Key Points
PDK1 is involved in thrombin-induced platelet activation and αIIbβ3-mediated outside-in signaling by regulating the downstream effector Gsk3β.
Abstract
The effects of phosphoinositide-dependent protein kinase 1 (PDK1), a master kinase in the phosphoinositide 3-kinase/Akt pathway, on platelet activation are unknown. Accordingly, platelet-specific PDK1-deficient mice were characterized to elucidate the platelet-related function(s) of PDK1. We found that PDK1 deficiency caused mild thrombocytopenia. The aggregation of PDK1−/− platelets was diminished in response to low levels of thrombin, U46619, and adenosine 5′-diphosphate. Further results demonstrated that PDK1 regulates thrombin-induced platelet activation by affecting αIIbβ3-mediated outside-in signaling. This result provided an explanation for the diminished spreading of PDK1−/− platelets on immobilized fibrinogen (Fg) and the decreased rate of clot retraction in platelet-rich plasma (PRP) containing PDK1−/− platelets. PDK1 deficiency diminished agonist-induced Akt Ser473 phosphorylation and thoroughly abolished Akt Thr308 and Gsk3β Ser9 phosphorylation in response to agonist treatment and platelet spreading, respectively. A Gsk3β inhibitor fully restored the aggregation of PDK1−/− platelets in response to low levels of thrombin, normal spreading of PDK1−/− platelets on Fg, and normal clot retraction in PRP containing PDK1−/− platelets. Those results indicated that Gsk3β is one of the major downstream effectors of PDK1 in thrombin-induced platelet activation and αIIbβ3-mediated outside-in signaling. In addition, in vivo data demonstrated that PDK1 is an important regulator in arterial thrombosis formation.
Introduction
Platelets, which are derived from megakaryocytes, circulate in mammalian blood and play essential roles in hemostasis, angiogenesis, inflammation, and metastasis.1-3 Phosphoinositide 3-kinases (PI3Ks) are a conserved family of enzymes, each having both protein and lipid kinase activities. PI3K-mediated signaling affects platelet adhesion and aggregation.4 PI3Ks are activated downstream of several membrane proteins, including G protein–coupled receptors (GPCRs)5 and the multifunctional platelet receptors αIIbβ3,6 GPIb/IX/V,7,8 and GPVI.9 PI3Ks facilitate thrombus formation by enhancing αIIbβ3 activation and calcium release.4
PI3Ks activate diverse substrates carrying the pleckstrin homology domain, which binds phosphorylated phosphatidylinositol and facilitates the recruitment of downstream effectors to the plasma membrane. A serine-threonine family kinase, protein kinase B (PKB/Akt), which includes three isoforms, Akt1, Akt2, and Akt3,10 is the primary enzyme activated by PI3Ks. All of these Akt isoforms are expressed in human and mouse platelets and play critical roles in platelet activation induced by αIIbβ3, GPIb/IX/V,8 the collagen receptor GPVI,11 and GPCRs.5 Recently, we reported that PI3K direct effector phosphatase and tensin homolog dephosphorylates PtdIns(3, 4, 5)P3 (PIP3), producing PtdIns(4, 5)P2, thereby negatively regulating Akt phosphorylation and collagen-induced platelet activation.12
Phosphoinositide-dependent protein kinase 1 (PDK1) is another cytoplasmic membrane–associated enzyme activated by PI3K. PDK1 plays essential roles in cell growth, metabolism, proliferation, and survival.13 PDK1 is activated by binding to the membrane-tethered PIP3, and the activated PDK1 phosphorylates Akt at Thr308 thereby activating its serine/threonine kinase activity.14 Although a study reported that integrin β3 Thr753 can be phosphorylated by PDK1 and Akt in vitro,15 the role of PDK1 in platelet activation and thrombus formation remains unknown. Here, we investigate the role of PDK1 in platelet activation and thrombus formation using mice with a platelet-specific PDK1 deletion and pharmacologic reagents. We found that platelet PDK1 activates Akt and inhibits Gsk3β, thereby enhancing thrombin-induced platelet aggregation, clot retraction, platelet spreading on immobilized fibrinogen (Fg), and thrombus formation.
Methods
Materials
Wortmannin, SH6, rapamycin, and 8-bromo-guanosine 3′,5′-cyclic monophosphate (cGMP) were purchased from Calbiochem (Darmstadt, Germany). Adenosine 5′-diphosphate (ADP), apyrase, PGE1, Fg, TXA2 analog U46619, mTORC2 inhibitor PP242, and the Gsk3β inhibitor SB216763 were purchased from Sigma-Aldrich (St. Louis, MO). α-Thrombin was from Enzyme Research Laboratories (South Bend, IN). The anti-Akt, anti–phospho-Akt (Ser473), anti–phospho-Akt (Thr308), anti-S6K, anti–phospho-S6K (Thr229), anti-Gsk3β, anti–phospho-Gsk3β (Ser9), anti-Raptor, and anti-actin antibodies were from Cell Signaling Technology (Danvers, MA). Hamster anti-mouse αIIbβ3 monoclonal antibody (mAb) 1B5 was a generous gift from Dr Barry Coller (Rockefeller University, New York, NY).
Generation of megakaryocyte/platelet-specific PDK1 knockout mice
PDK1-floxed mice in a C57BL/6 genetic background were provided by Dario Alessi (University of Dundee).16,17 To delete PDK1 specifically in platelets, PDK1-floxed mice (PDK1f/f) were crossed with PF4-Cre mice18 to obtain PDK1f/wt PF4-Cre+ mice. Further mating gave rise to PDK1f/f PF4-Cre+ (PDK1−/−) mice that have a PDK1 deficiency in platelets. Mice were genotyped by polymerase chain reaction, and PDK1 deficiency in platelets was confirmed by western blotting. The Shanghai Jiao Tong University School of Medicine Animal Care and Use Committee approved the animal research.
Platelet preparation and aggregation
Washed platelets were prepared from mice as described.12 An inhibitor was incubated with the platelets for 3 minutes before stimulation.
Analysis of annexin V binding to platelets
For detection of annexin V binding to platelets, washed platelets from PDK1f/f and PDK1−/− mice were resuspended in annexin V binding buffer, and preincubated with fluorescein isothiocyanate–conjugated annexin V for 15 minutes. Annexin V binding was analyzed using a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA).
Western blotting
For detection of target proteins by immunoblotting, samples of platelets were processed and developed as described.12 After detection of target proteins, the membranes were stripped and incubated with anti-actin antibodies to demonstrate the amount of protein present in each lane.
Fg binding assay
Washed, unstirred platelets were incubated for 20 minutes at room temperature with 150 μg/mL of Rhodamin-conjugated Fg and 0.05 U/mL α-thrombin in a final volume of 50 μL of Tyrode’s buffer containing 1 mM CaCl2, and Fg binding was measured by fluorescence-activated cell sorter analysis.
Platelet spreading on immobilized Fg
Analysis of platelet spreading on immobilized Fg was done as described.19 Images of spread platelet stained by Rhodamin-conjugated Phalloidin were captured with a microscope, and platelet size was quantified using the National Institutes of Health Image J software.
Clot retraction
Clot retraction using mouse platelets was processed as described.20 Clot size was quantified from photographs using National Institutes of Health Image J software, and retraction was expressed as retraction ratio [1 − (final clot size/initial clot size)].
Ferric chloride–induced carotid artery injury
A ferric chloride–induced carotid artery injury murine thrombosis model was processed as described.21 Monitoring of carotid artery blood flow was initiated at the time of FeCl3 treatment and continuously monitored for 13 minutes. Carotid artery blood flow <0.06 mL/min was scored as occlusion, allowing the time to first occlusion to be determined.
Results
Platelet-specific PDK1 deficiency causes thrombocytopenia
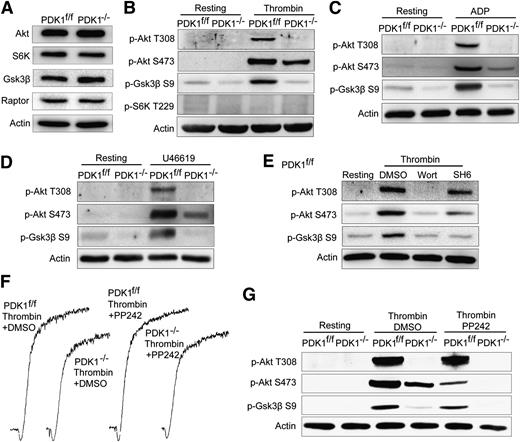
PDK1 knockout mice were severely growth retarded and died at around E9.0.16 Therefore, PDK1 was deleted specifically in platelets using Cre recombinase–mediated excision to study the function of PDK1 in platelet activation and thrombus formation. PDK1f/f and PDK1−/− mice were genotyped by polymerase chain reaction (Figure 1A), and PDK1 platelet-specific deficiency was confirmed by western blotting (Figure 1D). The results showed that PDK1 was totally ablated in PDK1−/− mouse platelets. Because the role of PDK1 in thrombopoiesis was not known, the platelet number in PDK1−/− mice was measured. Platelet counting revealed that PDK1f/f mice had 1092 ± 96.2 × 109 platelets/L, whereas the PDK1−/− mice had 833.3 ± 44.7 × 109 platelets/L (n = 20 mice/group). The blood of PDK1−/− mice contains ∼25% fewer platelets than that of PDK1f/f mice (P < .05; Figure 1B), demonstrating that megakaryocyte/platelet depletion of PDK1 causes mild thrombocytopenia. Recently, several groups reported that PDK1 is involved in cell apoptosis and plays an important role in cardiac development.17,22 Apoptosis in platelets is correlated with platelet life span and is a key factor for regulating peripheral blood platelet count.23-25 Annexin V binding to platelets was assayed to evaluate the role, if any, of apoptosis in thrombocytopenia correlated with PDK1 deficiency. The results indicated that PDK1 deficiency did not enhance annexin V binding to platelets (Figure 1C). This result supports the conclusion that impaired megakaryocyte differentiation into platelets instead of enhanced platelet apoptosis is the cause of PDK1 deficiency–induced thrombocytopenia.
Platelet-specific PDK1 deficiency causes thrombocytopenia, inhibits platelet aggregation in response to low doses of agonists, and delays FeCl3-induced occlusion of the carotid artery. (A) Genotyping results of PDK1f/wt, PDK1f/f, and PDK1−/− using polymerase chain reaction. (B) PDK1 deficiency causes a 25% decrease of peripheral blood platelet counts (P < .05. n = 20). (C) PDK1 deficiency did not enhance Annexin V binding to platelets. (D) Washed platelets were prepared from PDK1f/f and PDK1−/− mice; western blot results showed that PDK1 was depleted in PDK1−/− platelets. (E) The aggregation of PDK1-deficient platelets was diminished in response to low doses of thrombin. (F) The aggregation of PDK1-deficient platelets was diminished in response to low doses of U46619. (G) The aggregation of PDK1-deficient platelets was diminished in response to low doses of ADP. (H) The mouse carotid artery was treated with 10% FeCl3, as described. Traces of blood flow in the carotid arteries of PDK1−/− and PDK1f/f mice were presented, respectively. The times to occlusion were measured (n = 5).
Platelet-specific PDK1 deficiency causes thrombocytopenia, inhibits platelet aggregation in response to low doses of agonists, and delays FeCl3-induced occlusion of the carotid artery. (A) Genotyping results of PDK1f/wt, PDK1f/f, and PDK1−/− using polymerase chain reaction. (B) PDK1 deficiency causes a 25% decrease of peripheral blood platelet counts (P < .05. n = 20). (C) PDK1 deficiency did not enhance Annexin V binding to platelets. (D) Washed platelets were prepared from PDK1f/f and PDK1−/− mice; western blot results showed that PDK1 was depleted in PDK1−/− platelets. (E) The aggregation of PDK1-deficient platelets was diminished in response to low doses of thrombin. (F) The aggregation of PDK1-deficient platelets was diminished in response to low doses of U46619. (G) The aggregation of PDK1-deficient platelets was diminished in response to low doses of ADP. (H) The mouse carotid artery was treated with 10% FeCl3, as described. Traces of blood flow in the carotid arteries of PDK1−/− and PDK1f/f mice were presented, respectively. The times to occlusion were measured (n = 5).
Aggregation of PDK1-deficient platelets was diminished in response to low doses of α-thrombin, U46619, and ADP
The role of PDK1 in thrombin-elicited signaling was investigated by stimulating PDK1f/f and PDK1-deficient platelets with decreasing concentrations of α-thrombin. Compared with PDK1f/f platelets, the aggregation of PDK1-deficient platelets diminished in response to low (0.025 U/mL) and medium (0.05 U/mL) concentrations of α-thrombin (Figure 1E); however, a high level of α-thrombin (0.1 U/mL) caused equivalent levels of irreversible aggregation of both types of platelets. PDK1-deficient platelets were also tested with 2 other GPCR-activating agonists: U46619, a stable TxA2 analog, and ADP. As with α-thrombin, the aggregation of PDK1-deficient platelets was diminished in response to the lower concentrations of U46619 and ADP, and the high concentrations of U46619 and ADP produced normal aggregation of PDK1-deficient platelets (Figure 1F-G). Thus, the aggregation of PDK1-deficient platelets is abnormal in response to low levels of all the platelet agonists tested here that act through GPCRs.
PDK1 regulates arterial thrombosis
Stable thrombus formation in response to FeCl3-induced arterial injury provides an end point for evaluating the physiological role of signaling molecules in hemostasis and thrombosis in vivo.21,26 PDK1 plays an important role in agonist-induced platelet activation. Therefore, we examined thrombosis in PDK1f/f and PDK1−/− mice using the FeCl3-induced carotid artery thrombosis model. The average time to first occlusion for the PDK1−/− mice was 8.86 ± 0.796 minutes, in contrast to 6.626 ± 0.5645 minutes (P < .01) in the PDK1f/f mice (Figure 1H). These results suggest that platelet-derived PDK1 is important in arterial thrombus formation in vivo.
PDK1 deficiency, but not mammalian TOR complex 2 inhibitor, abolished agonist-induced the phosphorylation of Akt Thr308 and Gsk3β Ser9 in platelets
A number of previous studies showed that Akt and S6k are the signaling molecules downstream of PDK1.27 Akt and its downstream signaling molecules such as GSK3β,28 the mammalian target of rapamycin complex 1 (mTORC1), and S6k29 have been shown to play diverse roles in platelet activation. The activation status of several PDK1 downstream signaling molecules was investigated to elucidate the molecular mechanism of PDK1 function in platelet activation. First, we evaluated the expression levels of those signaling molecules in PDK1f/f and PDK1−/− platelets using western blot analyses. The results demonstrated that PDK1 deficiency had no effect on the expression of those signaling molecules in platelets (Figure 2A).
PDK1 deficiency abolished agonist-induced phosphorylation of Akt Thr308 and Gsk3β Ser9 in platelets, and the Akt Ser473 phosphorylation did not contribute to thrombin-induced platelets activation. (A) The expression levels of downstream molecules of PDK1. The phosphorylation of Akt at Ser473 and Thr308, Gsk3β at Ser9, and S6k at Thr229 in PDK1f/f and PDK1−/− platelets in response to low doses of (B) thrombin, (C) ADP, and (D) U46619. (E) The phosphorylation of Akt Ser473, Akt Thr308, and Gsk3β Ser9 in PDK1f/f platelets in response to a low dose of thrombin in the presence of dimethylsulfoxide, the PI3K inhibitor Wortmannin (Wort), and the Akt inhibitor SH6, respectively. (F) mTOR inhibitor PP242 (100nM) did not affect the aggregation of PDK1f/f and PDK1−/− platelets, respectively, in response to 0.05 U/mL of α-thrombin. (G) mTORC2 inhibitor PP242 inhibited most, but not all, the phosphorylation of Akt residue Ser473 in thrombin-treated PDK1f/f platelets, but totally inhibited Akt Ser473 phosphorylation in PDK1−/− platelets treated with α-thrombin. On the contrary, PP242 has no obvious effect on the phosphorylation of Akt Thr308 in PDK1f/f and PDK1−/− platelets in response to thrombin.
PDK1 deficiency abolished agonist-induced phosphorylation of Akt Thr308 and Gsk3β Ser9 in platelets, and the Akt Ser473 phosphorylation did not contribute to thrombin-induced platelets activation. (A) The expression levels of downstream molecules of PDK1. The phosphorylation of Akt at Ser473 and Thr308, Gsk3β at Ser9, and S6k at Thr229 in PDK1f/f and PDK1−/− platelets in response to low doses of (B) thrombin, (C) ADP, and (D) U46619. (E) The phosphorylation of Akt Ser473, Akt Thr308, and Gsk3β Ser9 in PDK1f/f platelets in response to a low dose of thrombin in the presence of dimethylsulfoxide, the PI3K inhibitor Wortmannin (Wort), and the Akt inhibitor SH6, respectively. (F) mTOR inhibitor PP242 (100nM) did not affect the aggregation of PDK1f/f and PDK1−/− platelets, respectively, in response to 0.05 U/mL of α-thrombin. (G) mTORC2 inhibitor PP242 inhibited most, but not all, the phosphorylation of Akt residue Ser473 in thrombin-treated PDK1f/f platelets, but totally inhibited Akt Ser473 phosphorylation in PDK1−/− platelets treated with α-thrombin. On the contrary, PP242 has no obvious effect on the phosphorylation of Akt Thr308 in PDK1f/f and PDK1−/− platelets in response to thrombin.
The Akt Thr308 site and the S6K Thr229 site can be phosphorylated by PDK1.14,30 Gsk3β, whose kinase activity is inhibited by Akt phosphorylation of Ser9, is an important negative regulator of platelet activation.5,28 Next, the phosphorylation of Akt Thr308, Akt Ser473, Gsk3β Ser9, and S6K Thr229 in response to 0.05 U/mL α-thrombin was evaluated in PDK1f/f and PDK1−/− mouse platelets to help clarify the role of PDK1 in platelet signaling. The results demonstrated that phosphorylation of Akt Thr308 and Gsk3β Ser9 was totally abolished in the PDK1-deficient platelets in response to α-thrombin, ADP, or U46619 (Figure 2B-D). However, Akt Ser473 phosphorylation levels were significantly diminished, but not ablated in PDK1-deficient platelets (Figure 2B-D). These results unequivocally demonstrated that PDK1-driven Akt phosphorylation shows a strong correlation between Akt Thr308 and Gsk3β Ser9 phosphorylation instead of a correlation between Akt Ser473 and Gsk3β Ser9 phosphorylation in activated platelets and that Gsk3β is downstream in the PI3K/Akt pathway (Figure 2E).
mTORC2 is a key downstream mediator of PI3K-dependent signaling.31 mTORC2 is able to phosphorylate Akt on Ser473 in human platelets in response to thrombin.32 The role of Akt phosphorylated at Ser473 in PDK1/Akt-mediated platelet activation was investigated using the mTORC2 inhibitor PP242. PP242 (100 nM) did not affect the aggregation of PDK1f/f and PDK1−/− mouse platelets in response to 0.05 U/mL of α-thrombin (Figure 2F). PP242 inhibited most, but not all, the phosphorylation of Akt residue Ser473 in thrombin-treated PDK1f/f platelets but totally inhibited Akt Ser473 phosphorylation in PDK1−/− platelets treated with α-thrombin. On the contrary, PP242 had no obvious effect on the phosphorylation of Akt Thr308 and Gsk3β Ser9 in PDK1f/f and PDK1−/− platelets in response to thrombin (Figure 2G). Therefore, phosphorylation of Akt Ser473 apparently does not play a role in thrombin induced platelet aggregation and the phosphorylation of Akt Thr308 and Gsk3β Ser9.
Gsk3β inhibition restored the aggregation of PDK1-deficient platelets in response to thrombin but diminished the extent of platelet aggregation caused by ADP
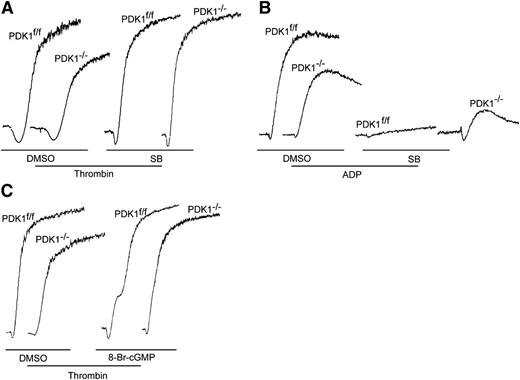
PDK1−/− platelets were preincubated with a 10-μM final concentration of the GSk3β inhibitor SB216763 before being stimulated with 0.05 U/mL α-thrombin to clarify the regulatory relationship between PDK1/Akt Thr308 phosphorylation and phosphorylation of Gsk3β Ser9 in thrombin-induced platelet activation. The results were illuminating: inhibition of GSk3β fully restored the aggregation of PDK1-deficient platelets in response to 0.05U/mL α-thrombin (Figure 3A). Therefore, phosphorylation of Gsk3β Ser9 in response to low levels of thrombin apparently causes the positive regulation of the signaling initiated by PDK1-mediated phosphorylation of Akt Thr308.
Gsk3β inhibition or cGMP restored the aggregation of PDK1-deficient platelets in response to thrombin, but Gsk3β inhibition diminished the extent of platelet aggregation caused by ADP. (A) A 10-μM final concentration of the Gsk3β inhibitor SB216763 restored full aggregation of PDK1-deficient platelets in response to 0.05 U/mL of thrombin. (B) SB216763 (10 μM) totally inhibited PDK1f/f platelet aggregation but only partially inhibited PDK1−/− platelet aggregation in response to 20 μM ADP. Therefore, Gsk3β plays a more complicated role in ADP-induced platelet activation. (C) Cyclic GMP analog 8-bromo-cGMP (8-Br-cGMP; 1 μM) partially restored the aggregation of PDK1-deficient platelets induced by 0.05 U/mL thrombin. These results suggested that NO/cGMP pathway also plays an important role in PDK1-mediated platelet activation.
Gsk3β inhibition or cGMP restored the aggregation of PDK1-deficient platelets in response to thrombin, but Gsk3β inhibition diminished the extent of platelet aggregation caused by ADP. (A) A 10-μM final concentration of the Gsk3β inhibitor SB216763 restored full aggregation of PDK1-deficient platelets in response to 0.05 U/mL of thrombin. (B) SB216763 (10 μM) totally inhibited PDK1f/f platelet aggregation but only partially inhibited PDK1−/− platelet aggregation in response to 20 μM ADP. Therefore, Gsk3β plays a more complicated role in ADP-induced platelet activation. (C) Cyclic GMP analog 8-bromo-cGMP (8-Br-cGMP; 1 μM) partially restored the aggregation of PDK1-deficient platelets induced by 0.05 U/mL thrombin. These results suggested that NO/cGMP pathway also plays an important role in PDK1-mediated platelet activation.
However, 10 μM SB216763 totally inhibited PDK1f/f platelet aggregation but only partially inhibited PDK1−/− platelet aggregation in response to 20 μM ADP (Figure 3B). These results suggested that Gsk3β also correlated with PDK1, although Gsk3β inhibition had opposing effects on ADP-induced platelet activation. Therefore, Gsk3β plays more complicated roles in ADP-induced platelet aggregation, and further experimentation is required to elucidate how Gsk3β plays such a different role on platelet activation.
cGMP restored the aggregation of PDK1-deficient platelets in response to thrombin
The Akt-induced nitric oxide (NO)/cGMP pathway has been shown to play very important roles in platelet activation.8,33 The function of the NO/cGMP pathway in PDK1/Akt-mediated platelet activation was investigated using the cGMP analog 8-bromo-cGMP. As shown in Figure 3C, the results demonstrated that 1 μM 8-bromo-cGMP partially rescued the aggregation of PDK1-deficient platelets induced by thrombin. These results suggest that the NO/cGMP pathway also plays a role in PDK1-mediated platelet activation.
PDK1 regulates platelet activation through integrin αIIbβ3–mediated outside-in signaling
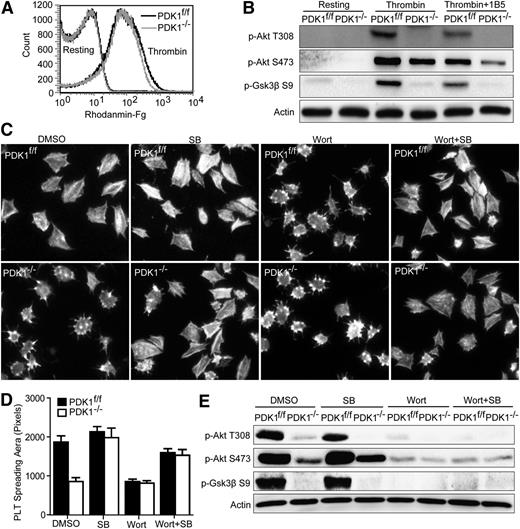
The most abundant platelet surface protein, integrin αIIbβ3, is required for a variety of aspects of hemostasis including platelet aggregation, clot retraction, and stable thrombus formation.6,34 The receptor αIIbβ3 mediates bidirectional signaling, an essential feature of its function. The PI3K/Akt pathway is involved in both integrin activation resulting from inside-out signaling and integrin-mediated outside-in signaling.5,6 However, the function of PDK1 in integrin-related signaling is poorly understood. Therefore, we evaluated the role(s) of PDK1 in αIIbβ3-mediated inside-out and outside-in signaling. We first evaluated the function of PDK1 in thrombin-induced αIIbβ3 activation using flow cytometry detection of Rhodanmine-conjugated Fg binding to platelets. The results demonstrated that PDK1 deficiency has no effect on Fg binding induced by a low level of thrombin under nonstirring conditions (Figure 4A). These results apparently mean that PDK1 probably does not affect αIIbβ3-mediated inside-out signaling. Next, we tested the role of PDK1 in αIIbβ3-mediated outside-in signaling. The phosphorylation of Akt residues Thr308 and Ser473 and Gsk3β residue Ser9 was measured in PDK1f/f and PDK1−/− mouse platelets in response to treatment with a low level of α-thrombin in the presence of mouse αIIbβ3 blocking mAb 1B5. The results demonstrated that 10 μg/mL mAb 1B5 totally inhibited thrombin-induced (0.05 U/mL) platelet aggregation as expected (not shown) and greatly reduced phosphorylation of Akt Thr308, Akt Ser473, and Gsk3β Ser9 in PDK1f/f mouse platelets. 1B5 also greatly reduced phosphorylation of Akt Ser473 in PDK1−/− mouse platelets (Figure 4B). That result was consistent with our observation that the phosphorylation of Akt Thr308 and Gsk3 Ser9 was totally abolished in PDK1−/− mouse platelets with or without 1B5 (Figure 4B). These results suggested that PDK1-induced Akt Thr308 phosphorylation and Gsk3β Ser9 phosphorylation were enhanced by integrin αIIbβ3–mediated outside-in signaling.
PDK1 is involved in integrin αIIbβ3-mediated outside-in signaling. (A) Binding of Rhodamine-Fg to PDK1f/f and PDK1−/− platelets stimulated by a low dose of thrombin. (B) Phosphorylation of Akt Thr308 (T308), Akt Ser473 (S473), and Gsk3β Ser9 (S9) in PDK1f/f and PDK1−/− platelets stimulated by 0.05 U/mL of thrombin in the absence or presence of αIIbβ3 blocking mAb 1B5. (C) Spreading of PDK1f/f and PDK1−/− platelets on immobilized Fg in the presence of dimethylsulfoxide, 10 μM SB, 100 nM Wort, or 10 μM SB plus 100 nM Wort. (D) Quantification of the areas (pixel number) of 4 random fields (mean ± standard error of the mean). Statistical analyses were performed using the Student t test. (E) Phosphorylation of Akt T308, Akt S473, and Gsk3β S9 in PDK1f/f and PDK1−/− platelets spread on Fg in the presence of dimethylsulfoxide, 10 μM SB, 100 nM Wort, or 10 μM SB plus 100 nM Wort.
PDK1 is involved in integrin αIIbβ3-mediated outside-in signaling. (A) Binding of Rhodamine-Fg to PDK1f/f and PDK1−/− platelets stimulated by a low dose of thrombin. (B) Phosphorylation of Akt Thr308 (T308), Akt Ser473 (S473), and Gsk3β Ser9 (S9) in PDK1f/f and PDK1−/− platelets stimulated by 0.05 U/mL of thrombin in the absence or presence of αIIbβ3 blocking mAb 1B5. (C) Spreading of PDK1f/f and PDK1−/− platelets on immobilized Fg in the presence of dimethylsulfoxide, 10 μM SB, 100 nM Wort, or 10 μM SB plus 100 nM Wort. (D) Quantification of the areas (pixel number) of 4 random fields (mean ± standard error of the mean). Statistical analyses were performed using the Student t test. (E) Phosphorylation of Akt T308, Akt S473, and Gsk3β S9 in PDK1f/f and PDK1−/− platelets spread on Fg in the presence of dimethylsulfoxide, 10 μM SB, 100 nM Wort, or 10 μM SB plus 100 nM Wort.
PDK1 and Gsk3β are key regulators of platelet spreading on immobilized Fg
Platelet spreading on immobilized Fg is dependent on cytoskeletal reorganization driven by αIIbβ3-mediated outside-in signaling. Therefore, platelet spreading on immobilized Fg was assessed to characterize the relationship between PDK1/Akt Thr308 phosphorylation and the phosphorylation of Gsk3β Ser9 in integrin αIIbβ3–mediated outside-in signaling. The results in Figure 4C showed that the average size of the platelets that spread on Fg was 1871 ± 159.0 pixels for PDK1f/f platelets vs 853.6 ± 99.32 pixels for the PDK1−/− platelets in the presence of dimethylsulfoxide, demonstrating that a platelet PDK1 deficiency severely interferes with platelet spreading on immobilized Fg (P < .005; Figure 4D). The role of Gsk3β on this effect of PDK1 deficiency was investigated by using the Gsk3β inhibitor SB216763. Interestingly, preincubation of the PDK1−/− platelets with the Gsk3β inhibitor rescued the spreading defect by increasing the area of the spread PDK1-deficient platelets to 1969 ± 208.6 pixels, from 853.6 ± 99.32 pixels in the absence of the inhibitor. In contrast, preincubation of the inhibitor with PDK1f/f platelets had no significant effect on platelet spreading (2130 ± 288.3 pixels). These results demonstrate that inhibition of Gsk3β fully eliminated the inhibition of platelet spreading caused by PDK1 deficiency (P < .005; Figure 4D).
Functions of PDK1 and Gsk3β in αIIbβ3-mediated outside-in signaling are PI3K dependent
The PI3K inhibitor wortmannin had no effect on PDK1−/− platelet spreading (804.6 ± 60.95 pixels) but decreased the area of the spread PDK1f/f platelets to about that of the spread PDK1−/− platelets (841.2 ± 63.85 pixels), indicating that the role of PDK1 is PI3K dependent. Interestingly, the Gsk3β inhibitor SB216763 rescued spreading of both PDK1f/f platelets treated with wortmannin (1676 ± 96.14 pixels) and that of PDK1−/− platelets treated with wortmannin (1508 ± 129.3 pixels), respectively (Figure 4D). These results demonstrated that inhibition of Gsk3β could reverse the defect in platelet spreading caused by PDK1 deficiency and inhibition of PI3K, respectively. These results were further supported by the fact that the spreading apparently driven by Akt phosphorylation at Thr308, but not Ser473, and Gsk3β phosphorylation at Ser9 were dependent on activity of both PI3K and PDK1 (Figure 4E). These results establish that PDK1 and Gsk3β play essential roles in integrin αIIbβ3–mediated platelet spreading on immobilized Fg.
PDK1 and Gsk3β are key regulators of clot retraction
Integrin αIIbβ3–mediated outside-in signaling can drive clot retraction. The PI3K inhibitor LY294002 inhibits clot retraction.6 However, the function of PDK1 in clot retraction is unknown. The results presented in Figure 5A demonstrated that the average ratio of clot retraction of platelet-rich plasma (PRP) containing PDK1f/f platelets was 0.8074 ± 0.0338 vs 0.3991 ± 0.0858 in PRP containing PDK1−/− platelets; therefore, PDK1 deficiency in platelets severely delayed clot retraction in PRP (P < .005; Figure 5B). Furthermore, preincubation with the Gsk3β inhibitor SB216763 completely rescued clot retraction in PRP containing PDK1-deficient platelets or platelets treated with wortmannin or SH6, an Akt inhibitor. These results suggested that clot retraction requires inhibition of Gsk3β function and that Gsk3β inhibition is dependent on activation of PI3K, PDK1, and Akt in platelets participating in clot retraction.
PDK1 regulates clot retraction. (A) Clot retraction of PRP containing PDK1f/f and PDK1−/− platelets in the presence of dimethylsulfoxide, 10 μM SB, 100 nM Wort, 1 μM SH6, or 10 μM SB combined with 100 nM Wort or 1 μM SH6. (B) Two-dimensional retraction of clots was measured using National Institutes of Health Image J software, and the data were expressed as retraction ratios (mean ± standard error of the mean from 3 separate experiments). Statistical significance was calculated using an independent sample Student t test.
PDK1 regulates clot retraction. (A) Clot retraction of PRP containing PDK1f/f and PDK1−/− platelets in the presence of dimethylsulfoxide, 10 μM SB, 100 nM Wort, 1 μM SH6, or 10 μM SB combined with 100 nM Wort or 1 μM SH6. (B) Two-dimensional retraction of clots was measured using National Institutes of Health Image J software, and the data were expressed as retraction ratios (mean ± standard error of the mean from 3 separate experiments). Statistical significance was calculated using an independent sample Student t test.
mTORC1 regulates platelet spreading but not clot retraction
mTORC1 is another Akt downstream signaling molecule with both ser/thr kinase and lipid kinase activities.35 mTORC1 is composed of mTOR, regulatory-associated protein of mTOR (Raptor), mammalian LST8/G-protein β-subunit like protein (mLST8/GβL), and partners PRAS40 and DEPTOR.36-38 This complex is a nutrient/energy/redox sensor with the ability to control protein synthesis that can be inhibited by rapamycin.38 The signaling molecule p70-S6 kinase 1 (S6K1) is one of the most thoroughly characterized targets of mTORC1.35 Aslan et al29 recently reported that the mTORC1–S6K signaling pathway downstream of Akt regulates Rac1-driven platelet spreading. The results presented in Figure 6A-B demonstrated that pretreatment of the platelets with the mTORC1 inhibitor rapamycin obviously decreased the spreading of PDK1f/f platelets (1108 ± 192.1 pixels), but did not inhibit the spreading of PDK1−/− platelets (788.6 ± 56.83 pixels). The Gsk3β inhibitor SB216763 enhanced spreading of both PDK1f/f (1830.5 ± 285.9 pixels) and PDK1−/− platelets (1599.6 ± 99.1 pixels) treated with rapamycin. These results demonstrated that the mTORC1-specific inhibitor rapamycin partially inhibits mouse platelet spreading on immobilized Fg and that the Gsk3β inhibitor SB216763 can overcome the inhibitory effects of rapamycin. Western blotting data demonstrated that rapamycin was able to partially inhibit spreading-driven Akt phosphorylation at Thr308 and Gsk3β phosphorylation at Ser9 (Figure 6C). Because mTORC1 is a downstream effector of Akt, mTORC1 presumably affects the PDK1/Akt/Gsk3β pathway through an unknown, complex positive feedback pathway.
Rapamycin inhibits platelet spreading but has no effect on clot retraction. (A) Spreading of PDK1f/f and PDK1−/− platelets on immobilized Fg in the presence of dimethylsulfoxide, 10 μM SB, 1 μM mTORC1 inhibitor rapamycin (Rapa), or 10 μM SB plus 1 μM Rapa. (B) Quantification of area (pixel number) in 4 random fields (mean ± standard error of the mean). Statistical analysis was performed using the Student t test. Rapa partially inhibited the spreading of PDK1f/f platelets, which was overcome by 10 μM Gsk3β inhibitor SB. Rapa had no further inhibitory effects on spreading of PDK1−/− platelets. (C) Phosphorylation of Akt T308, Akt S473, and Gsk3β S9 in PDK1f/f and PDK1−/− platelets spreading on Fg in the presence of dimethylsulfoxide, 10 μM SB, 1 μM Rapa, or 10 μM SB plus 1 μM Rapa. (D) Clot retraction of PRP containing PDK1f/f and PDK1−/− in the presence of dimethylsulfoxide, 1 μM Rapa, or 1 μM Rapa plus 10 μM SB. (E) Two-dimensional clot retraction was measured using National Institutes of Health Image J software, and the data were expressed as retraction ratios (mean ± standard error of the mean from 3 separate experiments). Statistical significance was calculated using a Student t test. Rapa did not inhibit clot retraction of PRP-containing PDK1f/f platelets and did not further inhibit clot retraction of PRP-containing PDK1−/− platelets.
Rapamycin inhibits platelet spreading but has no effect on clot retraction. (A) Spreading of PDK1f/f and PDK1−/− platelets on immobilized Fg in the presence of dimethylsulfoxide, 10 μM SB, 1 μM mTORC1 inhibitor rapamycin (Rapa), or 10 μM SB plus 1 μM Rapa. (B) Quantification of area (pixel number) in 4 random fields (mean ± standard error of the mean). Statistical analysis was performed using the Student t test. Rapa partially inhibited the spreading of PDK1f/f platelets, which was overcome by 10 μM Gsk3β inhibitor SB. Rapa had no further inhibitory effects on spreading of PDK1−/− platelets. (C) Phosphorylation of Akt T308, Akt S473, and Gsk3β S9 in PDK1f/f and PDK1−/− platelets spreading on Fg in the presence of dimethylsulfoxide, 10 μM SB, 1 μM Rapa, or 10 μM SB plus 1 μM Rapa. (D) Clot retraction of PRP containing PDK1f/f and PDK1−/− in the presence of dimethylsulfoxide, 1 μM Rapa, or 1 μM Rapa plus 10 μM SB. (E) Two-dimensional clot retraction was measured using National Institutes of Health Image J software, and the data were expressed as retraction ratios (mean ± standard error of the mean from 3 separate experiments). Statistical significance was calculated using a Student t test. Rapa did not inhibit clot retraction of PRP-containing PDK1f/f platelets and did not further inhibit clot retraction of PRP-containing PDK1−/− platelets.
The role of mTORC1 in clot retraction was also investigated. The data presented in Figure 6D-E demonstrated that the mTORC1 inhibitor rapamycin has no effect on clot retraction in PRP containing PDK1f/f or PDK1−/− platelets.
Discussion
The PI3k/Akt signaling pathway plays an important role in regulating platelet adhesion, spreading, and aggregation.4 The role of PDK1, an early component of the PI3K/Akt signaling pathway, in platelet activation is not described in the literature. In this study, we demonstrated an important role for PDK1 in platelet aggregation induced by thrombin, U46619, and ADP. The results of Fg binding (Figure 4A) and platelet aggregation studies (Figure 1E-G) indicated that PDK1 regulates platelet activation by partially enhancing αIIbβ3-mediated outside-in signaling. Also, the data shown in Figures 4C and 5A demonstrated that PDK1 is required in αIIbβ3-mediated platelet spreading and clot retraction.
The Akt isoforms Akt1, Akt2, and Akt3 are all downstream effectors of PI3K that are involved in the regulation of platelet activation and thrombus formation.10,39 Full activation of Akt requires phosphorylation of both Thr308 and Ser473.40 Phosphorylation of Akt Thr308 by PDK1 is dependent on its proximity to plasma membrane.14 Phosphorylation of Akt residue Ser473 also occurs close to plasma membrane.41 The integrin-linked kinase and the mTORC2 complex are each able to phosphorylate Akt residue Ser473.31,42 Apparently, phosphorylation of Akt Ser473 is a prerequisite for the phosphorylation of Thr308.43 However, a study recently showed that the mTORC2-specific inhibitors PP242 and Torin1 blocked thrombin-induced Akt Ser473 phosphorylation but had no effect on Akt Thr308 phosphorylation in human platelets.32 The results presented in Figure 2G confirmed this conclusion. Therefore, phosphorylation of either site apparently can be independent of phosphorylation of the other Akt site depending on the conditions of the study. The data in Figure 2F also demonstrated that PP242 had no effect on the aggregation of PDK1f/f or PDK1−/− platelets in response to thrombin, suggesting that Akt phosphorylated at Ser473 has no contribution to thrombin-induced platelet activation.
Gsk3β is a ser/thr kinase.44,45 The activity of Gsk3β is inhibited by Akt phosphorylation of Gsk3β at Ser9.46 Recently, a study suggested that Gsk3β negatively regulates platelet activation by blocking αIIbβ3-mediated outside-in signaling.5 In our study, we found that phosphorylation of Gsk3β at Ser9 did not occur in PDK1-deficient platelets stimulated with thrombin, U46619, and ADP or in PDK1-deficient platelets spreading on immobilized Fg (Figures 2B-D and 4E). Phosphorylation of Gsk3β at Ser9 is correlated with Akt Thr308 phosphorylation (Figures 2B-D and 4E) but not with phosphorylation of Akt Ser473 (Figures 2B-D,G and 4E). The results of platelet functional assays demonstrated that inhibition of Gsk3β activity was able to fully restore the ability of PDK1-deficient platelets to aggregate in response to thrombin, to spread on immobilized Fg, and to support clot retraction. Moreover, the results presented in Figure 3C showed that the cGMP analog 8-bromo-cGMP also partially restore the thrombin-induced PDK1-deficient platelet aggregation, suggesting that Gsk3β is the major, but not the only, downstream effector of PDK1 and Akt signaling.
However, there is a study showing that Gsk3β inhibition has an inhibitory effect on collagen-induced platelet activation.47 The results presented in Figure 3B extend that observation by demonstrating that the Gsk3β inhibitor SB216763 inhibits ADP-induced PDK1f/f platelet aggregation but only partially inhibited ADP-induced PDK1−/− platelet aggregation. Therefore, Gsk3β plays a complex role in platelet aggregation induced by ADP. Further experimentation is required to elucidate how the inhibition of Gsk3β can enhance the aggregation of PDK1−/− platelets stimulated with a low level of thrombin and enhance both the spreading on immobilized Fg and clot retraction by those platelets but inhibit the aggregation of PDK1f/f platelets in response to low levels of ADP or collagen.
mTORC1 is an Akt downstream effector35,48 that appears to affect the spreading of human platelets on Fg and collagen-induced human platelet aggregation through the regulation of Rac1 activation.29 S6K1, a substrate of mTORC1, has been reported to be phosphorylated at Thr229 by PDK1.30 However, we found that the phosphorylation of S6K1 at Thr229 did not occur in platelets in response to thrombin (Figure 2B). Therefore, the activation of S6K1 appears to be directly induced by mTOR but not by PDK1 in platelets. Our data presented in Figure 6A confirm that inhibition of mTORC1 by rapamycin blocked mouse platelet spreading on immobilized Fg and demonstrate that the Gsk3β inhibitor SB216763 can overcome the inhibitory effects of rapamycin. Western blotting data demonstrated that rapamycin can partially inhibit spreading-driven Akt phosphorylation at Thr308 and Gsk3β phosphorylation at Ser9, but not Akt phosphorylation at Ser473 (Figure 6C). These data may mean that mTORC1 regulates platelet spreading through an unknown feedback mechanism, apparently affecting Akt phosphorylation at Thr308 and Gsk3β phosphorylation at Ser9. However, the mTORC1 inhibitor rapamycin had no effect on clot retraction in PRP containing PDK1f/f or PDK1−/− platelets (Figure 6D-E). These results implied that maximal spreading of platelets on immobilized Fg requires full phosphorylation of Akt at Thr308 and Gsk3β at Ser9, but in contrast, probably a low level of phosphorylation of Akt at Thr308 and Gsk3β at Ser9 is enough to support platelet mediated clot retraction.
In summary, Fg binding to platelet αIIbβ3 caused outside-in signaling to activate PI3K. Presumably, the subsequent binding of PDK1 to the membrane-tethered PIP3 activates PDK1, and activated PDK1 phosphorylates Akt at Thr308, thereby activating platelets. Furthermore, the negative regulation of αIIbβ3-mediated outside-in signaling by Gsk3β was specifically prevented by PDK1-driven Akt Thr308 phosphorylation. Therefore, the current study identified some important aspects of PDK1 signaling that affect platelet activation and thrombus formation, but further work is required for a comprehensive understanding of Gsk3β in this process.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Key Basic Research Program of China (2012CB518000), Program of the National Natural Science Foundation of China (81170479 to J.L., 81000204 to D.L., 81030039 to J.L., and 81270278 to X.L.), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, Shanghai Municipal Education Commission and Shanghai Education Development Foundation (10SG21), and Shanghai Committee of Science and Technology (11DZ2260200).
Authorship
Contribution: X.C. and J.L. designed the experiments, analyzed data, and wrote the paper; X.C., Y.Z., Y.Wang., D.L., L.Z., and K.W. performed the experiments; and X.L., Z.Y., and Y.Wu. helped with the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junling Liu, Department of Biochemistry and Molecular Cell Biology, Institute of Medical Science, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China; e-mail: liujl@shsmu.edu.cn.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal