Key Points
Wogonoside induces cell cycle arrest and differentiation.
Wogonoside acts by changing PLSCR1 expression and subcellular localization in the nucleus and by PLSCR1-related molecular events.
Abstract
Wogonoside is the main flavonoid component derived from the root of Scutellaria baicalensis Georgi. It is a popular Chinese herbal medicine with the potential to treat hematologic malignancies. In this study, we investigated the anticancer effects of wogonoside in acute myeloid leukemia (AML) cell lines and primary patient-derived AML cells. Wogonoside exerted antiproliferative properties both in vitro and in vivo. Furthermore, it efficiently inhibited the proliferation of U937 and HL-60 cells through the induction of G1 phase arrest and the promotion of differentiation. We also demonstrated that wogonoside significantly increased the transcription of phospholipid scramblase 1 (PLSCR1) due to its influence on the expression of cell cycle– and differentiation-related genes, including the upregulation of p21waf1/cip1 and downregulation of the oncogenic protein c-Myc. Wogonoside also promoted PLSCR1 trafficking into the nucleus and facilitated its binding to the inositol 1,4,5-trisphosphate receptor 1 (IP3R1) promoter, thus increasing the expression of IP3R1. Finally, inhibition of PLSCR1 expression with small interfering RNA partially blocked wogonoside-induced cell cycle arrest and differentiation and disturbed the wogonoside-associated molecular events. The results of this study therefore suggest that wogonoside may represent a therapeutic candidate for the treatment of AML.
Introduction
Acute myeloid leukemia (AML) comprises a genetically and clinically heterogeneous group of aggressive hematological neoplasms.1 Continuing research into the pathogenesis and heterogeneity of AML has resulted in the development of several potentially useful therapeutic agents.2 However, despite some advances in the treatment of AML, therapies have not changed significantly in the past 20 years. Further research is thus warranted to identify effective agents and develop new therapeutic strategies for the treatment of this deadly disease. Flavonoids possess diverse biological and pharmaceutical properties and have been subjected to extensive investigations as likely candidates for cancer treatment.3
Scutellaria baicalensis Georgi (huang qin) is 1 of the most popular and multipurpose traditional Chinese medicinal herbs, and it has a high flavonoid content.4 Wogonin, a flavonoid extracted from S. baicalensis, has several biological effects including antioxidant, anti-inflammatory, antiviral, neuroprotective, anxiolytic, and anticancer activities.5 It has been shown to possess antitumor effects in various cancer cells,6 including antiproliferation, cell cycle arrest, induction of apoptosis and differentiation, inhibition of angiogenesis, anti-invasion, and increased sensitivity to apoptosis. Moreover, wogonin has shown therapeutic potential for the treatment of hematologic malignancies.7 Flavonoid aglycones undergo rapid and extensive metabolism after either oral or intravenous administration. Flavonoid aglycones enter the bloodstream in the form of glucuronide or sulfate conjugates, and very little unchanged aglycone can be found in the plasma, although levels of glucuronic acid conjugates are high. Glucuronidation is thought to affect the biological activity of aglycones by altering the physicochemical properties of flavonoids, which is seen as a detoxification process. The biological activities of these conjugates are thus important.8,9 The flavonoid wogonoside can also be derived from S. baicalensis, as it is a metabolite of wogonin. It is known to possess anti-inflammatory10 and anti-inflammation–induced angiogenic activities11 ; however, its antileukemic properties have not been explored.
Phospholipid scramblase 1 (PLSCR1) was originally identified as a type II transmembrane protein that mediates the calcium-dependent bidirectional movement of membrane phospholipids. It was also identified as a substrate for several kinases that participate in kinase signaling pathways, including c-Abl, c-Src, and protein kinase Cδ (PKCδ).12 Furthermore, PLSCR1 plays potential roles in hematopoiesis and leukemogenesis. PLSCR1−/− bone marrow cells exhibit defective myeloid proliferation and differentiation in response to stimulation by selected growth factors.13 Moreover, MmTRA1b/PLSCR1 messenger RNA (mRNA) levels correlate with significantly longer overall survival in AML, and PLSCR1 gene expression has been identified as a new prognostic factor in AML.14 PLSCR1 generally plays an antagonistic role in leukemia development. We previously found that wogonin induced granulocytic differentiation and upregulated PLSCR1 gene expression in NB4 cells.15 PLSCR1 also trafficked into the nucleus, and it may thus have a possible additional nuclear function. However, the ability of wogonoside to induce the differentiation of AML cells and promote the function of PLSCR1 requires further investigation.
This study investigated the antiproliferative activity of wogonoside in vitro and in vivo and also examined its effects on cell cycle progression and differentiation. Furthermore, we investigated the role of PLSCR1 in wogonoside-induced cell cycle arrest and differentiation, mainly with regard to changes in PLSCR1 expression, subcellular localization in the nucleus, and PLSCR1-related molecular events. The results suggest that wogonoside has potential antitumor activity in AML cells and provides information relevant to the clinical use of wogonin.
Materials and methods
Compounds and reagents
Wogonoside (>98% purity; Langze Pharmaceutical Co, Ltd, Nanjing, China), all-trans-retinoic acid (ATRA), and phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, St. Louis, MO) were dissolved in dimethylsulfoxide (DMSO) as stock solutions at 0.5 M, 20 mM, and 1.6 mM, respectively. All stock solutions were stored at −20°C.
PLSCR1 small interfering RNA (siRNA) was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA), and transfection was performed using Lipofectamine 2000 reagent (Invitrogen, San Diego, CA), according to the manufacturer’s instructions.16 A nuclear/cytosol fractionation kit (KeyGEN, Nanjing, China) was used according to the manufacturer’s directions.
Cell culture
Primary leukemic cells from AML patients without prior therapy (Zhongda Hospital of Southeast University, Nanjing, China) were collected using lymphocyte-monocyte separation medium (Jingmei, Nanjing, China), after obtaining informed consent, in agreement with Zhongda Hospital’s institutional review board and in accordance with the Declaration of Helsinki. The procedures were approved by the appropriate ethics committees. Primary leukemic cells and U937 and HL-60 human leukemic cell lines were cultured as described previously in Hussong et al.17
Cell proliferation assays in vitro and in vivo
Cell growth was assessed using the trypan blue dye exclusion method by manual cell counting using a hemocytometer (Qiujing, Shanghai, China).18 Cells (7 × 104 cells per mL) were incubated with or without wogonoside in 6-well plates for 5 days. Results were represented by cell number. Soft agar colony-formation assays were carried out after treatment with wogonoside for 4 days.19 The colonies were viewed at ×40 magnification to detect colony size and colony numbers, using an inverted microscope equipped with a color camera (Nikon Instruments Inc, Lewisville, TX). In vivo investigations were performed in BALB/c nude mice injected with U937 cells20 and in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) immunodeficient mice engrafted with primary human AML cells.21 (For more details, see supplemental Methods).
Cell cycle and differentiation analyses
The cell cycle was analyzed by propidium iodide staining and by using a FACSCalibur flow cytometer (Becton, Dickinson, San Jose, CA).22 The percentage of cells in each phase of the cell cycle was quantitated with Modfit software (Becton, Dickinson). Morphological assessment of differentiation was evaluated by Wright-Giemsa staining.23 The percentage of cells capable of reducing nitro blue tetrazolium (NBT) was measured as described earlier in Abaza et al.24 The results were determined by examining 200 cells per microscopic field and counting 5 times in each group. The data were expressed as the percentage of blue cells per (blue + white cells). Treated cells were tested using an α-naphthyl acetate esterase assay kit (Sigma-Aldrich) according to the manufacturer’s instructions. Expression of the cell surface differentiation markers CD11b and CD14 was determined by flow cytometry using a FACSCalibur flow cytometer (Becton, Dickinson).25
Western blot analysis
Western blot analysis was performed as described previously in Chen et al.26 (For more details, see supplemental Methods). Detection was performed using the Odyssey Infrared Imaging System (LI-COR Inc, Lincoln, NE).
Immunofluorescence microscopy
Subcellular localization of PLSCR1 was analyzed by immunofluorescence microscopy as described previously in Smith et al.27 (For more details, see supplemental Methods).
Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR)
For quantitative analysis of gene expression, total RNA was isolated using a TRIzol kit (Invitrogen, Carlsbad, CA). Complementary DNA was synthesized using a complementary DNA synthesis kit (Code DRR047A; TaKaRa, Kyoto, Japan) according to the manufacturer’s instructions.28 PLSCR1 primers (forward: 5′-CTGACTTCTGAGAAGGTTGC-3′, reverse: 5′-GAATGCTGTCGGTGGATACTG-3′)29 were synthesized by Invitrogen. Relative quantities of mRNA were measured using Applied Biosystems 7500 Fast Real-Time PCR System (PerkinElmer, Torrance, CA) and double-stranded DNA dye SYBR Green PCR core reagents (Code DRR081C; TaKaRa). Amplification was performed with 40 cycles at 95°C for 15 seconds and at 60°C for 30 seconds. Data were analyzed using 7500 system SDS software.
Electrophoretic mobility shift assay (EMSA)
Preparation of nuclear extracts and electrophoretic mobility shift assays were conducted according to the manufacturer’s instructions (EMSA kit; Beyotime, Nanjing, China).30 (For more details, see supplemental Methods).
Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM). The data shown were obtained from at least 3 independent experiments. Statistical analysis of multiple-group comparisons was performed by one-way analysis of variance (ANOVA) followed by the Bonferroni posthoc test. Comparisons between 2 groups were analyzed using 2-tailed Student t tests. The survival of NOD/SCID mice was evaluated by Kaplan-Meier analysis using the log-rank test to compare the difference. A P value < .05 was considered statistically significant.
Results
Antiproliferative effects of wogonoside in vitro and in vivo
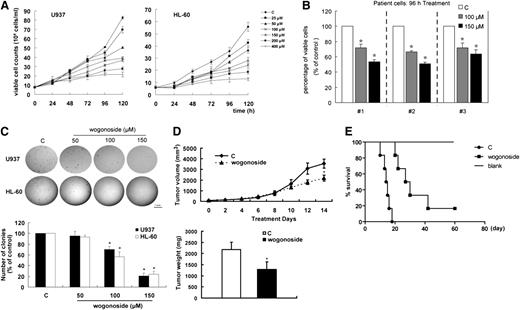
The antiproliferative effects of wogonoside in U937 and HL-60 cells and primary AML cells were examined by trypan blue dye exclusion (Figure 1A-B). Wogonoside inhibited the growth of these cells. The antiproliferative activity of wogonoside was further evaluated by soft agar colony-formation assay. Colony number and colony size were reduced in U937 and HL-60 cells treated with wogonoside compared with controls (Figure 1C). Moreover, the ability of wogonoside to inhibit the growth of U937 cells in vivo was investigated using a female nude mouse xenograft model (Figure 1D). At the end of the treatment, the mean volume of the U937 tumors in the treated mice (2185 ± 276 mm3) was 39% lower than that in control mice (3559 ± 421 mm3). The mean tumor weight in the wogonoside-treated group (1287 ± 340 mg) was reduced by 41% compared with the control group (2181 ± 330 mg). Wogonoside thus suppressed the growth and the weight of the tumors in the mice. In addition, wogonoside significantly prolonged survival in AML-bearing mice compared with the control group (Figure 1E, log-rank P value < .01); the median survival duration was 14.5 ± 2.7 days in the control group and 33.5 ± 15.1 days in the wogonoside-treated group. These results demonstrate that wogonoside possessed the antiproliferative capacity to inhibit the growth of human AML cells in vitro and in vivo.
Antiproliferative effect of wogonoside in vitro and in vivo. (A) Cell growth was measured by trypan blue exclusion assay. The cell growth curve represents the effect of wogonoside at different concentrations for 5 days. (B) Primary AML cells were incubated with wogonoside (100 μM and 150 μM) for 96 hours. Viability was measured by trypan blue exclusion assay. Statistical analysis shows the percentage of viable cells relative to the controls for every patient (control cells = 100%). (C) Cells were cloned in soft agar and cultured for 21 days after wogonoside treatment of 4 days. Colonies >50 μm in diameter were counted. The images of the representative colonies shown were taken using an inverted microscope (Nikon Instruments Inc) equipped with a color camera (Nikon) at ×40 magnification (objective lenses ×4) at room temperature. Statistical analysis shows the percentage of cells that formed colonies relative to the controls (control cells = 100%). Data represent mean ± SEM from 3 independent experiments. Asterisks denote statistically significant (P < .05) differences compared with controls by one-way ANOVA. (D) Examination was performed on tumor volumes and tumor weights to evaluate the effect of wogonoside on the proliferation of U937 cells in a xenograft model. U937 cells in Matrigel (Becton Dickinson, Bedford, MA) were injected bilaterally subcutaneously into BALB/c nude mice, forming 2 tumors per mouse. When U937 cells formed palpable tumors (50-100 mm3), mice were randomized into 2 groups (n = 5 per group) and treatment was initiated. Mice were treated with solvent or wogonoside (80 mg/kg) by intraperitoneal injection every other day for 14 days total. Tumor volumes were measured on alternate days during the experimental period. Mice were euthanized after 14 days of treatment, and the tumors were excised and weighed. Results are representative of 3 independent experiments. Results represent the mean ± SEM of tumor weights. Statistical significance was determined by 2-tailed Student t tests. Asterisks denote significant (P < .05) differences relative to controls. (E) A Kaplan-Meier survival plot for AML-bearing NOD/SCID mice is shown. Mice were sublethally irradiated (2.4 Gy), and primary AML cells were injected into the tail vein 24 hours later (2-5 × 106 cells per mouse, 6 mice per group). Starting the next day, mice were injected intraperitoneally with or without wogonoside (80 mg/kg) every other day for 14 days. The blank animal group, without primary cells treated with solvent, was used to evaluate survival ability. The results are representative of 2 separate experiments. Animals were observed for 60 days after cell injection. The survival curves differed significantly between the wogonoside-treated group and the control group (P < .001; log-rank test). C, control.
Antiproliferative effect of wogonoside in vitro and in vivo. (A) Cell growth was measured by trypan blue exclusion assay. The cell growth curve represents the effect of wogonoside at different concentrations for 5 days. (B) Primary AML cells were incubated with wogonoside (100 μM and 150 μM) for 96 hours. Viability was measured by trypan blue exclusion assay. Statistical analysis shows the percentage of viable cells relative to the controls for every patient (control cells = 100%). (C) Cells were cloned in soft agar and cultured for 21 days after wogonoside treatment of 4 days. Colonies >50 μm in diameter were counted. The images of the representative colonies shown were taken using an inverted microscope (Nikon Instruments Inc) equipped with a color camera (Nikon) at ×40 magnification (objective lenses ×4) at room temperature. Statistical analysis shows the percentage of cells that formed colonies relative to the controls (control cells = 100%). Data represent mean ± SEM from 3 independent experiments. Asterisks denote statistically significant (P < .05) differences compared with controls by one-way ANOVA. (D) Examination was performed on tumor volumes and tumor weights to evaluate the effect of wogonoside on the proliferation of U937 cells in a xenograft model. U937 cells in Matrigel (Becton Dickinson, Bedford, MA) were injected bilaterally subcutaneously into BALB/c nude mice, forming 2 tumors per mouse. When U937 cells formed palpable tumors (50-100 mm3), mice were randomized into 2 groups (n = 5 per group) and treatment was initiated. Mice were treated with solvent or wogonoside (80 mg/kg) by intraperitoneal injection every other day for 14 days total. Tumor volumes were measured on alternate days during the experimental period. Mice were euthanized after 14 days of treatment, and the tumors were excised and weighed. Results are representative of 3 independent experiments. Results represent the mean ± SEM of tumor weights. Statistical significance was determined by 2-tailed Student t tests. Asterisks denote significant (P < .05) differences relative to controls. (E) A Kaplan-Meier survival plot for AML-bearing NOD/SCID mice is shown. Mice were sublethally irradiated (2.4 Gy), and primary AML cells were injected into the tail vein 24 hours later (2-5 × 106 cells per mouse, 6 mice per group). Starting the next day, mice were injected intraperitoneally with or without wogonoside (80 mg/kg) every other day for 14 days. The blank animal group, without primary cells treated with solvent, was used to evaluate survival ability. The results are representative of 2 separate experiments. Animals were observed for 60 days after cell injection. The survival curves differed significantly between the wogonoside-treated group and the control group (P < .001; log-rank test). C, control.
Effect of wogonoside on cell cycle progression
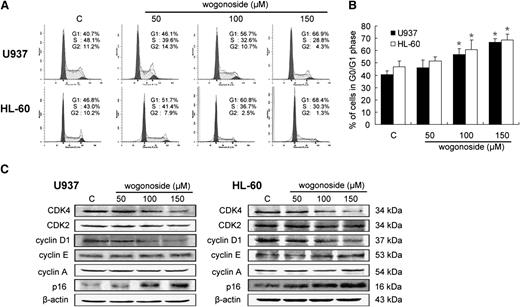
We examined the effect of wogonoside on cell cycle progression in U937 and HL-60 cells. Wogonoside arrested the cell cycle at the G0/G1 phase and decreased the G2/M and S phases in both cell lines in a dose-dependent manner (Figure 2A-B). To further investigate the molecules affected by wogonoside, we examined the expression levels of several G0/G1 to S phase–transition-related proteins under the same conditions. Cyclin-dependent kinase 4 (CDK4) and cyclin D1 appear to be necessary for transition through the early G1 phase, whereas CDK2, cyclin E, and cyclin A are necessary for the completion of the G1 phase and the initiation of the S phase.31 Wogonoside downregulated CDK4 and cyclin D1 protein levels, but it had little effect on CDK2, cyclin A, and cyclin E levels in both cell lines (Figure 2C). Meanwhile, expression of the CDK4 inhibitor p16 (INK4A) protein was increased by wogonoside treatment. These results indicate that wogonoside could induce G0/G1 cell cycle arrest in human AML cells.
Effects of wogonoside on cell cycle progression and related proteins. (A) Shown is a representative cell cycle analysis performed by flow cytometry of U937 and HL-60 cells treated with 50, 100, and 150 μM wogonoside for 48 hours. (B) The percentages of cells in the G0/G1 phases of the cell cycle after wogonoside treatment of 48 hours are shown. Data represent mean ± SEM from 3 independent experiments. Asterisks denote statistically significant (P < .05) differences compared with controls by one-way ANOVA. (C) Expression levels of CDK4, CDK2, cyclin D1, cyclin E, cyclin A, and p16 were analyzed by western blotting after treatment of 48 hours. β-actin was used as a loading control. Results are representative of 3 independent experiments.
Effects of wogonoside on cell cycle progression and related proteins. (A) Shown is a representative cell cycle analysis performed by flow cytometry of U937 and HL-60 cells treated with 50, 100, and 150 μM wogonoside for 48 hours. (B) The percentages of cells in the G0/G1 phases of the cell cycle after wogonoside treatment of 48 hours are shown. Data represent mean ± SEM from 3 independent experiments. Asterisks denote statistically significant (P < .05) differences compared with controls by one-way ANOVA. (C) Expression levels of CDK4, CDK2, cyclin D1, cyclin E, cyclin A, and p16 were analyzed by western blotting after treatment of 48 hours. β-actin was used as a loading control. Results are representative of 3 independent experiments.
Differentiation-inducing capacity of wogonoside
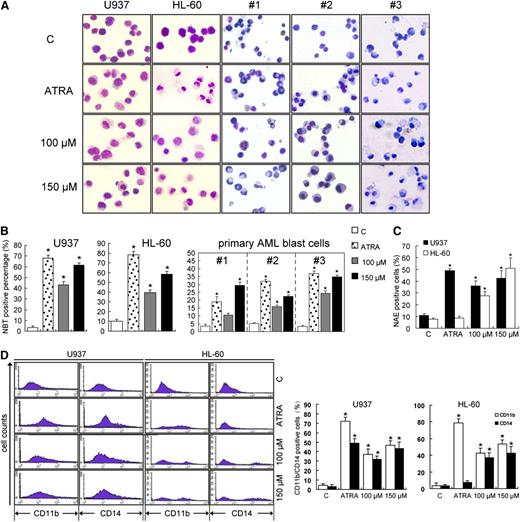
Wogonoside treatment of 96 hours influenced the morphology and NBT-reduction activity in U937 and HL-60 cells and in primary AML cells. Cells treated with wogonoside displayed morphological features of cell differentiation, such as a lower nucleocytoplasmic ratio and chromatin condensation (Figure 3A). HL-60 and patient 3# cells treated with ATRA showed granulocytic differentiation morphological features, but these cells treated with wogonoside appeared with monocytic phenotype features, including irregular cytoplasmic contours, a round or oval, peripherally located nucleus, and grayish cytoplasm. The reduction of NBT was used as another marker of differentiation of myeloid leukemia cells. The production of oxidative bursts in leukemic cells was increased by wogonoside treatment (Figure 3B). U937 and HL-60 cells treated with wogonoside showed increased α-naphthyl esterase activity (Figure 3C). To analyze the differentiation lineage induced by wogonoside, we investigated the expression of markers of myeloid differentiation (CD11b) and monocytic maturation (CD14). In U937 cells, the population of CD11b- and CD14-positive cells increased after 96 hours of incubation with wogonoside or ATRA, while in HL-60 cells, the percentage of CD11b-positive and CD14-negative cells increased after ATRA treatment, but both these antigens were upregulated by wogonoside treatment (Figure 3D). These results suggest that wogonoside induced differentiation along the monocytic lineage.
Wogonoside induces differentiation of U937, HL-60, and primary leukemic cells from AML patients. (A) Representative Wright-Giemsa staining for morphological examination is shown. Leukemic cells were treated with 1 μM ATRA, 100 μM wogonoside, or 150 μM wogonoside for 96 hours. Original magnification was ×400 (objective lenses ×40) under a light microscope (IX51; Olympus, Tokyo, Japan), and images were captured using DP2-BSW software (Olympus) at room temperature. (B) Detection of NBT-reduction activity of the leukemic cells is shown. NBT-positive cells with purple-black color were counted, and the overall percentage was calculated based on 200 total cells per microscopic field and counting 5 times in each group. (C) The effect of wogonoside on α-naphthyl acetate esterase activity is shown. (D) The expression of CD11b/CD14 in U937 and HL-60 cells is shown. The percentages of cells expressing CD11b and CD14 were detected by flow cytometry analyses. The data represent the mean ± SEM of 3 different experiments. Asterisks denote statistically significant (P < .05) differences compared with controls by one-way ANOVA.
Wogonoside induces differentiation of U937, HL-60, and primary leukemic cells from AML patients. (A) Representative Wright-Giemsa staining for morphological examination is shown. Leukemic cells were treated with 1 μM ATRA, 100 μM wogonoside, or 150 μM wogonoside for 96 hours. Original magnification was ×400 (objective lenses ×40) under a light microscope (IX51; Olympus, Tokyo, Japan), and images were captured using DP2-BSW software (Olympus) at room temperature. (B) Detection of NBT-reduction activity of the leukemic cells is shown. NBT-positive cells with purple-black color were counted, and the overall percentage was calculated based on 200 total cells per microscopic field and counting 5 times in each group. (C) The effect of wogonoside on α-naphthyl acetate esterase activity is shown. (D) The expression of CD11b/CD14 in U937 and HL-60 cells is shown. The percentages of cells expressing CD11b and CD14 were detected by flow cytometry analyses. The data represent the mean ± SEM of 3 different experiments. Asterisks denote statistically significant (P < .05) differences compared with controls by one-way ANOVA.
Effects of wogonoside on PLSCR1 expression and its related cell cycle and differentiation proteins
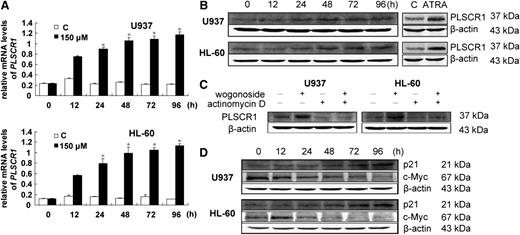
Wogonoside at the differentiation-inducing concentration of 150 μM increased PLSCR1 mRNA levels in both U937 and HL-60 cells, as evidenced by RT-PCR (Figure 4A). PLSCR1 expression was upregulated starting at 24 hours and reached a plateau after 48 hours of wogonoside treatment (Figure 4B). To determine if the increase in PLSCR1 mRNA levels was due to the upregulation of gene expression or an increase in mRNA stability, cells were pretreated with 4 μg/mL of the transcription inhibitor actinomycin D, followed by 150 μM wogonoside for 48 hours. Wogonoside-induced PLSCR1 expression became barely detectable after incubation with actinomycin D (Figure 4C), suggesting that the elevated PLSCR1 protein levels in U937 and HL-60 cells exposed to wogonoside were the result of transcriptional activation. We also analyzed some critical cell cycle– and differentiation-related genes regulated by PLSCR1, including p21waf1/cip1 and c-Myc. Induction of PLSCR1 expression has been reported to increase p21waf1/cip1 protein by increasing transcription and reducing degradation, as well as significantly inhibiting c-Myc mRNA and protein expression.32 We similarly observed the downregulation of c-Myc in U937 and HL-60 cells starting at 24 hours after exposure to wogonoside. p21waf1/cip1 was increased approximately 48 hours after wogonoside treatment in U937 and HL-60 cells (Figure 4D). The wogonoside-induced changes in c-Myc, p21waf1/cip1, and PLSCR1 observed in the current study were in accord with the results of previous research.32
Wogonoside increases PLSCR1 transcription and influences the expression of cell cycle– and differentiation-related proteins. (A) Total RNAs were extracted at the indicated time points. PLSCR1 mRNA levels were detected by quantitative real-time reverse transcription-PCR, and fold changes were assessed and shown normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA level. For analysis of RT-PCR results, asterisks denote significant (P < .05) differences relative to controls by 2-tailed Student t tests. (B) Wogonoside induced PLSCR1 expression. Whole cell extracts were analyzed by western blotting for PLSCR1 protein, using β-actin as a loading control. Cells treated with ATRA for 96 hours were used as a positive control. (C) Cells were cultured for 48 hours with or without 150 μM wogonoside after a 4-hour preincubation period with 4 μg/mL actinomycin D, and then analyzed for PLSCR1 and β-actin protein expression by western blotting. (D) The effects of wogonoside on the expression of p21cip and c-Myc proteins are shown.
Wogonoside increases PLSCR1 transcription and influences the expression of cell cycle– and differentiation-related proteins. (A) Total RNAs were extracted at the indicated time points. PLSCR1 mRNA levels were detected by quantitative real-time reverse transcription-PCR, and fold changes were assessed and shown normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA level. For analysis of RT-PCR results, asterisks denote significant (P < .05) differences relative to controls by 2-tailed Student t tests. (B) Wogonoside induced PLSCR1 expression. Whole cell extracts were analyzed by western blotting for PLSCR1 protein, using β-actin as a loading control. Cells treated with ATRA for 96 hours were used as a positive control. (C) Cells were cultured for 48 hours with or without 150 μM wogonoside after a 4-hour preincubation period with 4 μg/mL actinomycin D, and then analyzed for PLSCR1 and β-actin protein expression by western blotting. (D) The effects of wogonoside on the expression of p21cip and c-Myc proteins are shown.
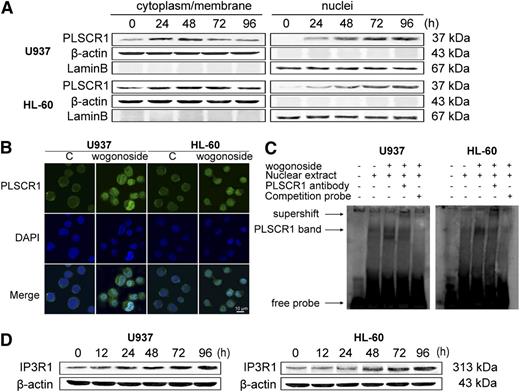
Wogonoside promotes PLSCR1 transport into the nucleus and facilitates its binding to the IP3R1 promoter
PLSCR1 has a biological function at the plasma membrane and cytoplasm, and recent results have suggested that this protein plays an additional role in the nucleus by binding to genomic DNA.33 To understand the subcellular distribution of PLSCR1 after induction by wogonoside, we fractionated nuclei and cytoplasm/membrane proteins in the presence of wogonoside for 0, 24, 48, 72, and 96 hours. Western blot analyses of fractionated cells showed that nuclear PLSCR1 was scarcely detectable before treatment, but nuclear localization increased after treatment with wogonoside (Figure 5A). Nuclear localization of PLSCR1 by wogonoside was confirmed by immunofluorescence analysis. PLSCR1 antigen was detected using fluorescein isothiocyanate (FITC)–labeled secondary goat anti-rabbit antibody (Zhongshan Golden Bridge Biotechnolog, Beijing, China; Figure 5B green channel), and nuclear DNA was stained with DAPI (4,6 diamidino-2-phenylindole, Santa Cruz, CA; Figure 5B blue channel). PLSCR1 trafficking into the nucleus was observed following treatment with wogonoside for 48 hours (Figure 5B). PLSCR1 was previously reported to traffic into the nucleus and bind to a specific segment of the IP3R1 5′-promoter and enhance transcription of this gene.34 We therefore investigated the promotion of PLSCR1 binding to the IP3R1 gene promoter by wogonoside using EMSA. PLSCR1 binding to double-stranded oligonucleotides containing the IP3R1 promoter was upregulated after treatment with 150 μM wogonoside for 48 hours, while untreated cells showed little or no DNA binding in the promoter (Figure 5C). It is also noted that IP3R1 expression increased in U937 and HL-60 cells exposed to 150 μM wogonoside (Figure 5D). These studies revealed that wogonoside promoted PLSCR1 translocation into the nucleus. They also suggest that wogonoside may augment the biological functions of PLSCR1 in the nucleus.
Wogonoside promotes translocation of PLSCR1 into the nucleus and facilitates its binding to the IP3R1 promoter. (A) The cytoplasmic/membrane and nuclear fractions of the cells were analyzed by western blotting for the PLSCR1 protein, with β-actin and lamin B as cytoplasmic and nuclear loading controls, respectively. (B) U937 and HL-60 cells were incubated with wogonoside (150 μM) for 48 hours. Cells were then incubated with primary anti-PLSCR1 antibody (1:50) at 37°C for 1 hour and then at 4°C overnight, followed by incubation with FITC-labeled secondary goat anti-rabbit antibody (1:100) for 1 hour at 37°C. The coverslips were washed and counterstained with DAPI working solution (100 μg/mL) for 20 minutes. PLSCR1 antigen (green fluorescence) and cell nuclei stained with DAPI (blue fluorescence) were detected by confocal microscopy (FV1000; Olympus, Tokyo, Japan) with FV10-ASW2.1 acquisition software (Olympus) at room temperature. (Original magnification ×1000; immersion objective ×100 with immersion oil type F). Images are representative of 3 independent experiments. (C) EMSA assay to detect PLSCR1 binding to its consensus site in the IP3R1 promoter is shown. Cells were incubated with wogonoside (150 μM) for 48 hours, and DNA binding was determined in nuclear extracts using EMSA. To determine the composition of the DNA-binding complex, the anti-PLSCR1 antibody was used for supershift experiments. These figures are representative of 3 separate experiments. (D) The effect of wogonoside on IP3R1 expression was analyzed by western blotting.
Wogonoside promotes translocation of PLSCR1 into the nucleus and facilitates its binding to the IP3R1 promoter. (A) The cytoplasmic/membrane and nuclear fractions of the cells were analyzed by western blotting for the PLSCR1 protein, with β-actin and lamin B as cytoplasmic and nuclear loading controls, respectively. (B) U937 and HL-60 cells were incubated with wogonoside (150 μM) for 48 hours. Cells were then incubated with primary anti-PLSCR1 antibody (1:50) at 37°C for 1 hour and then at 4°C overnight, followed by incubation with FITC-labeled secondary goat anti-rabbit antibody (1:100) for 1 hour at 37°C. The coverslips were washed and counterstained with DAPI working solution (100 μg/mL) for 20 minutes. PLSCR1 antigen (green fluorescence) and cell nuclei stained with DAPI (blue fluorescence) were detected by confocal microscopy (FV1000; Olympus, Tokyo, Japan) with FV10-ASW2.1 acquisition software (Olympus) at room temperature. (Original magnification ×1000; immersion objective ×100 with immersion oil type F). Images are representative of 3 independent experiments. (C) EMSA assay to detect PLSCR1 binding to its consensus site in the IP3R1 promoter is shown. Cells were incubated with wogonoside (150 μM) for 48 hours, and DNA binding was determined in nuclear extracts using EMSA. To determine the composition of the DNA-binding complex, the anti-PLSCR1 antibody was used for supershift experiments. These figures are representative of 3 separate experiments. (D) The effect of wogonoside on IP3R1 expression was analyzed by western blotting.
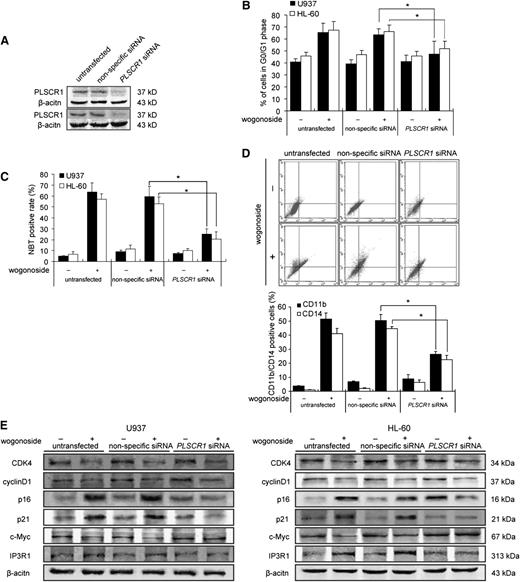
PLSCR1 is required for wogonoside-induced cell cycle arrest and differentiation
To determine the role of PLSCR1 in wogonoside-induced cell cycle arrest and differentiation, U937 and HL-60 cells were transfected with siRNA against PLSCR1. The efficacy of siRNA to inhibit PLSCR1 is shown in Figure 6A. After transfection, wogonoside-induced G0/G1 phase arrest was decreased, as observed previously (Figure 6B). Compared with nonspecific RNA groups, NBT-reducing activity in U937 and HL-60 cells (Figure 6C) and CD11b/CD14 expression in U937 cells (Figure 6D) were decreased in PLSCR1 siRNA groups after wogonoside treatment. To investigate the role of PLSCR1 in the wogonoside-induced cell cycle changes and differentiation-associated molecular events, the expression levels of associated proteins were detected by western blotting after transfection. The upregulation of p16 (INK4A), p21waf1/cip1, and IP3R1 by wogonoside was inhibited in PLSCR1 siRNA cells, compared with nonspecific-transfected cells. c-Myc expression was increased in PLSCR1 siRNA cells and was unchanged by wogonoside treatment, while CDK4 and cyclin D1 were decreased by wogonoside in cells transfected with PLSCR1 siRNA or nonspecific siRNA. These results suggest that transfection with PLSCR1 siRNA partially inhibited the effects of wogonoside on cell cycle arrest and differentiation.
PLSCR1 is involved in wogonoside-induced differentiation. U937 and HL-60 cells were transfected with nonspecific siRNA and PLSCR1 siRNA treated with or without 150 μM wogonoside for 96 hours. (A) Confirmation of the silencing of PLSCR1 expression was detected by western blotting with β-actin as a loading control. (B) The percentages of cells in G0/G1 phases of the cell cycle are shown. (C) Detection of NBT-reduction activity is shown. (D) CD11b-/CD14-positive cells were measured by flow cytometry. (E) Shown are the effects of silencing PLSCR1 on the expression of cell cycle– and differentiation-related proteins, which could be influenced by wogonoside. These figures are representative of 3 separate experiments.
PLSCR1 is involved in wogonoside-induced differentiation. U937 and HL-60 cells were transfected with nonspecific siRNA and PLSCR1 siRNA treated with or without 150 μM wogonoside for 96 hours. (A) Confirmation of the silencing of PLSCR1 expression was detected by western blotting with β-actin as a loading control. (B) The percentages of cells in G0/G1 phases of the cell cycle are shown. (C) Detection of NBT-reduction activity is shown. (D) CD11b-/CD14-positive cells were measured by flow cytometry. (E) Shown are the effects of silencing PLSCR1 on the expression of cell cycle– and differentiation-related proteins, which could be influenced by wogonoside. These figures are representative of 3 separate experiments.
Discussion
This study demonstrated that wogonoside had antiproliferative activity in U937, HL-60, and primary AML cells in vitro, and it inhibited the proliferation of U937 xenografts and prolonged survival in AML-bearing mice in vivo. The results of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide] assays showed that wogonoside could not completely inhibit cell viability, and cell viability was inhibited up to 80% by 400 μM wogonoside (supplemental Data 1). Meanwhile, 50, 100, and 150 μM wogonoside scarcely induced apoptosis in U937 and HL-60 cells after 96 hours of treatment (supplemental Data 2). This suggests that the antiproliferative effect of wogonoside is not directly related to cell death but involves another biological phenomenon. Treating leukemia by forcing malignant cells to undergo terminal differentiation instead of killing them through cytotoxicity is a promising alternative therapeutic strategy for AML. Terminal differentiation into diverse cell types results in an irreversible loss of proliferative potential, and cell cycle arrest in cell proliferation inhibition is an essential early event in cell differentiation. We therefore investigated the potential therapeutic mechanisms of wogonoside, including the blockade of cell cycle progression and the induction of differentiation. Wogonoside caused cell cycle arrest associated with the downregulation of cyclin D1 and CDK4, and it induced the differentiation of AML cell lines and primary AML cells.
The expression of PLSCR1 protein in several myeloid leukemia cell lines and primary leukemia cells is markedly increased in response to some differentiation-inducing agents such as ATRA, PMA, and interferon (IFN), and it is required for these agents to trigger leukemic cell differentiation.29,35 Suppression of PLSCR1 expression by siRNA inhibits ATRA- and PMA-induced leukemic cell differentiation.36 Moreover, induction of PLSCR1 expression arrested proliferation at the G1 phase and prompted differentiation toward granulocyte-like cells of myeloid leukemic U937 cells (U937plscr1 cells).32 These results demonstrated that PLSCR1 expression could be induced during wogonoside-induced differentiation of AML-derived cell lines (U937 and HL-60). Wogonoside also increased PLSCR1 mRNA levels through transcriptional control, which could be blocked by the transcription inhibitor actinomycin D. Exogenous transfection of PLSCR1 siRNA into the cells reduced their differentiation, as well as G1 phase arrest, in the presence of wogonoside. Overall, these results suggest that transcriptional upregulation of the PLSCR1 gene may play an important role in the mechanism of wogonoside-induced cell cycle arrest and differentiation in leukemia cells. We previously showed that wogonin induced differentiation of NB4 and U937 cells, and it also induced G1 phase arrest in U937 cells via PKCδ phosphorylation. However, PKCδ phosphorylation played a minor role in the wogonin-induced differentiation of U937 cells.15,25 ATRA and IFN have been reported to upregulate PLSCR1 expression by the PKCδ-c-Jun N-terminal kinases-signal transducer and activator of transcription 1 pathway.29,37 We therefore investigated if wogonoside increased PLSCR1 expression through PKCδ phosphorylation by treating U937 and HL-60 cells with 150 μM wogonoside in the absence or presence of the specific PKCδ inhibitor rottlerin. The expression of phosphorylated PKCδ increased in U937 cells from 4 to 12 hours, and in HL-60 cells from 8 to 16 hours, after wogonoside treatment (supplemental Data 3). Pretreatment with rottlerin inhibited wogonoside-increased PLSCR1 expression in U937 cells, but it only slightly inhibited it in HL-60 cells. It is possible that the regulation of PLSCR1 expression and PKCδ phosphorylation, and the role of these 2 molecules in differentiation, is complex and varies among different cell types and conditions.
p21waf1/cip1 is not only an negative regulator of G1-phase cell cycle progression, but it is also involved in the regulation of the progression to terminal differentiation.38 The oncogenic protein c-Myc elicits a variety of biological responses related to cell cycle distribution, differentiation, and apoptosis, and it is frequently activated in AML and plays an important role in the induction of leukemogenesis.39,40 To gain further insight into the wogonoside-induced events leading to G0/G1 arrest during terminal differentiation, we analyzed the expression of p21waf1/cip1 and c-Myc, which have been reported to be regulated by PLSCR1. p21waf1/cip1 expression in wogonoside-treated cells increased starting at 48 hours after treatment, while levels of the c-Myc protein were conversely decreased by exposure to wogonoside. Transfection with PLSCR1 siRNA blocked the wogonoside-induced increase in p21waf1/cip1 and decrease in c-Myc. The changes in c-Myc, p21waf1/cip1, and PLSCR1 induction by wogonoside observed in the current study were in accord with the results of Huang et al’s study.32 However, further studies are needed to determine how PLSCR1 regulates the expression of p21waf1/cip1 and c-Myc after wogonoside treatment and to clarify the relationships between these molecular events and cell signaling processes involved in wogonoside treatment.
PLSCR1 expression is transcriptionally upregulated by cytokines and growth factors, and nuclear trafficking of newly expressed PLSCR1 has been observed following transcriptional activation by IFN.41 The cysteine-rich sequence 184CCCPCC189 is required for palmitoylation of PLSCR1, which determines whether it inserts into the plasma membrane or localizes in the nucleus.41 PLSCR1 contains a nonclassic nuclear localization signal (257GKISKHWTGI266) with a unique binding site in importin-α.33 In the absence of palmitoylation, PLSCR1 import into the nucleus is mediated by the importin α/β nucleopore transport system, where it binds to DNA.42 PLSCR1 within the nucleus is involved in responding to cytokine induction and precursor maturation. PLSCR1 has been shown to traffic into the nucleus in the human ovarian tumor cell line HEY1B in response to IFN induction.41 Nuclear-localized PLSCR1 enhanced granulopoiesis in mice that were stimulated by granulocyte-colony stimulating factor; this resulted in the production of neutrophils from hematopoietic precursors and led to an increase in the number of mature blood neutrophils.43 In the present study, PLSCR1 was found inside the nucleus in U937 and HL-60 cells after treatment with wogonoside. Expression of the protein in the nucleus may be the result of transcriptional upregulation induced by wogonoside. The nuclear-localized PLSCR1 binds to DNA in a nucleotide-sequence-specific manner and is capable of activating targeted gene expression. PLSCR1 specifically binds to a segment of the 5′-promoter of IP3R1 (−101GTAACCATGTGGA−89), enhancing transcription of this gene.34 IP3R1 is known to play a key role in IP3-mediated mobilization of intracellular calcium ion Ca2+ stores from the endoplasmic reticulum in a variety of cells and tissues, and it is also essential for cell growth and differentiation.44,45 IP3R1 expression in many cells is upregulated in response to ATRA, DMSO, and interleukin-1β.46-48 The effects of PLSCR1 on cell proliferation and maturation may also be related to alterations in the expression of cellular IP3R1.49 The results showed that wogonoside promoted nuclear PLSCR1 binding to the transcriptional activation domain of IP3R1 and increased IP3R1 protein expression. siRNA against PLSCR1 clearly blocked the increased expression of IP3R1 when cells were cultured with wogonoside. Wogonoside thus appeared to augment PLSCR1 expression at the transcriptional level and promoted its localization in the nucleus and its binding to the IP3R1 promoter, thus influencing the expression of this receptor.
We also examined other cell cycle modulators affected by wogonoside after PLSCR1 siRNA transfection. The upregulation of p16 (INK4A) caused by wogonoside was inhibited by transfection with siRNA against PLSCR1, but p16 expression was increased in PLSCR1 siRNA-transfected HL-60 cells. This suggests that other molecular relationships may exist between PLSCR1 and p16 in different cell lines. However, the downregulation of cyclin D1 and CDK4 by wogonoside was unaffected by PLSCR1 siRNA transfection. These results indicate that some other signaling pathways are involved in wogonoside activity, and the exact molecular mechanisms whereby wogonoside increases p16 (INK4A) expression through PLSCR1 remain to be resolved in future studies. We need to explain why the ability of wogonoside to affect the posttranscriptional modification of PLSCR1 depends on its localization in the cell membrane or nucleus, which depends in turn on its palmitoylated state. Furthermore, the contribution of PLSCR1 nuclear localization induced by wogonoside to cell differentiation needs further investigation.
Wogonin has been widely investigated as an anticancer drug. It undergoes rapid metabolism and enters the bloodstream mainly in the form of the glucuronide wogonoside. Glucuronidaton can lead to increased solubility and a higher molecular weight, and it is known as a detoxification process for toxic xenobiotics, suggesting that wogonin will have limited clinical use. Wogonoside shows less toxicity to AML cells than wogonin does in vitro, but it shows no obvious difference in terms of acute toxicity in vivo (data not shown). Our study confirmed that wogonoside possesses the abilities to induce cycle arrest and differentiation of AML cells and to prolong the survival of AML-bearing mice, indicating that the glucuronide metabolite wogonoside, like its aglycone wogonin, has biological activity. These results suggest that wogonoside may be a promising agent for the treatment of AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Project Program of the State Key Laboratory of Natural Medicines, China Pharmaceutical University (JKGZ201101), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT-IRT1193), and the National Science and Technology Major Project (2012ZX09304-001).
Authorship
Contribution: Y.C. and H.H. designed and performed research, analyzed data, and wrote the paper; H.Y. performed research and analyzed data; K.Z. performed research; Y.Q. collected data and performed statistical analysis; C.G. collected and analyzed data; X.W. corrected grammatical and typing errors; and N.L. and Q.G. conceptualized the project and directed the experimental design and data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qinglong Guo, State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Carcinogenesis and Intervention, China Pharmaceutical University, Tongjiaxiang 24, Nanjing 210009, China; e-mail: anticancer_drug@yahoo.com.cn; and Na Lu, State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of Carcinogenesis and Intervention, China Pharmaceutical University, Tongjiaxiang 24, Nanjing 210009, China; e-mail: luna555@163.com.
References
Author notes
Y.C. and H.H. contributed equally to this article.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal