Key Points
CCR7 uptake by NK cells can be strongly induced by major histocompatibility complex–specific activating KIRs, in particular by KIR2DS1 (specific for HLA-C2).
The KIR2DS1-induced CCR7 expression on NK cells may expand greatly the contingent of alloreactive NK cells migrating to secondary lymphoid compartments after hematopoietic stem cell transplantation.
Abstract
Natural killer (NK) cells may capture the CCR7 chemokine receptor from allogeneic CCR7+ cells by trogocytosis and acquire migrating properties in response to lymph node chemokines. This event is negatively regulated by inhibitory killer Ig-like receptors (KIRs) and NKG2A. In this study, we analyzed the role of the HLA-C2–specific activating receptor KIR2DS1 in the process of CCR7 uptake by NK cells interacting with different allogeneic CCR7+ cells. Co-incubation of KIR2DS1+ fresh NK cells or NK-cell clones with HLA-C2+ CCR7+ lymphoblastoid cell lines resulted in increased CCR7 uptake. Remarkably, KIR2DS1 expression represented a major advantage for acquiring CCR7 from HLA-C2+ allogeneic dendritic cells (DCs) and T-cell blasts. These findings have important implications in haploidentical hematopoietic stem cell transplantation in which donor-derived (alloreactive) KIR2DS1+ NK cells, upon CCR7 acquisition, become capable of migrating toward lymph nodes, where they may kill patient DCs and T cells, preventing graft-versus-host and host-versus-graft reactions.
Introduction
Human natural killer (NK) cell function is regulated by a balance between activating and inhibitory receptors. The HLA-specific inhibitory receptors, including killer Ig-like receptors (KIRs) (specific for allotypic determinants of HLA-C, -B and -A molecules)1-3 and the HLA-E–specific CD94/NKG2A,4 monitor the level of HLA class I molecules expressed on autologous cells. The lack of appropriate receptor/HLA interactions may lead to the NK-mediated killing of cells with defective HLA class I expression. In T-cell–depleted haploidentical hematopoietic stem cell transplantation (haplo-HSCT), donor-derived alloreactive NK cells, which express inhibitory KIRs (iKIRs), kill KIR-ligand mismatched leukemic blasts (the C1 and C2 epitopes of HLA-C represent dominant KIR ligands)3 and play a crucial role in eradicating high-risk leukemia. Remarkably, patients who receive transplants from an NK alloreactive donor also benefit from higher rates of engraftment and reduced incidence of graft-versus-host disease.5-7 Both clinical and experimental data in mice suggest that this is due to inefficient priming of donor T cells due to NK-mediated clearance of the recipient’s myeloid dendritic cells (DCs) in lymph nodes.8 However, because KIR+ NK cells do not express CCR7,9,10 it was unclear how the donor’s alloreactive NK cells could reach lymph nodes and kill recipient mature DCs (mDCs). We have recently shown that NK cells acquire surface CCR7 upon interaction with CCR7+ cells10 and that the mechanism underlying this event is due to a phenomenon termed trogocytosis.10-13 The uptake of CCR7 by NK cells occurs within a few minutes, a time interval incompatible with either exosome secretion12 or de novo transcription/synthesis (as revealed by the absence of CCR7 messenger RNA).10 In addition, CCR7 could be acquired from cells transfected with CCR7 but not from their untransfected counterpart. The acquired CCR7 was expressed at the NK cell surface with the proper reorientation (similar to other surface molecules14 ), as it could bind specific monoclonal antibodies (mAbs) and promote NK-cell migration in response to CCL19 and/or CCL21.10
Trogocytosis involves membrane fragments derived from the immune synapses where certain molecules are accumulated.15 Described both in humans and in mice, both in vitro11,12,15 and in vivo,16 trogocytosis may involve several cell surface molecules and different cell types, including T and B lymphocytes, NK cells, DCs, and tumor cells. We found that, upon coculture with the CCR7+ 221 cell line, NK cells acquired, together with CCR7, CD19 and CD86 (a marker commonly used to assess trogocytosis).12 It has been proposed that the formation of the immune synapse for capturing target cell membrane fragments by NK cells may be promoted by the interaction of NK receptors with their specific ligands.12,15 Indeed, we showed that both inhibitory and activating receptors influence this process. Thus, CCR7 uptake was inhibited by the interaction between iKIRs or NKG2A and HLA class I molecules expressed on CCR7+ cells. Accordingly, in an allogeneic setting, only iKIR+ NKG2A− alloreactive NK cells can acquire CCR7 (eg, from CCR7+ mDCs). In addition, CCR7 uptake is regulated by non-major histocompatibility complex (non-MHC)–specific receptors including LFA-1, CD2, and NKp46.10
We show that the CCR7 uptake by NK cells also can be strongly induced by major histocompatibility complex (MHC)–specific activating KIRs, in particular by KIR2DS1 (specific for HLA-C2). Remarkably, unlike the non-MHC–specific receptors, KIR2DS1 could promote CCR7 uptake even in NKG2A+ NK cells, thus expanding considerably the proportion of NK cells capable of migrating to lymph nodes. These data provide valuable novel insight into the role of activating KIRs in hematopoietic stem cell transplantation.
Study design
Antibodies
Anti-hCCR7 (IgG2a, clone 150503) and anti-KIR2DL1 fluorescein isothiocyanate–conjugated (clone 143211)6 mAbs were from R&D Systems Inc (Abingdon, United Kingdom). Anti-CD16 allophycocyanin-cyanine 7 (APC-Cy7)–conjugated (clone 3G8) mAb was from BD Pharmingen (San Diego, CA). Anti-CD56 phycoerythrin-cyanine 7 (PC7)–conjugated (clone C218), anti-NKG2A allophycocyanin–conjugated (clone Z199), and anti-KIR2DL2/L3 PC7–conjugated (clone GL183) mAbs were from Beckman Coulter, Immunotech (Marseille, France). Anti-KIR3DL1 biotin–conjugated (clone DX9), anti-biotin Vio700, anti-KIR2DL1/S1 biotin–conjugated (clone 11PB6), and anti-biotin Vioblue mAbs were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). A6/136 and F(ab′)2 6A4 (IgM and IgG1, respectively, anti–HLA-I),6,10,17,18 Y9 (IgM, anti-CD94),19 and DF200 (IgG1, anti-KIR)20 were produced in our laboratory. For masking experiments, NK cells were pre-incubated with mAbs specific for the various NK receptors (anti-NKG2A and anti-KIR) or with anti–HLA class I mAbs (A6/136 or F(ab′)2 6A4, IgM, and IgG1, respectively) 10 minutes before the addition of target cells; mAb concentration was 10 μg/mL. The 6A4 mAb was previously shown to affect recognition by C2-specific KIRs but not by C1-specific KIRs.17
Cell preparations, functional experiments
Fresh NK cells, NK-cell clones, mDCs, and T-cell blasts were derived from healthy donors as described in Marcenaro et al10 and Sivori et al.18 Approval was obtained from the review board, Azienda Ospedaliera Universitaria S. Martino of Genova, Italy. Informed consent was provided according to the Declaration of Helsinki. We cultured 1 × 105 NK cells for 15 minutes at 37°C in the presence of different CCR7+ cells (1 × 105) in round-bottom, 96-well microplates. NK cell surface phenotype was assessed by flow cytometry (FACSVerse; Becton, Dickinson).
Statistical analysis
The independent sample Student t test was employed for evaluating quantitative variables. The statistical level of significance was present at 0.05. Graphic representation and statistical analyses were performed using PASW Statistic version 18.0 software (formerly SPSS Statistics; IBM, Italy).
Migration assay
Chemotaxis of NK cells was measured by migration through a polycarbonate filter with a 3.0-µm pore size in 96-well transwell chambers (Corning Costar) with the use of RPMI/10% fetal calf serum as assay medium. We added 150 µL assay medium containing 250 ng/mL of CCL19 and CCL21 (PeproTech, London, United Kingdom) to the lower chamber. We used 150 µL assay medium alone as a control for spontaneous migration. Then 7 × 104 NK cells, stimulated or not, were added to the upper chamber in a total volume of 50 µL. After 3 hours of incubation at 37°C, cells that had migrated to the bottom chamber were counted by optical microscopy (Carl Zeiss Microscopy GmbH) with a 25× objective. The number of spontaneously migrated cells was subtracted from the total number of migrated cells. Values are given as the chemotactic index compared with the migration of unstimulated NK cells.10,21 As further confirmation, migrated cells were counted also by flow cytometry (absolute count) (BD Flow Sensor option, FACSVerse) (not shown).
Results and discussion
KIR2DS1 induces the expression of CCR7 and the acquisition of migratory properties in alloreactive NK cells upon interaction with HLA-C2+ CCR7+ cell transfectants
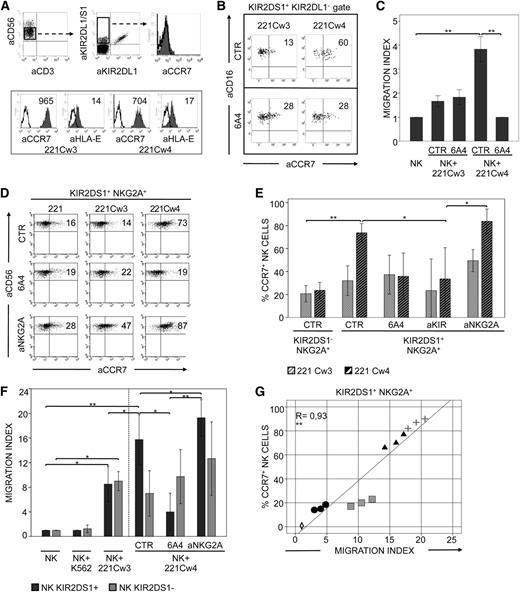
Purified peripheral blood NK cells, derived from C1/C1 KIR2DS1+ donors, included a subset of KIR2DS1+/KIR2DL1− cells. These KIR+ cells were confined to CD56dull cells and displayed a homogeneous CCR7− phenotype (Figure 1A). Fresh NK cells were cocultured with 221 cells transfected with either HLA-Cw3 (C1 epitope) or HLA-Cw4 (C2 epitope). Notably, 221 cells expressed high levels of CCR7 (Figure 1A). After 15 minutes of incubation, cells were analyzed for CCR7 expression. KIR2DS1+/KIR2DL1− NK cells, cocultured with Cw4+ transfectants, expressed higher levels of CCR7 than those cocultured with Cw3+ transfectants (Figure 1B). Capture of CCR7 from C2+ target cells was inhibited by the disruption of the KIR2DS1/HLA-C interaction by using the anti–HLA class I–specific 6A4 mAb, known to prevent HLA-C2 recognition by both KIR2DS1+ and KIR2DL1+ cells (Figure 1B). In addition, these CCR7+ NK cells displayed a more efficient capability of migrating in response to CCL19/CCL21. Migration was strongly reduced by anti–HLA class I mAbs added to cocultures (Figure 1C). Thus, the expression of KIR2DS1 may be important for the acquisition of migratory properties in NK cells interacting with HLA-C2+ CCR7+ cells.
KIR2DS1 induces uptake of CCR7 and acquisition of migratory properties in NK cells interacting with C2+ cell transfectants. (A) Freshly isolated CD56dull NK cells (from a representative C1/C1 KIR2DS1+ donor) contained a subset of KIR2DS1+/KIR2DL1− cells that did not express CCR7 (upper panels). The level of expression (median values) of CCR7 and HLA-E molecules on 221 cells transfected with either HLA-Cw3 (C1 epitope) or HLA-Cw4 (C2 epitope) is also shown (lower panels). (B) The same NK cells as in (A) were incubated for 15 minutes at 37°C with either 221-Cw3 or 221-Cw4 transfectants, in the absence or presence of specific anti–HLA-I mAbs (6A4). Then cells were harvested and stained with anti-CD16 (to distinguish NK cells from the 221 cells), different anti-KIRs (to select and gate on the KIR2DS1+/KIR2DL1− cell subset), and anti-CCR7 mAbs for cytofluorimetric analysis. Percentages of CCR7+ cells are indicated. (C) Freshly isolated NK cells from 4 C1/C1 KIR2DS1+ donors were incubated alone (NK) or with 221-Cw3 or 221-Cw4 cells, in the absence or presence of anti–HLA-I mAbs (6A4). After 15 minutes at 37°C, NK cells were analyzed for their migratory properties in response to CCL19/CCL21 chemokines. The average of 4 independent experiments is shown. The histogram refers to the migration index of NK cells after incubation. (D) A representative KIR2DS1+ NKG2A+ NK-cell clone (from a C1/C1 donor) was incubated at 37°C with untransfected 221 cells or 221-Cw3 or 221-Cw4 transfectants in the absence or presence of anti–HLA-I (6A4) or anti-NKG2A mAbs. After 15 minutes, NK cells were harvested and stained with anti-CD56 and anti-CCR7 mAbs for cytofluorimetric analysis. The percentages of CCR7+ cells are indicated. (E) The average of 6 independent experiments of KIR2DS1+ or KIR2DS1− NK-cell clones incubated with 221-Cw3 or 221-Cw4 is shown. NK clones (6 for each type) were derived from 3 different C1/C1 donors. All NK-cell clones expressed NKG2A as the only inhibitory receptor. Co-incubation was performed in the absence or presence of anti–HLA-I (6A4), anti-KIR, or anti-NKG2A mAbs, as indicated. The histogram represents the percentage of CCR7+ NK cells after incubation with 221 cell transfectants. (F) The same KIR2DS1+ or KIR2DS1− NK-cell clones, which had been incubated alone (NK) or with 221 cell transfectants in the absence or presence of anti–HLA-I (6A4) or anti-NKG2A mAbs, were analyzed for their migratory properties in response to CCL19/CCL21 chemokines. NK cells incubated with K562 were used as a control. The average of 6 independent experiments is shown. The histogram refers to the migration index of NK cells after coculture. (G) The relationship between the migration index and the CCR7 expression on 3 representative KIR2DS1+ NKG2A+ NK-cell clones (from 3 C1/C1 donors) was analyzed according to a linear regression model (R = 0.93) and confirmed by analyzing the Pearson correlation coefficient (=0.93). Shown are NK alone (empty rhombus); NK plus 221-Cw3 (black circle), NK plus 221-Cw4 (black triangle), NK plus 221-Cw4 in the presence of anti-NKG2A mAbs (gray cross), and NK plus 221-Cw4 in the presence of anti–HLA-I mAbs (6A4) (gray square). *P < .05; **P < .01.
KIR2DS1 induces uptake of CCR7 and acquisition of migratory properties in NK cells interacting with C2+ cell transfectants. (A) Freshly isolated CD56dull NK cells (from a representative C1/C1 KIR2DS1+ donor) contained a subset of KIR2DS1+/KIR2DL1− cells that did not express CCR7 (upper panels). The level of expression (median values) of CCR7 and HLA-E molecules on 221 cells transfected with either HLA-Cw3 (C1 epitope) or HLA-Cw4 (C2 epitope) is also shown (lower panels). (B) The same NK cells as in (A) were incubated for 15 minutes at 37°C with either 221-Cw3 or 221-Cw4 transfectants, in the absence or presence of specific anti–HLA-I mAbs (6A4). Then cells were harvested and stained with anti-CD16 (to distinguish NK cells from the 221 cells), different anti-KIRs (to select and gate on the KIR2DS1+/KIR2DL1− cell subset), and anti-CCR7 mAbs for cytofluorimetric analysis. Percentages of CCR7+ cells are indicated. (C) Freshly isolated NK cells from 4 C1/C1 KIR2DS1+ donors were incubated alone (NK) or with 221-Cw3 or 221-Cw4 cells, in the absence or presence of anti–HLA-I mAbs (6A4). After 15 minutes at 37°C, NK cells were analyzed for their migratory properties in response to CCL19/CCL21 chemokines. The average of 4 independent experiments is shown. The histogram refers to the migration index of NK cells after incubation. (D) A representative KIR2DS1+ NKG2A+ NK-cell clone (from a C1/C1 donor) was incubated at 37°C with untransfected 221 cells or 221-Cw3 or 221-Cw4 transfectants in the absence or presence of anti–HLA-I (6A4) or anti-NKG2A mAbs. After 15 minutes, NK cells were harvested and stained with anti-CD56 and anti-CCR7 mAbs for cytofluorimetric analysis. The percentages of CCR7+ cells are indicated. (E) The average of 6 independent experiments of KIR2DS1+ or KIR2DS1− NK-cell clones incubated with 221-Cw3 or 221-Cw4 is shown. NK clones (6 for each type) were derived from 3 different C1/C1 donors. All NK-cell clones expressed NKG2A as the only inhibitory receptor. Co-incubation was performed in the absence or presence of anti–HLA-I (6A4), anti-KIR, or anti-NKG2A mAbs, as indicated. The histogram represents the percentage of CCR7+ NK cells after incubation with 221 cell transfectants. (F) The same KIR2DS1+ or KIR2DS1− NK-cell clones, which had been incubated alone (NK) or with 221 cell transfectants in the absence or presence of anti–HLA-I (6A4) or anti-NKG2A mAbs, were analyzed for their migratory properties in response to CCL19/CCL21 chemokines. NK cells incubated with K562 were used as a control. The average of 6 independent experiments is shown. The histogram refers to the migration index of NK cells after coculture. (G) The relationship between the migration index and the CCR7 expression on 3 representative KIR2DS1+ NKG2A+ NK-cell clones (from 3 C1/C1 donors) was analyzed according to a linear regression model (R = 0.93) and confirmed by analyzing the Pearson correlation coefficient (=0.93). Shown are NK alone (empty rhombus); NK plus 221-Cw3 (black circle), NK plus 221-Cw4 (black triangle), NK plus 221-Cw4 in the presence of anti-NKG2A mAbs (gray cross), and NK plus 221-Cw4 in the presence of anti–HLA-I mAbs (6A4) (gray square). *P < .05; **P < .01.
KIR2DS1+ NK cells from C1/C1 individuals usually coexpress iKIRs and/or NKG2A (which is required for their process of “licensing”22 ). Analysis of NK-cell clones revealed that certain iKIRs, but not NKG2A, block KIR2DS1-dependent cytotoxicity.6,18 Thus, in order to avoid possible interferences, only KIR2DS1+ NKG2A+ NK-cell clones were selected and analyzed for CCR7 uptake. Similar to freshly isolated NK cells, KIR2DS1+ NKG2A+ clones also acquired CCR7, primarily when cocultured with Cw4+ transfectants (Figure 1D), and the uptake was inhibited by anti–HLA class I (Figure 1D-E) or anti-KIR mAbs (Figure 1E). Importantly, these experiments demonstrate that, in the mechanism of CCR7 uptake, the activating signals generated by KIR2DS1/Cw4 interactions override the inhibitory signals generated by the interaction between NKG2A and its ligand HLA-E (which is expressed by 221 transfectants, as shown in Figure 1A). However, a further increase in CCR7 uptake was detectable after the disruption of this inhibitory interaction in the presence of anti-NKG2A mAbs (Figure 1D-E). In agreement with these data, KIR2DS1+/NKG2A+ NK-cell clones acquired substantial migratory activity in response to CCL19/CCL21, whereas, in contrast, KIR2DS1-/NKG2A+ NK-cell clones displayed low migratory properties (Figure 1F). The migratory activity of KIR2DS1+/NKG2A+ NK-cell clones was proportional to the expression of CCR7, was inhibited by anti–HLA class I, and was increased by anti-NKG2A mAbs (Figure 1F-G).
KIR2DS1 induces the uptake of CCR7 upon NK-cell interaction with allogeneic C2+ T-cell blasts or mDCs
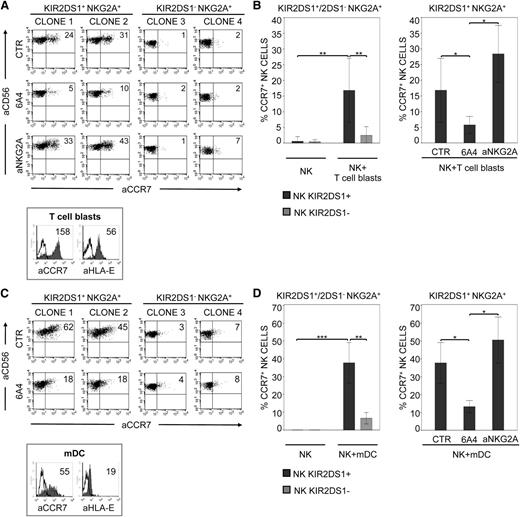
The same set of NK-cell clones was also assessed for the capability of acquiring CCR7 from allogeneic (C2/C2) T-cell blasts expressing CCR7 and HLA-E (Figure 2A). As shown in Figure 2A-B, CCR7 uptake was essentially restricted to KIR2DS1+/NKG2A+ clones, was inhibited by anti–HLA class I, and was increased by anti-NKG2A mAbs. In contrast, KIR2DS1−/NKG2A+ clones did not acquire (or acquired low levels of) CCR7, even in the presence of anti-NKG2A mAbs (Figure 2A). This indicates that the uptake of CCR7 from T-cell blasts is mainly dependent on the activating signals delivered by KIR2DS1 (Figure 2A-B). KIR2DS1 also played a prominent role in the killing of C2/C2 T-cell blasts, as revealed by the ability of anti-KIR2DS1 mAbs to abrogate lysis (not shown).18
KIR2DS1-induced uptake of CCR7 in NK cells interacting with C2+ T-cell blasts or mDCs. (A) Two representative KIR2DS1+ NKG2A+ NK-cell clones (derived from 2 different C1/C1 donors) and 2 KIR2DS1− NKG2A+ (from the same donors) were incubated at 37°C with CCR7+ allogeneic T-cell blasts derived from a C2/C2 donor. Co-incubation was performed in the absence or presence of specific anti–HLA-I (6A4) or anti-NKG2A mAbs. After 15 minutes, NK cells were harvested and stained with anti-CD56 and anti-CCR7 mAbs for cytofluorimetric analysis. The percentages of CCR7+ cells are indicated. Histogram plots (lower panel) illustrate the expression of CCR7 and HLA-E on T-cell blasts (median values are indicated). (B) The average of 6 independent experiments of KIR2DS1+ NKG2A+ or KIR2DS1− NKG2A+ NK-cell clones incubated alone (NK) or with T-cell blasts is shown (left panel). NK clones (6 for each type) were derived from 3 different C1/C1 donors. The average of 6 independent experiments of KIR2DS1+ NKG2A+ NK-cell clones incubated with T-cell blasts, performed either in the absence or presence of specific anti–HLA-I (6A4) and anti-NKG2A mAbs, is shown (right panel). The histograms refer to the percentage of CCR7+ NK cells detectable after incubation with T-cell blasts under the indicated conditions. (C) Two representative KIR2DS1+ NKG2A+ NK-cell clones (derived from 2 different C1/C1 donors) and 2 KIR2DS1− NKG2A+ clones (from the same donors) were incubated at 37°C in the absence or presence of anti–HLA-I (6A4) mAbs with allogeneic mDCs derived from a C2/C2 donor. After 15 minutes, NK cells were harvested and stained with anti-CD56 and anti-CCR7 mAbs for cytofluorimetric analysis. Percentages of CCR7+ cells are indicated. The histogram plots (lower panel) illustrate the expression of CCR7 and HLA-E on mDCs (median values are indicated). (D) The average of 6 independent experiments of KIR2DS1+ NKG2A+ or KIR2DS1− NKG2A+ NK-cell clones incubated alone (NK) or with mDCs is shown (left panel). NK clones (6 for each type) were derived from 3 different C1/C1 donors. The average of 6 independent experiments of KIR2DS1+ NKG2A+ NK-cell clones incubated with mDCs in the absence or presence of specific anti–HLA-I (6A4) or anti-NKG2A mAbs is shown (right panel). The histograms indicate the percentage of CCR7+ NK cells under the indicated conditions. *P < .05; **P < .01; ***P < .001.
KIR2DS1-induced uptake of CCR7 in NK cells interacting with C2+ T-cell blasts or mDCs. (A) Two representative KIR2DS1+ NKG2A+ NK-cell clones (derived from 2 different C1/C1 donors) and 2 KIR2DS1− NKG2A+ (from the same donors) were incubated at 37°C with CCR7+ allogeneic T-cell blasts derived from a C2/C2 donor. Co-incubation was performed in the absence or presence of specific anti–HLA-I (6A4) or anti-NKG2A mAbs. After 15 minutes, NK cells were harvested and stained with anti-CD56 and anti-CCR7 mAbs for cytofluorimetric analysis. The percentages of CCR7+ cells are indicated. Histogram plots (lower panel) illustrate the expression of CCR7 and HLA-E on T-cell blasts (median values are indicated). (B) The average of 6 independent experiments of KIR2DS1+ NKG2A+ or KIR2DS1− NKG2A+ NK-cell clones incubated alone (NK) or with T-cell blasts is shown (left panel). NK clones (6 for each type) were derived from 3 different C1/C1 donors. The average of 6 independent experiments of KIR2DS1+ NKG2A+ NK-cell clones incubated with T-cell blasts, performed either in the absence or presence of specific anti–HLA-I (6A4) and anti-NKG2A mAbs, is shown (right panel). The histograms refer to the percentage of CCR7+ NK cells detectable after incubation with T-cell blasts under the indicated conditions. (C) Two representative KIR2DS1+ NKG2A+ NK-cell clones (derived from 2 different C1/C1 donors) and 2 KIR2DS1− NKG2A+ clones (from the same donors) were incubated at 37°C in the absence or presence of anti–HLA-I (6A4) mAbs with allogeneic mDCs derived from a C2/C2 donor. After 15 minutes, NK cells were harvested and stained with anti-CD56 and anti-CCR7 mAbs for cytofluorimetric analysis. Percentages of CCR7+ cells are indicated. The histogram plots (lower panel) illustrate the expression of CCR7 and HLA-E on mDCs (median values are indicated). (D) The average of 6 independent experiments of KIR2DS1+ NKG2A+ or KIR2DS1− NKG2A+ NK-cell clones incubated alone (NK) or with mDCs is shown (left panel). NK clones (6 for each type) were derived from 3 different C1/C1 donors. The average of 6 independent experiments of KIR2DS1+ NKG2A+ NK-cell clones incubated with mDCs in the absence or presence of specific anti–HLA-I (6A4) or anti-NKG2A mAbs is shown (right panel). The histograms indicate the percentage of CCR7+ NK cells under the indicated conditions. *P < .05; **P < .01; ***P < .001.
KIR2DS1+ (but not KIR2DS1−) NK-cell clones efficiently acquired CCR7 also upon coculture with C2/C2 CCR7+ allogeneic mDCs (Figure 2C-D). Also in this case (Figure 2D, right panel), the uptake was inhibited by anti–HLA class I and increased by anti-NKG2A mAbs (as shown in Figure 2C, mDCs express HLA-E). Thus, KIR2DS1+ NK cells not only kill C2+ mDCs,18 but they also acquire CCR7 from them, an early event preceding killing.10 This finding is of particular interest in view of the crucial role of allogeneic DCs in the induction of graft-versus-host (GvH) reactions.8
Our data further support the notion that the expression of KIR2DS1 by alloreactive NK cells is of great relevance in haplo-HSCT to cure high-risk leukemia. Previous studies indicated that the expression of activating KIRs, particularly KIR2DS1, may improve the clinical outcome in haplo-HSCT.6,18,23,24 In this context, KIR2DS1+ NK-cell clones from C1/C1 donors could kill both C2/C2 and C1/C2 leukemic blasts6 as well as mDCs and T-cell blasts.18 Importantly, the expression of KIR2DS1 can render alloreactive a subset of NK cells expressing NKG2A. Thus, in appropriate donor/recipient pairs, KIR2DS1 expression may considerably increase the size of the alloreactive NK subset. However, the effectiveness of alloreactive NK cells could be limited by the lack of CCR7 expression (a common feature of KIR+ cells)9,10 and by the consequent inability to migrate to lymph nodes. We show that KIR2DS1 strongly potentiates the uptake of CCR7 and the migratory properties of both fresh and activated NK cells in response to CCL19/CCL21. Thus, the KIR2DS1+/NKG2A+ subset of alloreactive NK cells can migrate to lymph nodes and prevent GvH and host-versus-graft reactions by killing recipient DCs and T cells at these sites. The uptake of CCR7 from DCs may occur first in peripheral tissues, where these cells undergo maturation (and upregulation of CCR7 expression) in response to various stimuli. In this context, a number of studies highlighted the importance of NK/DC crosstalk for the induction of optimal DC maturation/selection before their migration into secondary lymphoid compartments (SLCs).25 However, because NK cells recruited into lymph nodes may downregulate their CCR7 expression within a few hours,10 it is conceivable that they require further CCR7 uptake in order to prevent their early release from lymph nodes. Thus, thanks to the acquisition of CCR7 from allogeneic mDCs and T cells within lymph nodes, alloreactive NK cells can be retained in a microenvironment rich in SLC chemokines and can exert their beneficial effect by killing cells involved in the induction of GvH and host-versus-graft responses.
The induction of CCR7 expression in donor-alloreactive peripheral NK cells can be exploited to further increase the effectiveness of alloreactive NK cells in protocols of adoptive immunotherapy associated with haplo-HSCT. Along this line, Somanchi et al16 showed that the CCR7 acquired by NK cells (after in vitro incubation with CCR7+ cells) could not only mediate the in vitro migration of NK cells in response to CCL19/CCL21 but could also increase their in vivo migration toward lymph nodes.
In conclusion, our study supports the notion that, in haplo-HSCT, the improved clinical outcome related to the presence of donor NK cells expressing activating KIRs (in particular KIR2DS1)6,18,23,24 may reflect the increased ability of these cells to reach the site where they can kill mDCs and T-cell blasts. In this context, the unique capability of KIR2DS1 to override the inhibition mediated by NKG2A appears to play a crucial role that greatly increases the contingent of alloreactive NK cells migrating to SLCs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank G. Reggiardo (Medi Service, Genova, Italy) for his valuable help in statistical analysis.
This work was supported by grants from Associazione Italiana Ricerca sul Cancro: (IG project 10643) (A.M.), (IG project 10225) (L.M.), and (special project 5×1000 9962) (A.M. and L.M.); Ministero dell’Istruzione, Università e Ricerca (MIUR-FIRB 2003 project RBLA039LSF-001/003) (L.M. and A.M.); Ministero della Salute (RF2006-Ricerca Oncologica-Project of Integrated Program 2006-08, agreements RO strategici 3/07) (L.M. and A.M.); and Progetto Ricerca Ateneo 2011 (E.M.). S.P. and S.C. are recipients of fellowships awarded by Associazione Italiana Ricerca sul Cancro (IG project 10643) and Federazione Italiana Ricerca sul Cancro, respectively.
Authorship
Contribution: E.M. designed and performed research, interpreted data, and wrote the article; S.P. designed and performed research and interpreted data; S.S. and S.C. generated NK-cell clones; L.M. revised the article; and A.M. designed and performed research, interpreted data, and wrote the article.
Conflict-of-interest disclosure: A.M. is a founder and shareholder of Innate-Pharma (Marseille, France). The remaining authors declare no competing financial interests.
Correspondence: Alessandro Moretta, MD, Dipartimento di Medicina Sperimentale, Sezione di Istologia, Via G.B. Marsano 10, 16132 Genova, Italy; e-mail: alemoret@unige.it.
References
Author notes
E.M. and S.P. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal