The results showed that PDI can directly bind to Mac-1. However, as exogenous PDI still binds to αMβ2 integrin-deficient neutrophils, it is likely that the regulatory role of PDI in integrin function is not limited to Mac-1.
Integrins contain several highly conserved cysteine residues. Some of the cysteines are disulfide-bonded and some exist as free thiols. Cleavage of disulfide bonds appears to be involved in the activation of integrins.3,9 The reducing agent dithiothreitol activates integrins on platelets and neutrophils by reduction of disulfide bonds within the integrin’s cysteine-rich repeat.3,9 The study by Hahm et al1 demonstrates that PDI on the cell surface of neutrophils interacts with activated Mac-1 within lipid rafts via electrostatic interactions and catalyzes thiol exchange on the αM subunit of Mac-1 (see figure), thereby regulating Mac-1 adhesiveness during neutrophil activation. Integrin adhesiveness is regulated by modulating integrin affinity and avidity.10 Blocking of PDI in human neutrophils did not reduce the binding of the reporter antibody CBRM 1/5, which only detects the activated form of Mac-1, but abolished Mac-1 clustering,1 suggesting that PDI does not affect inside-out–mediated affinity regulation of Mac-1, but rather the stabilization of the integrin in the activated conformation. The results of this study stand in contrast to another study showing that modification of disulfide bonds in the I domain of the αM subunit alters the affinity of ligand binding.9 These data suggest that the modification of some cysteine residues regulates integrin affinity, whereas modification of other cysteine residues modulates integrin avidity. Future studies are necessary to examine whether PDI has a binding specificity to a specific integrin subunit and determine which cysteine residue on Mac-1 is modified by PDI.
In summary, these findings put forward a novel and important role for PDI in regulating neutrophil recruitment during inflammation. An important aspect of these findings is that PDI interacts with activated Mac-1 in lipid rafts and modifies thiol exchange on Mac-1, thereby regulating Mac-1 adhesiveness and neutrophil recruitment. Further studies are anticipated that will investigate the specific mechanisms underlying the regulation of β2-integrin activation on leukocytes, which remain unknown.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal