Key Points
Astatination of anti-CD45 antibody via a closo-decaborate compound yields a stable conjugate that targets radiation to hematologic organs.
211At-anti-CD45 radioimmunotherapy combined with bone marrow transplantation prolongs survival in a disseminated murine leukemia model.
Abstract
Despite aggressive chemotherapy combined with hematopoietic stem cell transplantation (HSCT), many patients with acute myeloid leukemia (AML) relapse. Radioimmunotherapy (RIT) using monoclonal antibodies labeled with β-emitting radionuclides has been explored to reduce relapse. β emitters are limited by lower energies and nonspecific cytotoxicity from longer path lengths compared with α emitters such as 211At, which has a higher energy profile and shorter path length. We evaluated the efficacy and toxicity of anti-CD45 RIT using 211At in a disseminated murine AML model. Biodistribution studies in leukemic SJL/J mice showed excellent localization of 211At-anti-murine CD45 mAb (30F11) to marrow and spleen within 24 hours (18% and 79% injected dose per gram of tissue [ID/g], respectively), with lower kidney and lung uptake (8.4% and 14% ID/g, respectively). In syngeneic HSCT studies, 211At-B10-30F11 RIT improved the median survival of leukemic mice in a dose-dependent fashion (123, 101, 61, and 37 days given 24, 20, 12, and 0 µCi, respectively). This approach had minimal toxicity with nadir white blood cell counts >2.7 K/µL 2 weeks after HSCT and recovery by 4 weeks. These data suggest that 211At-anti-CD45 RIT in conjunction with HSCT may be a promising therapeutic option for AML.
Introduction
Acute myeloid leukemia (AML) is an aggressive malignancy with few treatments producing prolonged remissions in high-risk patients. Hematopoietic stem cell transplantation (HSCT) may offer the best chance for a cure, but it has been associated with high rates of treatment-related mortality and relapse. Investigators have escalated chemotherapy and/or radiation doses to decrease relapse, but this strategy has been associated with substantial toxicity yielding no significant improvement in overall survival.1 Monoclonal antibodies (mAbs) targeting hematologic-specific antigens have been used in radioimmunotherapy (RIT) studies as a means to deliver higher radiation doses prior to HSCT.2-6 One such target is CD45, a cell surface antigen highly expressed on hematologic tissues (∼200 000 binding sites per cell) with minimal expression on nonhematologic tissues.7,8 CD45 is not extensively internalized after mAb binding,9,10 further making anti-CD45 RIT a viable approach for therapy of high-risk AML. In particular, anti-CD45 mAb coupled to 131I has been shown to deliver an average twofold to threefold higher radiation-absorbed dose to spleen and bone marrow than to nonleukemic normal organs, and it can be safely administered to high-risk patients with acute leukemia or myelodysplastic syndrome in conjunction with standard high-dose chemotherapy and 12Gy total-body irradiation.5,11 Favorable results suggesting improvements in survival have also been shown using this anti-CD45 RIT approach as part of a reduced-intensity HSCT regimen in older relapsed/refractory AML patients with high disease burdens.6
Despite these advances, limitations inherent to the radionuclides investigated thus far hinder more widespread use of RIT as an adjunct to HSCT. The majority of RIT studies have used mAbs labeled with β emitters such as 131I and 90Y. However, these radionuclides may generate nonspecific cytotoxicity due to a cross-fire effect from their relatively longer path length (0.3-2.3 mm), and for 131I, its associated γ-emissions. Conversely, α-emitting radionuclides have the potential to deliver improved therapeutic ratios of absorbed radioactivity with less nonspecific toxicity because of their very short path lengths (∼60-80 µm), making them attractive candidates for therapy of leukemias and elimination of minimal residual disease.12-16 Differences in path lengths dictate that β emitters rely on the cross-fire effect, irradiating bystander cells without the need to deliver the radionuclide directly to each malignant cell. The cross-fire effect from α emitters is confined to smaller volumes, resulting in a cytotoxic effect in short-range tracks near cells binding the radionuclide via the mAb. Moreover, α-emitting reagents have a high linear energy transfer (LET) because of the high decay energy (5-8 MeV) deposited over short distances that allows for potent and efficient cell kill compared with β emitters such as 131I and 90Y with lower decay energies (0.66-2.3 MeV) and longer path lengths.2
Only a few α-emitting radionuclides, however, are currently suitable for clinical application.17 For the α emitters with favorable radiobiologic characteristics, other issues such as availability, labeling chemistry, and in vivo stability, especially of astatinated macromolecules, have had an impact on their use in RIT.14,18,19 Astatinated mAbs exhibit in vivo dehalogenation of mAbs when labeled via conventional approaches, effectively hindering development of 211At-based α-targeted therapy. By using a novel approach to 211At-label mAb, we have thus used the α-emitting radionuclide 211At for anti-CD45 RIT of AML because it has a reasonable half-life (t1/2 = 7.2 hours), a favorable energy profile (6.8 MeV; averages of two α decays, 5.9 and 7.5 MeV), and a short path length (average range, 55-70 µm). In this report, we describe the efficacy and toxicity of 211At-labeled-anti-CD45 RIT combined with HSCT in a mouse model of syngeneic disseminated AML. These studies show that 211At-anti-CD45 mAb can efficiently localize to sites of leukemia, and when used in lieu of total-body irradiation, this approach facilitates engraftment of donor bone marrow with minimal toxicity and significantly prolongs survival.
Methods
Mice
Female SJL/J mice, 6 to 12 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME) and housed at the Fred Hutchinson Cancer Research Center in a pathogen-free environment under protocols approved by the Institutional Animal Care and Use Committee.
Cell lines and antibodies
Conjugation of 30F11 and rat IgG with B10
Anti-CD45 mAb (30F11) or rat IgG antibody was diluted 1:1 with 100 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer to yield 50 mM (with 150 mM NaCl [pH 8.6]). Isothiocyantophenethyl-ureido-closo-decaborate(2-) (B10-NCS) at 10 mg/mL in dimethylsulfoxide was added to antibody solution,22 and the reaction proceeded overnight with gentle tumbling at room temperature. The next morning, the mixture was split into two equal fractions and passed over two PD-10 columns and collected in phosphate-buffered saline. Protein fractions were combined, concentrated, and sterile filtered to yield B10-30F11 or B10-rat IgG.
211At-labeling of B10-30F11 and B10-rat IgG
211At was isolated from an irradiated bismuth target by a wet chemistry approach as previously described.22 The isolated 211At solution was used directly in the labeling procedure. Briefly, a 250-µL solution of 500 mM sodium phosphate (pH 6.8) was combined with 250 µL B10-30F11 or B10-rat IgG. To this solution, 500 µL of Na[211At]At (3.5 mCi in water [pH 7]) was added to the B10-30F11 reaction, or 350 µL of Na[211At]At (2.1 mCi in water [pH 7]) was added to the B10-rat IgG reaction, followed by 10 µL of an aqueous Chloramine-T solution (10 mg/mL). After 2 minutes at room temperature, the reaction was quenched by adding 10 µL of sodium metabisulfite solution (10 mg/mL in water) to the B10-30F11 or B10-rat IgG reaction. Each mixture was then placed on a PD-10 column and eluted with phosphate-buffered saline. The protein-containing fractions were combined to give 211At-B10-30F11 (75% radiochemical yield and 79% protein recovery) or 211At-B10-rat IgG (72% radiochemical yield and 62% protein recovery). Purity of 211At-labeled injectate was >99% by thin-layer chromatography.
Biodistribution studies
Mice were injected intravenously with 1 × 105 SJL leukemia cells . Two days after injection of leukemia cells, 100 μg (0.67 nmol) of B10-30F11 and B10-rat IgG were trace-labeled with either 125I, as previously described,20 or with 211At and injected into groups of 5 mice. Mice were then euthanized 8 and 24 hours after injection of 125I-B10-mAb conjugate or 1, 3, 7, and 24 hours after injection of 211At-B10-mAb conjugate. Organs were excised and weighed, and the 125I or 211At activity was counted on a Packard 5000 γ counter (Packard Instrument Company, Meriden, CT), with correction for radioactive decay during the counting process, to determine the percent injected dose of radiation activity per gram of organ (% ID/g).
Radiation dosimetry
RIT of leukemic mice
RIT studies were performed by using 211At-B10-mAb conjugates in groups of 10 mice; 5 days prior to study onset, the mice were placed on a diet containing Uniprim antibiotic (irradiated, 4100 ppm from Harlan Laboratories, Indianapolis, IN). Mice were injected intravenously with 1 × 105 SJL leukemia cells, and 2 days later, the mice received 100 µg (0.67 nmol) of either B10-30F11 or B10-rat IgG labeled with 12, 20, or 24 µCi 211At. Two days after injection of 211At-B10-mAb, mice underwent syngeneic bone marrow transplantation (BMT), receiving 15 × 106 donor bone marrow cells without T-cell depletion, as described previously.24 Mice were monitored daily for changes in appearance and body weight. Mice were euthanized if they became moribund from progressive leukemia or if they lost more than 30% of baseline weight. Survival curves were compared by the log-rank test (Mantel-Cox test) and were reported only when they were statistically significant (when less than the Bonferroni-corrected threshold for multiple comparisons).
Toxicity assessments
Groups of 10 non–leukemia-bearing mice were treated with 12 or 24 µCi 211At-B10-mAb conjugate and rescued via BMT as described above. At 1, 2, 3, 4, 6, 8, and 25 weeks after BMT, blood was drawn via the retro-orbital plexus, and complete blood counts were obtained. Serum was assayed for kidney function by measuring blood urea nitrogen (BUN) and creatinine levels, and for liver function by measuring the enzymes alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate transaminase (AST). All toxicity tests were performed by Phoenix Central Laboratory (Everett, WA).25 Values were compared with those obtained at the same time points from untreated age-matched control SJL/J mice.
Results
Biodistribution of radioactivity using 211At-B10 anti-CD45 mAb in SJL/J leukemic mice
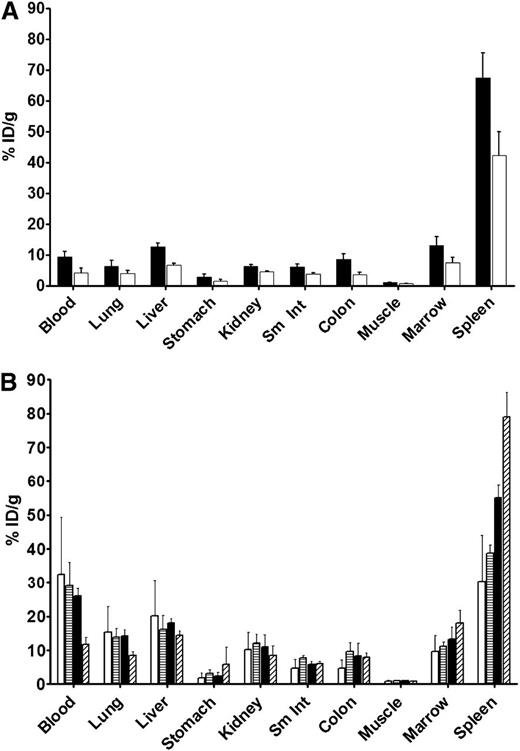
To assess the ability of the B10-mAb constructs to localize to leukemia targets, we performed biodistribution studies by using radiolabeled 30F11-B10 conjugates. Because radioiodine has been reproducibly used in prior biodistribution studies,20,25 we labeled B10-30F11 with 125I and 211At for comparison. Uptake of 125I-B10-30F11 in the spleen was 67.6% ± 8% ID/g by 8 hours, and 24 hours after injection of 125I-B10-30F11, the spleen retained more activity (42.3% ± 8% ID/g) than any other tissue at this same time point (Figure 1A). Other nontargeted tissues, such as liver and kidney, had significantly less radioactivity at 24 hours, with 6.7% ± 0.5% and 4.6% ± 0.1% ID/g, respectively.
Biodistribution of radioactivity in AML-bearing SJL/J mice. Mice received 0.67 nmol of B10-30F11 conjugate trace-labeled with 125I or 211At and were then euthanized at stated time points. Their organs were harvested, and radioactivity was measured for (A) 125I at 8 (▪) and 24 hours (□) or for (B) 211At at 1 (□), 3 (▤), 7 (▪), and 24 (▨) hours after injection of radioactivity. Counts were decay-corrected and expressed as a percentage of the injected dose per gram of tissue (% ID/g). Sm Int, small intestine.
Biodistribution of radioactivity in AML-bearing SJL/J mice. Mice received 0.67 nmol of B10-30F11 conjugate trace-labeled with 125I or 211At and were then euthanized at stated time points. Their organs were harvested, and radioactivity was measured for (A) 125I at 8 (▪) and 24 hours (□) or for (B) 211At at 1 (□), 3 (▤), 7 (▪), and 24 (▨) hours after injection of radioactivity. Counts were decay-corrected and expressed as a percentage of the injected dose per gram of tissue (% ID/g). Sm Int, small intestine.
Since the novel α-labeling construct B10 did not appear to impede targeting given the excellent localization of 125I-B10-30F11 to bone marrow and spleen, we then performed biodistribution studies using the α emitter 211At. One hour after 211At-B10-30F11 injection, blood and spleen displayed the highest concentrations of radioactivity (32.3% ± 17% and 30.3% ± 14% ID/g, respectively [Figure 1B]). In addition, blood clearance of 211At-B10-30F11 was rapid, decreasing from 32.3% ± 17% ID/g 1 hour after injection to 11.7% ± 2% ID/g after 24 hours; the spleen retained 79% ± 7.3% ID/g and the bone marrow retained 18% ± 3.8% ID/g after 24 hours. Importantly, 211At-B10-30F11 did not exhibit excessive nonspecific uptake in the kidneys, even without use of renal protective agents, with 10.2% ± 5.1%, 12.2% ± 2.6%, 10.9% ± 3.6%, and 8.4% ± 2.9% ID/g retained in kidneys 1, 3, 7, and 24 hours after injection of 211At-B10-30F11 respectively. Relatively low concentrations of radioactivity were delivered to nontarget organs such as liver, lung, small intestine, and colon after 24 hours (14.3% ± 1.3%, 8.4% ± 1.2%, 6.02% ± 0.7%, and 7.93% ± 1.3% ID/g, respectively). These biodistribution studies showed that both 125I and 211At localized to target tissues of bone marrow and spleen, with less nonspecific uptake in nonhematologic tissues.
211At-B10-anti-CD45 mAb dosimetry
Using standard medical internal radiation dose methods26 and biodistribution data of 211At-B10-30F11, radiation absorbed doses (cGy) for organs harvested were calculated per µCi of 211At injected. Values shown are for 0.19 µCi of 211At injected to yield a liver dose of 5 cGy (Table 1). CD45+ tissues targeted by B10-30F11 had the highest total absorbed dose at 18 and 7.5 cGy per 0.19 µCi of 211At administered for spleen and blood, respectively. Nontarget organs had lower radiation absorbed doses, with kidneys and lungs having 2.9 and 4.1 cGy absorbed dose per 0.19 µCi of 211At injected, respectively.
Absorbed dose per 0.19 µCi 211At activity injected (to dose 5 cGy to liver)
| Tissue . | Absorbed dose (cGy) . |
|---|---|
| Spleen | 18 |
| Marrow | 1.7 |
| Blood | 7.5 |
| Kidney | 2.9 |
| Liver | 5.0 |
| Stomach | 0.96 |
| Small intestine | 1.8 |
| Colon | 2.4 |
| Lung | 4.1 |
| Tissue . | Absorbed dose (cGy) . |
|---|---|
| Spleen | 18 |
| Marrow | 1.7 |
| Blood | 7.5 |
| Kidney | 2.9 |
| Liver | 5.0 |
| Stomach | 0.96 |
| Small intestine | 1.8 |
| Colon | 2.4 |
| Lung | 4.1 |
α-Camera imaging
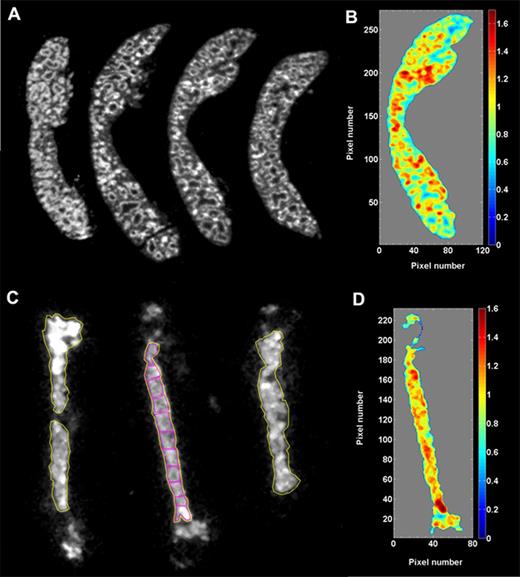
α-Camera imaging described previously was used to further characterize the distribution of 211At-B10-30F11 conjugate because some α emitters have been known to exhibit heterogeneous distributions in targeted tissues at the suborgan level.27 Cryosections of spleen and femur from leukemia-bearing mice treated with 211At-B10-30F11 were imaged with the α-camera technique at various time points after radiolabeled injections. At the 3-hour time point, α-camera imaging revealed that 211At was distributed throughout the spleen and bone marrow, with some variability that correlates with suborgan architecture. By imaging, the activity uptake in spleen (Figure 2A) was 33% ± 2% ID/g at 3 hours after injection of 80 µCi 211At-B10-30F11, an uptake value in good agreement with the biodistribution data. Figure 2B presents a color-coded histogram of the activity distribution in a spleen section, in which the activity uptake of the different subareas was normalized to the mean uptake for the whole spleen. More than 80% of the total section area was within a factor of 0.7 to 1.3 of the mean activity. The highest concentrations were noted in areas corresponding to the marginal zone between red and white pulp. α-Camera imaging of cryosectioned femurs (Figure 2C; yellow regions of interest [ROIs]) quantified the uptake in bone marrow to be 11% ± 2% ID/g at 3 hours after injection of 211At-B10-30F11. These results correlated well with biodistribution studies (Figure 1A) in which 211At-B10-30F11 demonstrated 11.2% ± 1.3% ID/g at the 3-hour time point. The activity distribution (normalized to the mean) along the bone marrow cavity of a femur was similar, in which 85% of the area was within a factor of 0.7 to 1.3 of the mean (Figure 2D). To further analyze activity distribution in the femur, 11 small ROIs were placed along the marrow cavity to quantify how their activity concentration varied compared with the mean activity of the whole femur (Figure 2C; magenta ROIs). The variations in activity between the ROIs were small, ranging from 0.9 to 1.25. The highest uptake was seen in the bone epiphyses.
α-Camera imaging. (A) Cryosections (10 µm) of spleen imaged 3 hours after intravenous injection of 211At-B10-30F11. (B) The third section from the left in panel A was used for quantitative analysis of the activity distribution of 211At within the spleen. Each pixel intensity value (activity) was normalized to the mean pixel intensity of the whole spleen. The normalized data were divided in 10 bins (0 to max) and plotted as a color-coded histogram (1.0 = mean activity of the whole section). (C) Cryosections (10 µm) of femur imaged 3 hours after intravenous injection of 211At-B10-30F11. The large ROIs (yellow) outline the areas used for quantification of % ID/g in bone marrow by α-camera imaging. The small ROIs (magenta) in the middle femur image were used to compare the activity uptake (% ID/g) along the bone marrow cavity. (D) Quantitative histogram of 211At distribution of the middle femur section in panel C.
α-Camera imaging. (A) Cryosections (10 µm) of spleen imaged 3 hours after intravenous injection of 211At-B10-30F11. (B) The third section from the left in panel A was used for quantitative analysis of the activity distribution of 211At within the spleen. Each pixel intensity value (activity) was normalized to the mean pixel intensity of the whole spleen. The normalized data were divided in 10 bins (0 to max) and plotted as a color-coded histogram (1.0 = mean activity of the whole section). (C) Cryosections (10 µm) of femur imaged 3 hours after intravenous injection of 211At-B10-30F11. The large ROIs (yellow) outline the areas used for quantification of % ID/g in bone marrow by α-camera imaging. The small ROIs (magenta) in the middle femur image were used to compare the activity uptake (% ID/g) along the bone marrow cavity. (D) Quantitative histogram of 211At distribution of the middle femur section in panel C.
211At-anti-CD45 RIT of disseminated murine leukemia
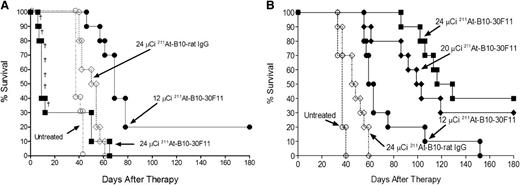
Having demonstrated excellent localization of the B10-anti-murine CD45 mAb conjugate in biodistribution and α-camera imaging studies, we assessed the therapeutic efficacy of 211At-B10-30F11 in a clinically relevant disseminated murine myeloid leukemia model. SJL/J mice were injected with 1 × 105 SJL leukemic cells as previously described.20,21 Two days later groups of 10 mice per dose were treated with 12 or 24 µCi 211At-B10-30F11 or 24 µCi 211At-B10-rat IgG. Studies in the absence of stem cell rescue (Figure 3A) demonstrated a survival benefit for the mice treated with 12 µCi 211At-B10-30F11 with a statistically significant improvement in median overall survival (OS) of 69 days (with 2 treated mice surviving for >180 days) compared with a median survival of 36 days for untreated control mice (P < .0001) and 54 days for mice that received 211At-B10-rat IgG (P = .0003). The marginal improvement in OS seen in the rat IgG control group was likely due to a nonspecific radiation effect from circulating radiolabeled B10-rat IgG. Seventy percent of the mice treated with 24 µCi 211At-B10-30F11 died within 12 days after injection of 211At-B10-30F11 (median OS of 11 days) due to toxicity related to marrow aplasia and probable infection; these mice had normal liver and kidney function assessed prior to euthanasia and did not have evidence of leukemic blasts in tissues by gross histology or in blood as assayed by flow cytometric analysis.
Anti-CD45 RIT of AML-bearing SJL/J mice with and without BMT. (A) Mice were injected with SJL leukemia, followed 2 days later by 0.67 nmol of B10-30F11 labeled with 12 (•) or 24 (▪) µCi 211At. Control mice received 24 µCi 211At-B10-rat IgG (♢) or no treatment (○). Mice did not receive BMT in this study, and (†) denotes individual murine deaths due to toxicity. (B) Mice were injected with SJL leukemia and 2 days later with 0.67 nmol of 211At-B10-30F11 with 12 (•), 20 (♦) or 24 (▪) µCi 211At. Control mice received 24 µCi 211At-B10-rat IgG (♢) or no treatment (○). On day +2, mice were given 15 × 106 bone marrow cells via tail vein injection.
Anti-CD45 RIT of AML-bearing SJL/J mice with and without BMT. (A) Mice were injected with SJL leukemia, followed 2 days later by 0.67 nmol of B10-30F11 labeled with 12 (•) or 24 (▪) µCi 211At. Control mice received 24 µCi 211At-B10-rat IgG (♢) or no treatment (○). Mice did not receive BMT in this study, and (†) denotes individual murine deaths due to toxicity. (B) Mice were injected with SJL leukemia and 2 days later with 0.67 nmol of 211At-B10-30F11 with 12 (•), 20 (♦) or 24 (▪) µCi 211At. Control mice received 24 µCi 211At-B10-rat IgG (♢) or no treatment (○). On day +2, mice were given 15 × 106 bone marrow cells via tail vein injection.
211At-anti-CD45 RIT combined with BMT for leukemia
Survival among animals treated with escalated doses of 211At-B10-30F11 was limited by radiation-induced marrow toxicity that resulted in early nonleukemic deaths, suggesting that improved outcomes could be obtained with BMT. We therefore evaluated the efficacy of 211At-B10-30F11 RIT in conjunction with BMT in syngeneic leukemic mice. Groups of 10 SJL/J mice were injected with 1 × 105 SJL leukemic cells followed 2 days later by injection of 12, 20, or 24 µCi 211At-B10-30F11 or 24 µCi 211At-B10-rat IgG. Two days after 211At-B10-mAb injection, at a time when 99% of 211At had decayed, mice received 15 × 106 bone marrow cells intravenously from syngeneic donors. Mice that received 211At-B10-30F11 showed a dose-dependent improvement in median OS, with increased long-term survival rates seen after BMT (Figure 3B). Mice treated with 12, 20, or 24 µCi 211At-B10-30F11 had a median OS of 61, 101, and 123 days (P < .001 for all three when compared with untreated control mice), respectively. Seven of the 20 mice treated with the higher doses (either 20 or 24 µCi 211At-B10-30F11) survived to euthanasia at day 180 postinjection without evidence of recurrent leukemia. For comparison, untreated control leukemic mice had a median OS of 37 days, and mice treated with 211At-B10-rat IgG conjugate had a median OS of 46.5 days (P = .0023). Overall, these data suggest that the hematologic toxicity from anti-CD45 RIT using 211At may be overcome by hematopoietic cell rescue.
Assessment of toxicity after 211At-anti-CD45 RIT
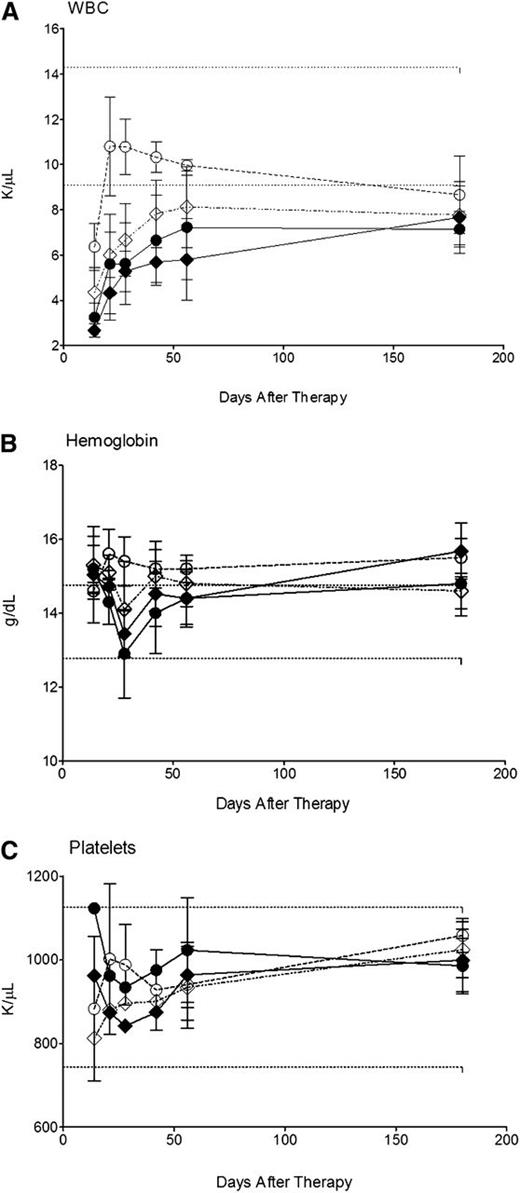
To evaluate the tolerability and systemic toxicity of 211At-labeled anti-CD45 RIT with BMT, 10 mice per group were treated with 24 µCi 211At-B10-rat IgG or either 12 or 24 µCi 211At-B10-30F11 2 days prior to BMT. Blood, renal and hepatic studies were performed at multiple time points after delivery of each radiolabeled B10-mAb conjugate. Each laboratory test was compared with untreated age-matched control mice. These studies demonstrated that doses up to 24 µCi 211At-B10-30F11 with BMT were minimally toxic, with all mice developing a dose-dependent leukopenia that improved by 4 weeks after transplantation (Figure 4A). White blood cell counts had nadirs between 2.66 and 4.34 K/µL at 2 weeks after BMT for mice treated with 24 and 12 µCi 211At-B10-30F11, respectively; they stabilized (5.80-7.22 K/µL) in all mice by day 56 after infusion of the radiolabeled B10-Ab conjugate and remained in this range out to 180 days. Hemoglobin and platelet levels were minimally affected by either dose of 211At-B10-30F11 (ranging between 12.9 and15.7 g/dL and 812 and 1120 K/µL, respectively; Figure 4B-C).
Hematologic toxicity using 211At-B10-30F11 and BMT. Nonleukemic SJL/J mice were injected with 0.67 nmol of B10-30F11 labeled with either 12 (●) or 24 (♦) µCi 211At. Control mice were given 24 µCi 211At-B10-rat IgG (♢) or BMT alone (○). Mice were bled at 1, 2, 3, 4, 6, 8, and 26 weeks to assay for (A) white blood cells (WBCs), (B) hemoglobin, and (C) platelets. Dashed lines indicate the range of normal control values.
Hematologic toxicity using 211At-B10-30F11 and BMT. Nonleukemic SJL/J mice were injected with 0.67 nmol of B10-30F11 labeled with either 12 (●) or 24 (♦) µCi 211At. Control mice were given 24 µCi 211At-B10-rat IgG (♢) or BMT alone (○). Mice were bled at 1, 2, 3, 4, 6, 8, and 26 weeks to assay for (A) white blood cells (WBCs), (B) hemoglobin, and (C) platelets. Dashed lines indicate the range of normal control values.
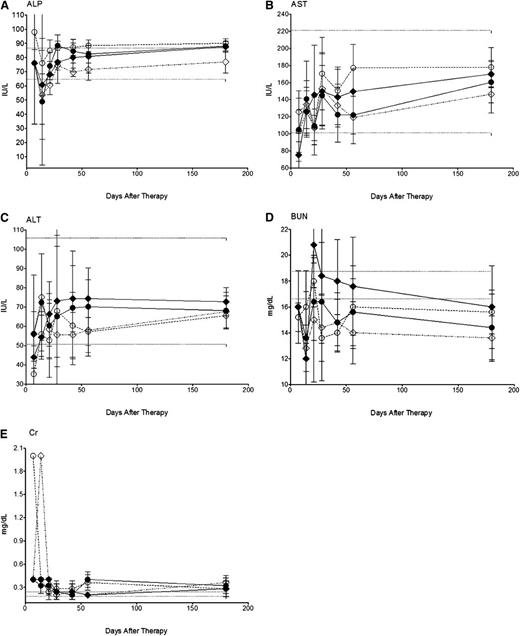
Renal and hepatic function tests for mice treated with 24 µCi 211At-B10-30F11 did not significantly deviate from the normal range of untreated controls for at least 180 days after BMT (Figure 5). Despite a modest initial decrease in ALP levels during the first 4 weeks to 76.0, 43.1, and 43.0 IU/L in mice receiving 24 µCi 211At-B10-rat IgG, and 12 or 24 µCi 211At-B10-30F11, respectively, ALP levels remained stable thereafter (69.6-88.1 IU/L), which was comparable to the normal range of 64.8 to 86.8 IU/L (Figure 5A). Mild increases in AST levels were seen in mice that received 211At-B10-30F11, yet these values remained within normal range throughout the study (ALT, 50.7-105.9 IU/L; AST, 101-221 IU/L; Figure 5B-C). BUN levels were within normal range at 15.6 and 17.6 mg/dL 6 weeks after therapy for mice treated with 12 and 24 µCi 211At-B10-30F11; this was in comparison with BUN levels of 12.8 to 18.0 mg/dL in mice treated with control 211At-B10-rat IgG or BMT alone at the same time point (Figure 5D). Serum creatinine levels remained stable throughout the monitored period, with baseline levels between 0.2 and 0.4 mg/dL for all 211At-B10-30F11 mice (Figure 5E). These data suggest that minimal toxicity is associated with anti-CD45 RIT using 211At and BMT in this model.
Nonhematologic toxicity using 211At-B10-30F11 and BMT. Nonleukemic SJL/J mice were injected with 0.67 nmol of B10-30F11 labeled with either 12 (●) or 24 (♦) µCi 211At. Control mice were given 24 µCi 211At-B10-rat IgG (♢) or BMT alone (○). Mice were bled at 1, 2, 3, 4, 6, 8, and 26 weeks to assay for (A) ALP, (B) AST, (C) ALT, (D) BUN, and (E) creatinine. Dashed lines indicate the range of normal control values.
Nonhematologic toxicity using 211At-B10-30F11 and BMT. Nonleukemic SJL/J mice were injected with 0.67 nmol of B10-30F11 labeled with either 12 (●) or 24 (♦) µCi 211At. Control mice were given 24 µCi 211At-B10-rat IgG (♢) or BMT alone (○). Mice were bled at 1, 2, 3, 4, 6, 8, and 26 weeks to assay for (A) ALP, (B) AST, (C) ALT, (D) BUN, and (E) creatinine. Dashed lines indicate the range of normal control values.
Discussion
In this study, we have demonstrated that anti-CD45 RIT using the α-emitting radionuclide 211At in conjunction with BMT can prolong survival in mice with disseminated murine leukemia with minimal renal or hepatic toxicity. Biodistribution studies showed that the anti-CD45 B10-30F11 conjugate provides excellent localization of radioactivity to sites of leukemia (spleen and marrow), which was confirmed by using α-camera imaging. Dosimetry estimates using 211At-B10-30F11 revealed maximum absorbed doses delivered to splenic tissues harboring a high leukemic cell burden, with ratios of absorbed dose per unit injected for target-to-nontarget organs of 3.6, 4.4, and 6.2 for spleen-to-liver, spleen-to-lung, and spleen-to-kidney, respectively. Importantly, dosimetric calculations demonstrated that a minimal radiation dose was delivered to the kidneys using this approach. When 24 µCi 211At-B10-30F11 was injected into mice, the kidneys were estimated to have an absorbed dose of 4.3 Gy, well within the tolerable safe dose of 10 Gy from previous studies on 211At-based RIT using renal filtration capacity in mice.28 These results may lessen concerns for radiation-induced nephritis, which has been linked to α-emitting radionuclides and their associated daughter radionuclides.12,14,29 Minimal renal uptake also supports the viability, stability, and targeted delivery of astatinated macromolecules via the conjugation chemistry of the B10-NCS reagent, corroborating earlier studies that showed comparable in vivo stability of 211At- and 125I-labeled mAb at 24 hours in canine biodistribution studies that used 211At-labeled mAb via our B10 chemistry.22 In these RIT studies, the delivery of 211At-anti-CD45 RIT resulted in improved OS for leukemic mice that was most significant when combined with BMT, in which 40% of mice treated at the highest dose (ie, 24 µCi 211At-B10-30F11) survived at least 180 days. Moreover, 211At-anti-CD45 RIT was well tolerated, with relatively minimal leukopenia that resolved by 4 weeks after BMT.
In addition to tolerability, several other features suggest that α-emitting radionuclides may be better suited than the traditionally investigated β-emitting radionuclides for certain clinical situations. Short effective path lengths and higher decay energies are 2 of the differentiating characteristics of α-emitting radionuclides compared with β or γ emitters. α-Emitting radionuclides decay with energies on the order of 4 to 8 MeV, which are deposited over their path length, usually on the order of 50 to 80 µm. Consequently, the LET of α particles approaches 100 KeV/µm, around which the radiobiological effectiveness has been shown to reach a maximum. Conversely, radionuclides that decay via β particles, such as 90Y with its mean energy of 2.4 MeV and significantly longer path length (2.7 mm) yields a lower LET of 0.22 KeV/µm. Other β-emitting radionuclides studied in RIT, such as 131I or 177Lu, also have lower energies (0.66 and 0.5 MeV, respectively) and lower LET compared with α emitters.
Dose calculations by other investigators have predicted differences in efficacy between α and β emitters, largely from differences in their energy deposition. Energy from α emitters is high, and their short range is such that only a few nearby cells are killed, whereas the energy deposition from β emitters is mostly deposited at further distances, usually not killing the cell the radionuclide is attached to.30,31 Because β emitters will deposit most of the energy away from the site of decay, 211At could be more favorable than 90Y for small tumor cell clusters, given more favorable tumor:normal tissue mean absorbed dose analysis when comparing 90Y and 211At distributed uniformly in spheres.32 Thus, α-emitting radionuclides may be best suited for scenarios of circulating disease, such as leukemia, or in settings of minimum residual disease because their effective range of energy deposition is on the order of a few cell diameters. Unfortunately, these α-emitting radionuclides may be less effective in large, bulky tumors that require homogeneous distribution throughout the target volume.
RIT using α-emitting radionuclides has been investigated in a small number of human AML studies. Jurcic et al33 treated 18 AML patients by using an anti-CD33 antibody (HuM195) labeled with 213Bi, with 14 patients achieving a significant reduction in bone marrow leukemic blast cells. Subsequently, the use of chemotherapy up front to reduce the leukemic burden prior to administration of 213Bi-HuM195 in an attempt to eliminate minimal residual disease showed encouraging improvements.3,34 Given the short half-life of 213Bi (t1/2 = 46 minutes), which can have an impact on the logistics and limit widespread use of this approach, 225Ac (t1/2 = 10 days) has more recently been investigated as an alternative radionuclide for anti-CD33 (HuM195) RIT.35
Prior α-emitter–based RIT studies that used 213Bi to treat lymphomas in preclinical models have been associated with nephrotoxicity, likely from daughter radionuclides that have accumulated in the kidneys. Blocking agents, such as the heavy-metal–chelating reagent dimercaptopropane-sulfonic acid, have significantly lowered the 213Bi absorbed dose to kidneys, in which kidney doses have been decreased by as much as 60%, which may help render mAb labeled with α-emitting radionuclides useful for future clinical applications.12,21,29 In fact, even 211At-labeled B10-mAb using a different B10 conjugation chemistry resulted in two cases of canine renal toxicity in a dose-escalation study.22 In this study, 211At was linked to the anti-CD45 mAb via a lysine amine reaction with B10-NCS; this 211At-labeling method did not result in high absorbed doses to the kidney and did not produce renal dysfunction, even without the administration of renal protective agents. The renal safety profile of 211At may be advantageous compared with that of other α emitters that may decay into short-lived nephrotoxic daughter α emitters such as 221Fr, 217At, and 213Bi.15
In summary, our studies have demonstrated that the α-emitter 211At, when used with anti-CD45 RIT, can be targeted successfully to leukemia-bearing organs, with associated improvements in OS when combined with BMT in a disseminated model of murine leukemia. Dosimetry estimates showed maximum radiation delivery to target tissues (spleen and bone marrow), with minimal accumulation in the kidneys and, consequently, minimal renal toxicity. In view of the myeloablative potential of 211At, 211At-anti-CD45 RIT may serve as a beneficial adjunct to HSCT in the treatment of AML and should be considered for clinical trials in settings of minimal residual disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (R01 CA138720, R01 CA109663, R01 CA076287, R01 CA136639, P01 CA044991 and awards from the Lymphoma Research Foundation (O.W.P and J.M.P), Damon Runyon Cancer Foundation (J.M.P.), Leukemia and Lymphoma Society (O.W.P., J.M.P., A.K.G., and J.J.O.), and the American Society of Blood and Marrow Transplantation (J.J.O.), as well as Frederick Kullman (J.M.P.) and the Penny E. Petersen Endowed Chair (O.W.P.), funded by James and Sherry Raisbeck.
Authorship
Contribution: J.J.O., T.B., A.K., E.R.B., D.S.W., D.R.F., M.D.H., D.J.G., A.K.G., O.W.P., and J.M.P. contributed to the conception, design, analysis, and interpretation of the research; J.J.O. and J.M.P. wrote the manuscript; J.J.O., A.K., S.L.F., and M.D.H. performed research and collected and analyzed data; and D.K.H. contributed vital reagents. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John M. Pagel, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, M/S D3-190, Seattle, WA 98109; e-mail: jpagel@fhcrc.org.