Key Points
S1P1 activity in human T cells can be reliably measured by assessing downstream signaling events induced upon S1P1 ligation.
S1P1 activity is impaired in T cells from HIV-1+ lymph nodes.
Abstract
The determinants of HIV-1-associated lymphadenopathy are poorly understood. We hypothesized that lymphocytes could be sequestered in the HIV-1+ lymph node (LN) through impairments in sphingosine-1-phosphate (S1P) responsiveness. To test this hypothesis, we developed novel assays for S1P-induced Akt phosphorylation and actin polymerization. In the HIV-1+ LN, naïve CD4 T cells and central memory CD4 and CD8 T cells had impaired Akt phosphorylation in response to S1P, whereas actin polymerization responses to S1P were impaired dramatically in all LN maturation subsets. These defects were improved with antiretroviral therapy. LN T cells expressing CD69 were unable to respond to S1P in either assay, yet impaired S1P responses were also seen in HIV-1+ LN T cells lacking CD69 expression. Microbial elements, HIV-1, and interferon α – putative drivers of HIV-1associated immune activation all tended to increase CD69 expression and reduce T-cell responses to S1P in vitro. Impairment in T-cell egress from lymph nodes through decreased S1P responsiveness may contribute to HIV-1-associated LN enlargement and to immune dysregulation in a key organ of immune homeostasis.
Introduction
In HIV-1 infection, lymphoid tissues are important sites of disease pathogenesis,1,2 and in the early years, generalized lymphadenopathy was a sentinel manifestation of infection.3,4 In the HIV-1-infected lymph node (LN), effector T cells are typically overrepresented and there is concurrent overexpression of a variety of inflammatory cytokines.5-7 This contrasts with the LN environment in health where highly ordered processes are necessary to facilitate T-cell homeostasis and antigen-driven T-cell maturation and expansion.8 In untreated HIV-1 infection, these processes are compromised but can be improved with antiretroviral therapy.9-12 In untreated HIV-1 infection, normal lymphocyte trafficking is impaired, with abnormal T-cell sequestration in lymphoid tissues.13,14 Very early after antiretroviral therapy (ART) initiation, there are both dramatic reductions in lymph node size and rapid increases in numbers of circulating lymphocytes. Replenishment of circulating lymphocytes during this period is suggestive of cellular redistribution rather than de novo production,15 as there is no evidence of increased cell cycling16 and increases in circulating lymphocytes are related to reductions in T-cell densities within lymphoid tissues.17 Thus, inappropriate retention of lymphocytes within inflammatory LNs is thought to be a characteristic of untreated HIV-1 infection, but the determinants of this retention are poorly understood.
The principal route of T-cell entry into lymphoid tissue involves CD62L selectin-dependent tethering along high endothelial venules,18 facilitating T-cell entry into the lymphoid interstitial space through CCL21-CCR7 interaction.19 Egress of T cells from the lymph node is also receptor-mediated through activation of the G protein-coupled20 sphingosine-1-phosphate receptor 1 (S1P1) by tightly regulated gradients of the extracellular lipid mediator sphingosine-1-phosphate (S1P),21 resulting in lymphocyte chemotaxis through efferent lymphatic vessels.22,23 S1P1 is transcriptionally regulated by Kruppel-like factor 2 (KLF2).24 Upon T-cell activation, KLF2 is rapidly downregulated and subsequently reexpressed with further T-cell maturation.25,26 Furthermore, S1P1 expression can be posttranslationally antagonized by the C-type lectin, CD69.27 CD69 is expressed very early upon T-cell activation and directly binds S1P1, inducing its intracytoplasmic retention and subsequent degradation.28,29
This pathway of S1P1-dependent cellular egress from lymphoid tissues has been illuminated and characterized extensively in mice. There is reason to suspect that similar control of lymph node trafficking is operative in humans, as an S1P analog FTY 720 that has been approved for the treatment of multiple sclerosis promotes receptor internalization and durable unresponsiveness to S1P, resulting in profound lymphopenia that is attributed to blockade of cellular egress from lymphoid tissues.30,31 With this stated, there has been only limited study of the role of SIP and its receptors in the human system.
We hypothesized that impaired S1P1-mediated signaling and chemotactic egress from lymph nodes might underlie the excessive lymphocyte sequestration in lymphoid tissues seen in HIV-1 infection. To test this hypothesis, we developed novel methods to evaluate this system in human cells. Herein, we characterize S1P1 bioactivity in human T cells and show that this activity is impaired in lymph node T cells in HIV-1 infection.
Methods
Study subjects
All procedures were approved by the institutional review boards at the relevant institutions, and all participants gave written informed consent before all procedures. Whole pelvic lymph nodes were obtained from adult women not known to be HIV-1 infected who were undergoing medically indicated surgery at University Hospitals of Cleveland. Their median age was 63 years (range, 43-82 years). ART-naïve HIV-1-infected patients were recruited at the Drexel University College of Medicine, Philadelphia, Pennsylvania. This group consisted of 5 males, 2 females, and 1 transgender subject. Median age of these subjects was 35 years (range, 27-48 years). All were naïve to ART and had HIV-1 levels in plasma ≥ 2000 copies/mL and a median CD4 count of 558. Lymph nodes were also obtained from 5 HIV-1 infected subjects (from University Hospitals in Cleveland, Ohio) who had been receiving suppressive combination antiretroviral therapy. The median age of the treated HIV-1 infected group was 50 years (range, 42-54 years). This group consisted of 5 males, and their median CD4 cell count was 665. Lymph nodes were excised from the inguinal region under local anesthesia as previously described.14 Surgeries were performed in the morning and the excised nodes were then placed in RPMI 1640 and shipped to Case Western Reserve University at 4°C via same day air along with matching peripheral blood. The time between biopsy and subsequent specimen processing was no more than 7 hours.
Blood and lymph node processing
Upon receipt of tissue, LNs were washed once in ice cold phosphate-buffered saline (PBS). After careful removal of surrounding fatty tissue, LNs were cut into 1 mm × 1 mm tissue blocks and digested with collagenase IV (5 mg/mL in RPMI 1640) (GIBCO Life Technologies, Grand Island, NY) supplemented with 0.5% fatty acid-free bovine serum albumin (Sigma-Aldrich, St. Louis, MO) and 200 μg/mL DNAse I (Roche, Basel, Switzerland). Collagenase-digested tissues were then mechanically digested with a motorized pestle, and the liberated cell suspensions were passed through a 40-micron nylon mesh and washed once in RPMI 1640.
Flow cytometry
Viable cells were gated using LIVE/DEAD-Aqua viability dye (Invitrogen, Grand Island, NY). Lymphocytes were identified by light-scatter properties and then were analyzed for surface marker expression using the following fluorochrome-labeled monoclonal antibodies: anti-CD3–peridinin chlorophyll protein, anti-CD8–alexa fluor 700, anti-CD27–allophycocyanin, anti-CD45RA–phycoerythrin, anti-CD69–phycoerythrin cy7 (all from Becton Dickinson, San Jose, CA), and anti-CD4–Pacific Blue (Biolegend, San Diego, CA). Cells were then washed, fixed in PBS containing 4% formaldehyde, and acquired on an LSRII flow cytometer (Becton Dickinson) equipped with lasers emitting at wavelengths 355, 488, 532, 407, and 638 nm. Data were analyzed using DIVA (BD Biosciences, San Jose, CA), version 6.2 software. Additional analysis was performed using Flowjo software (Tree Star, San Carlos, CA). T-cell maturation phenotypes were defined by CD45RA and CD27 expression. For detection of S1P1 surface expression on HTC-4 cell lines, cells were stained with anti-Edg1–phycoerythrin (R & D Systems, Minneapolis, MN).
Real-time quantitative RT-PCR
Fresh LN tissue sections were placed in 500 µL RNAlater (Ambion, Grand Island, NY) and stored at 4°C for 24 hours. Specimens were then transferred to a −80°C freezer. Thawed samples were immediately disrupted in lysis buffer (Qiagen, Valencia, CA) using a homogenizer (Powergen, Pittsburgh, PA) and were subsequently passed through a Qiashredder microfuge spin column (Qiagen, City, State). RNA was then isolated from tissue lysates via silica-membrane RNeasy spin columns (Qiagen, Valencia, CA). In concurrent experiments, T cells were purified from the LN cell suspensions by negative selection through incubation with a cocktail of antibodies targeting CD14, CD16, CD19, CD36, CD56, CD123, and Glycophorin A (Miltenyi, Auburn, CA) followed by magnetic bead separation (Miltenyi). Purified LN T cells were then lysed in Trizol and RNA was extracted with isopropanol. After purification, 1 μg of total RNA was reverse-transcribed using M-MuLV reverse transcriptase in the presence of oligo32 primers (Invitrogen). Complementary DNA was amplified by SteponePlus (Applied Biosystems, Carlsbad, CA) real-time quantitative polymerase chain reaction using pre-designed TaqMan Gene Expression assays for S1P1 (Assay ID; Hs01922614_s1) and KLF2 (Assay ID: Hs00360439_g1) (Applied Biosystems, Carlsbad, CA). Target gene expression was normalized to primers amplifying 18S rRNA (part number 4308329; Applied Biosystems).
Measurement of S1P-induced bioactivity in human T cells
Peripheral blood T cells were negatively selected by incubation with a cocktail of antibodies targeting CD14, CD16, CD19, CD36, CD56, CD123, and Glycophorin A (Miltenyi) followed by magnetic bead separation (Miltenyi). Separated PB T cells or unpurified LN cell suspensions were cultured in X-VIVO chemically defined serum-free medium (Lonza, Allendale, NJ) at a density of 0.5 × 106 cells/mL for approximately 18 hours at 37°C in 5% CO2.
To assess ligand-induced Akt phosphorylation and actin polymerization, cells were then stimulated with 50 nM S1P (Sigma-Aldrich) or 0.1 ng/mL stromal cell-derived factor-alpha (SDF-1α) (Sigma-Aldrich), dissolved in PBS containing 0.5% fatty acid-free bovine serum albumin (Sigma-Aldrich) for 30 seconds, and immediately fixed in 4% paraformaldehyde. Preliminary experiments identified these conditions as optimal for induction of Akt phosphorylation and actin polymerization. Cells were then permeabilized on ice with a saponin-based permeabilization buffer (Becton Dickinson) for actin polymerization or a methanol-based buffer for Akt phosphorylation (Becton Dickinson) and subsequently incubated with either anti-phospho Akt-phycoerythrin (anti-ser473) (Becton Dickinson) to assess ligand-induced Akt activation, or fluorescein isothiocyanate-labeled phalloidin (Invitrogen) to measure actin polymerization. Cells were additionally stained with anti-CD3–PercP, anti-CD4–Pacific Blue, anti-CD8–PEcy5, anti-CD45RA–PEcy7, and anti-CD27–allophycocyanin after permeabilization. In some experiments, T cells were pre-treated with SIP receptor inhibitors FTY720 (Cayman, Ann Arbor, MI) or W146 (Avanti Polar Lipids, Alabaster, AL) for 18 hours or for 1 hour, respectively, prior to stimulation. To assess CD69 surface expression on S1P-stimulated T cells, the cells were pre-stained with anti-CD69–Alexa Fluor 700 (Becton Dickenson) prior to ligand exposure.
Measurement of S1P responses in T cells pre-activated in vitro
Platelets were depleted by incubation of human peripheral blood mononuclear cells with anti-CD61 microbeads (Miltenyi) and passage of cells through an Automacs Pro separator (Miltenyi). The negative fraction was collected and incubated in X-VIVO chemically defined serum-free medium for 48 hours or medium supplemented with plate-bound anti-CD3 (Becton Dickenson), 1000 U/mL interferon-α subtype 2a (Pestka Biomedical Laboratories, Piscataway, NJ), 100 ng/mL LPS (InvivoGen, San Diego, CA), 1 µg/mL Bacillus Subtillis Flagellin (InvivoGen), 3 ug/mL synthetic CpG oligonucleotides (CpG 7909, Coley Pharmaceuticals, Wellesley, MA), 25 ng/mL poly I:C, 1 ug/mL R848 (InvivoGen), 150 ng/mL aldrithiol-2 (AT-2) inactivated CL.4/SUPT1 CXCR4-tropic, and ADA-M/SUPT1 CCR5-tropic HIV-1 strains kindly provided by Dr. Jeffrey Lifson (National Cancer Institute, Frederick, MD). S1P and SDF-1α induced Akt phosphorylation and actin polymerization, which was then assessed by flow cytometry as previously described.
Triple immunoflouresence assay in LN tissue sections
Slides containing 5-μm LN tissue sections were incubated for 1 hour at 60°C. Slides were then placed in Xylene for 2 (5-minute) intervals, followed by dehydration in decreasing concentrations of ethanol at 5-minute intervals. After washing slides in dionized water, antigen retrieval was performed on slides in citrate buffer (10 mM) pH 5.0 in a pressure cooker for 30 seconds at 125°C. Tissue sections were encircled with a Papanicolaou pen and subsequently immersed in Sudan Black B (0.1% in 70% EtOH) (Sigma) for 30 minutes. Slides were then washed in TBS-T and blocked with Sniper Blocking reagent (Biocare Medical, Concord CA) for 60 minutes. Tissue sections were then incubated with mouse anti-CD3 (Dako 1:50) and rabbit anti-Edg1 clone H-60 (1:20) (Santa Cruz Biotechnology) in TBST +2% Sniper at 4°C overnight. Tissue sections were then washed in TBS-T three times and subsequently incubated with anti-mouse-Alexa 488, anti-rabbit-Alexa 555 (Invitrogen, 1:400), and DAPI (Invitrogen) in the dark at room temperature for 60 minutes. Tissue sections were washed in TBS-T 3 times and mounted with Aqua Poly/Mount (Polysciences Inc., Warrington, PA) Tissue sections were captured on an Aperio ScanScope (Vista, CA) slide scanner at 20×.
Statistics
Comparisons were made using the Mann-Whitney U test.
Ethics statement
All investigation was carried out according to Declaration of Helsinki principles.
Results
Human T-cell responses to S1P can be measured in vitro
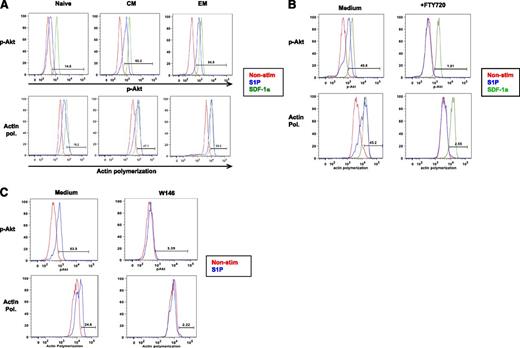
A major limitation to the study of S1P1 in humans has been its low level surface expression on human T cells. Using a murine monoclonal antibody reactive with the extracellular amino terminus of S1P1, we were unable to detect S1P1 on primary human lymphocyte surfaces by flow cytometry, although we could detect cell surface S1P1 on a transfected cell line (supplemental Figure 1A). Additionally, an antibody specific to the intracellular C-terminus of S1P1 was reactive with endothelial cells but not T cells on immunofluorescence staining of fixed human LN tissue sections (supplemental Figure 1B). Therefore, we developed assays to characterize S1P1 bioactivity in peripheral blood (PB) T cells by taking advantage of distinct signaling events induced after S1P1 ligation. S1P1 is thought to be exclusively coupled to G α i G-protein subunits, and in murine lymphocytes, S1P1 ligation drives polymerization of the actin cytoskeleton and phosphorylation of Akt.33 We assessed S1P-induced Akt phosphorylation at the Ser473 residue using a fluorochrome-labeled monoclonal antibody and monitored actin polymerization by staining with fluorochrome-tagged phalloidin, a molecule that binds to polymerized actin but not to actin monomers.34 To assess S1P1 responses relative to the activity of a chemotactic receptor expressed at detectable levels on T cells, CXCR4-dependent signaling was assessed after stimulation with SDF-1α. We found that freshly prepared peripheral blood T cells are unresponsive to S1P (not shown), however, after an 18-hour incubation period in a serum-free medium that contains no S1P, all circulating T-cell maturation subsets contained cells responsive to S1P, with both S1P-induced Akt phosphorylation and actin polymerization particularly robust among memory T cells (Figure 1A). As Akt phosphorylation and actin polymerization can be induced by signaling through multiple receptors, we next sought to determine if these readouts were specific for S1P1 after S1P exposure. Pre-treatment of PB T cells with the S1P-receptor functional antagonist, FTY720,35 completely abrogated both S1P-induced Akt phosphorylation and actin polymerization (Figure 1B). Similar results were obtained using the S1P1-specific competitive antagonist, W14636 (Figure 1C). Thus in this system, S1P responses reflect activity mediated solely by S1P1.
Monitoring S1P1 activity in human T-cells. S1P responses were measured by flow cytometric analysis in separated PB T-cells after 18 hours of incubation in serum-free medium. (A) PB T-cell subpopulations: naïve (CD45RA+, CD27+), central memory (CM) (CD45RA−, CD27+), effector memory (EM) (CD45RA−CD27−) cells identified after permeabilization with a PE-labeled phospho-Akt monoclonal antibody or fluorescein isothiocyanate-labeled phalloidin to detect actin polymerization. Responses to SDF-1a were assessed as a positive control. (B) PB T-cells were pre-treated with 250 nM FTY720 prior to assessment of S1P-induced actin polymerization and S1P-induced Akt phosphorylation. This experiment is representative of 3. (C) PB T-cells were pre-treated with 10 uM W146 for 1 hour prior to measurement of S1P-induced Akt phosphorylation and actin polymerization. This experiment is representative of 2. Actin pol., actin polymerization.
Monitoring S1P1 activity in human T-cells. S1P responses were measured by flow cytometric analysis in separated PB T-cells after 18 hours of incubation in serum-free medium. (A) PB T-cell subpopulations: naïve (CD45RA+, CD27+), central memory (CM) (CD45RA−, CD27+), effector memory (EM) (CD45RA−CD27−) cells identified after permeabilization with a PE-labeled phospho-Akt monoclonal antibody or fluorescein isothiocyanate-labeled phalloidin to detect actin polymerization. Responses to SDF-1a were assessed as a positive control. (B) PB T-cells were pre-treated with 250 nM FTY720 prior to assessment of S1P-induced actin polymerization and S1P-induced Akt phosphorylation. This experiment is representative of 3. (C) PB T-cells were pre-treated with 10 uM W146 for 1 hour prior to measurement of S1P-induced Akt phosphorylation and actin polymerization. This experiment is representative of 2. Actin pol., actin polymerization.
Lymph node T cells from HIV-1+ donors are less sensitive to S1P-mediated lymph node egress signals
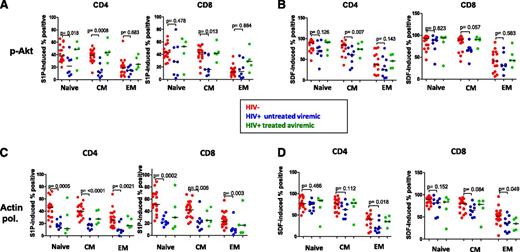
Next we prepared single cell suspensions from surgically removed lymph nodes of HIV-1− infected patients on or off therapy and uninfected controls. The cell suspensions were incubated for 18 hours in serum-free medium and were then examined for their responsiveness to S1P. In untreated, viremic HIV-1+ patients’ lymph nodes both CD4+ naïve and central memory T cells, as well as CD8+ central memory T cells had significantly diminished Akt phosphorylation responses after S1P exposure (Figure 2A). Diminished Akt phosphorylation was also seen in untreated viremic HIV-1+ patients’ central memory CD4+ T cells in response to SDF-1α (Figure 2B). On the other hand, the actin polymerization response to S1P was significantly impaired in all T-cell subpopulations in the untreated viremic HIV-1+ LNs (Figure 2C), whereas more subtle impairments in the SDF-1α response were demonstrable only in CD4 and CD8 EM T cells. This dissociation between Akt phosphorylation and actin polymerization responses to S1P indicates that a relatively preserved Akt phosphorylation response (such as that seen for example among EM cells) may not assure “normal” actin polymerization in response to S1P. Thus, LN T cells in uncontrolled HIV-1 infection demonstrate impairments in response to chemotactic signals provided by the interaction between S1P and its type 1 (S1P1) receptor, and this may especially compromise the efficiency with which these cells can exit inflammatory LNs. As can be seen in this figure, these impairments largely resolve or improve after control of HIV-1 replication with suppressive antiretroviral therapies.
Blunted responses to S1P in the HIV-1+ lymph node. S1P responses were assessed in cell suspensions prepared from lymph nodes of HIV-1+ patients and uninfected controls. (A) S1P-induced Akt phosphorylation. (B) SDF-induced Akt phosphorylation. (C) S1P-induced actin polymerization. (D) SDF-induced actin polymerization. Actin pol., actin polymerization; CM, central memory; EM effector memory,
Blunted responses to S1P in the HIV-1+ lymph node. S1P responses were assessed in cell suspensions prepared from lymph nodes of HIV-1+ patients and uninfected controls. (A) S1P-induced Akt phosphorylation. (B) SDF-induced Akt phosphorylation. (C) S1P-induced actin polymerization. (D) SDF-induced actin polymerization. Actin pol., actin polymerization; CM, central memory; EM effector memory,
The C-type lectin CD69 antagonizes S1P responsiveness in human LN T cells
In mice, CD69 can bind to S1P1, inducing its intracytoplasmic retention and degradation.28 To determine if this interaction might also take place in human T cells, we assessed S1P responses in CD69-negative and CD69-positive LN T cells. We find that despite strong responses of both CD69+ and CD69 cell populations to SDF-1α, CD69-expressing T cells fail to phosphorylate Akt and also fail to polymerize the actin cytoskeleton in response to S1P (Figure 3A). Thus, we find that human T cells expressing CD69 fail to respond to S1P mimicking findings in mice.27 Next we asked whether S1P responsiveness was also impaired in HIV-1+ LN T cells that lacked surface expression of CD69. In both HIV-1+ and uninfected control LNs, both CD4 and CD8+ T cells expressing CD69 failed to polymerize actin or phosphorylate Akt in response to S1P (Figure 3B). Yet even among the CD69 negative T-cell populations, both CD4+ and CD8+ T cells from the LNs of HIV-1+ viremic subjects had impaired Akt phosphorylation and actin polymerization in response to S1P (Figure 3B). In 3 treated patients who had controlled viremia, the responses to S1P among CD69 negative LN T cells were largely improved. Thus, although CD69 can antagonize S1P responsiveness in human LN T cells, these data suggest that mechanisms independent of CD69 expression also may contribute to reduced T-cell sensitivity to S1P in the LN in the setting of HIV-1 viremia.
CD69 expression and impaired T-cell responsiveness to S1P. Cell surface expression of CD69 and its relationship to S1P responsiveness was assessed in LN T-cells. (A) Impaired S1P induction of Akt phosphorylation and actin polymerization in CD69+ LN T-cells. This is representative of 13 samples from both HIV− and HIV+ donors. (B) S1P-induced Akt phosphorylation and actin polymerization in CD69 positive and CD69 negative LN cells. Actin pol., actin polymerization.
CD69 expression and impaired T-cell responsiveness to S1P. Cell surface expression of CD69 and its relationship to S1P responsiveness was assessed in LN T-cells. (A) Impaired S1P induction of Akt phosphorylation and actin polymerization in CD69+ LN T-cells. This is representative of 13 samples from both HIV− and HIV+ donors. (B) S1P-induced Akt phosphorylation and actin polymerization in CD69 positive and CD69 negative LN cells. Actin pol., actin polymerization.
In vitro immune activation attenuates T-cell responses to S1P
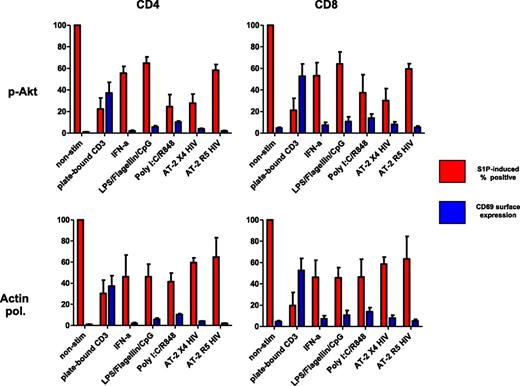
Because immune activation in HIV-1 infection has been linked to systemic exposure to both viral and bacterial elements,37,38 we asked whether in vitro exposure of PB cells to bacterial toll-like receptor (TLR) ligands, or to HIV-1, or to the type 1 interferons that these microbial products induce could affect T-cell sensitivity to S1P. As a positive control, cells were also activated with agonistic antibodies to CD3 and CD28. After exposure of peripheral blood cells to these agents, CD4 and CD8 T-cell Akt phosphorylation and actin polymerization in response to S1P were inhibited and CD69 surface expression tended to increase (Figure 4), suggesting that in vivo exposure to these factors might perturb the ability of T cells to exit lymphoid tissues efficiently. Notably, these same stimuli all failed to significantly affect responsiveness to SDF-1α (not shown).
Induction of CD69 and inhibition of S1P responses in human T cells in vitro. Platelet-depleted peripheral blood mononuclear cells were treated for 48 hours as noted and S1P-induced bioactivity and CD69 surface expression were assessed (data shown represent means and SD of 3 separate experiments). S1P responses are reported as percentage change relative to nonstimulated conditions. Actin pol., actin polymerization.
Induction of CD69 and inhibition of S1P responses in human T cells in vitro. Platelet-depleted peripheral blood mononuclear cells were treated for 48 hours as noted and S1P-induced bioactivity and CD69 surface expression were assessed (data shown represent means and SD of 3 separate experiments). S1P responses are reported as percentage change relative to nonstimulated conditions. Actin pol., actin polymerization.
Decreased expression of S1P1 in HIV-1+ LN tissues
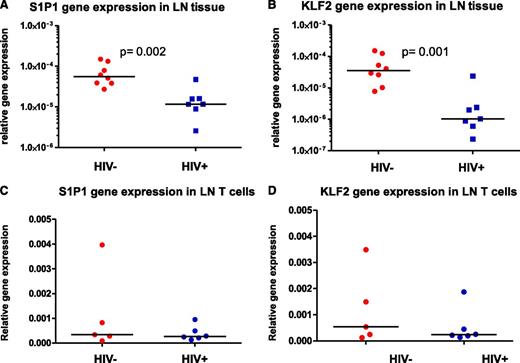
As our data indicated that T cells from the LNs of untreated, viremic subjects that lacked CD69 expression also showed impaired responses to S1P, we sought additional explanations for the defects we observed. We measured levels of S1P1 mRNA and found that S1P1 transcript levels were significantly decreased in HIV-1+ LN tissue sections from patients with uncontrolled viral replication when compared with levels in controls’ samples (Figure 5A). Also, because S1P1 transcription is controlled by KLF2, we also asked whether reduced KLF2 gene expression might underlie the diminished S1P1 mRNA levels we observed. This could be the case as KLF2 mRNA levels were also diminished in LNs from untreated, viremic HIV-1+ patients (Figure 5B). LN Tissue is comprised of a heterogenous mixture of cell types. To determine whether our findings were representative of gene expression in LN T cells, we assessed S1P1 and KLF2 mRNA levels in purified LN T cells. Despite reductions in S1P1 and KLF2 mRNA in the LN tissue, S1P1 and KLF2 mRNA levels in purified LN T cells of untreated, viremic HIV-1+ patients, and uninfected controls were not different (Figure 5C-D). According to our data, it is suggested that there is a reduction of S1P1 and KLF2 mRNA levels in lymph nodes of HIV-1+ patients with uncontrolled viral replication, but these reductions may not necessarily reflect mRNA levels of S1P1 and KLF2 in T cells and may reflect reductions in other cell types.
Diminished levels of S1P1 and KLF2 RNAs in HIV-1 + LNs. (A) S1P1 and (B) KLF2 gene expression in LN tissues was assessed by real-time PCR and normalized to levels of 18S rRNA. (C) S1P1 and (D) KLF2 gene expression in purified T cells was assessed by real-time PCR and normalized to levels of 18S rRNA.
Diminished levels of S1P1 and KLF2 RNAs in HIV-1 + LNs. (A) S1P1 and (B) KLF2 gene expression in LN tissues was assessed by real-time PCR and normalized to levels of 18S rRNA. (C) S1P1 and (D) KLF2 gene expression in purified T cells was assessed by real-time PCR and normalized to levels of 18S rRNA.
Discussion
The lymph node is an important site of immunopathology in HIV-1 infection. Generalized lymph node enlargement is common and this typically diminishes with disease progression as the inflammatory environment results in the deposition of collagen, disruption of lymph node architecture, and fibrosis.7,39 Even in the absence of clinically detectable lymph node enlargement, with application of antiretroviral therapies, there is rapid redistribution of lymphocytes from inflammatory LNs to the systemic circulation indicating that HIV-1 replication is associated with lymphocyte sequestration within these inflammatory sites.17 Yet the precise mechanisms of lymphocyte sequestration in the HIV-1+ nodes are not well characterized. Other viral infections can involve a transient shutdown of T-cell egress from lymphoid tissues27 that in humans is associated with lymph node enlargement, yet in these settings the determinants of lymphadenopathy are also not well characterized. Temporary egress inhibition is thought to facilitate immune priming, increasing the likelihood of antigen encounter.40 Yet sustained dysregulated lymphocyte sequestration in lymph nodes could be detrimental in chronic HIV-1 infection, in which lymphoid tissues are a major site of viral replication, and the inflammatory environment may disrupt immune homeostasis. Thus, we set out to explore possible determinants of lymphocyte sequestration within HIV-1+ LNs.
T-cell egress from lymphoid tissues is thought to be governed principally by S1P1 ligation. Yet, there are not any robust methods to study S1P1 activity in humans. Immunologic methods to identify cell surface expression are limited by low expression levels and chemotaxis assays have been reported,9 but migratory responses to S1P have been low and difficult to quantify, perhaps because the key T-lymphocyte S1P receptor, S1P1 is desensitized immediately after ligation.41 We report here the application of novel assays that allow robust, rapid, and reliable detection of S1P1-dependent responses to S1P in human T cells. We show here that we can measure bioactivity of S1P1 through the phosphorylation of Akt and polymerization of the actin cytoskeleton.
In these studies, responses to S1P can be demonstrated in all T-cell maturation subsets, but demonstrating this response typically requires ex vivo incubation of these cells in S1P-free medium. In blood samples, this is likely a consequence of sustained exposure to plasma levels of S1P prior to preparation; it is less clear why human lymph node cells also characteristically fail to respond to S1P immediately after preparation. In mice, freshly-isolated LN T cells are also typically unresponsive to S1P stimulation but regain responsiveness after a period of incubation in S1P-deprived medium.42 It has been suggested that subnanomolar concentrations of S1P are found within lymphoid interstitial spaces,40 and this may suffice to desensitize lymph node T cells to S1P during the isolation process. In our culture conditions, we did not measure S1P in culture supernatants; therefore, we cannot rule out the possibility that S1P may be present still within the medium during this incubation period. However, we can be confident that our culture conditions are sufficient to allow T cells to become resensitized to S1P after this culture period.
Here we show that T-cell responses to S1P are diminished in the HIV-1+ lymph node in uncontrolled infection. Although both CD4+ and CD8+ central memory T cells and naïve CD4+ T cells have diminished Akt phosphorylation responses to S1P, modest impairments are also seen in response to SDF-1 among naïve and central memory CD4+ T cells. In untreated infection, actin polymerization responses to S1P are impaired substantially in all LN T-cell maturation subsets, whereas SDF-1-induced actin polymerization is decreased only modestly and only within the effector memory compartment. It is not clear why these 2 readouts of S1P1 activity were not completely concordant. They might be differentially regulated after S1P1 ligation or additional signals may be necessary to optimally polymerize actin. Further studies will be necessary to define this defect in more detail. Nonetheless, our data demonstrate that in the HIV-1 infected lymph node, T cells have demonstrable impairment in the activity of a chemotactic receptor that in murine systems is necessary for return of lymphocytes to the systemic circulation. We propose that this defect underlies the recognized sequestration of T cells within the inflammatory nodes in HIV-1 infection.
Trafficking of T cells in and out of lymphoid tissues is kept at a delicate balance between signals that mediate T-cell ingress and signals that mediate T-cell egress. It is conceivable that impairments in lymphocyte ingress could also contribute to the lymphadenopathy seen in HIV-1 infection. The CCL19 and CCL21 chemokines promote T-cell entry into lymphoid tissues in a CCR7 dependent manner, and these chemokines have been reported to be increased in untreated HIV-1+ patients and treated patients with immune failure.43 Additionally, in untreated HIV-1 infection, lymphoid tissues are enriched for CXCR3+ effector CD8 T cells,44 and the infiltration of these inflammatory CD8 T cells into sites from which they are normally excluded may also contribute to HIV-1 associated lymphadenopathy.
S1P1 activity can be regulated transcriptionally and posttranslationally. Here, we confirm that in human lymphoid tissues, the observation made in murine systems that CD69 expression identifies cells that cannot respond to S1P. Yet, we also find that even CD69 negative T cells from HIV-1 infected lymph nodes demonstrate an impaired response to S1P. Although we observe decreased RNA levels of S1P1 and its transcription factor KLF2 in whole LN tissues from HIV-1 infected subjects, we could not confirm these findings in purified T cells from these nodes Thus, we do not have a clear understanding as to why there is an impairment in S1P1 function in CD69− cells in the HIV-1 infected LN. We also do not know yet which cell types within the HIV-1+ LN tissue are responsible for the reductions of S1P1 and KLF2 gene expression that we observed. IHC assays demonstrate S1P1 expression by endothelial cells within lymphatic tissues (supplemental Figure 1), and in mice endothelial cell S1P1 dampened cytokine storm and innate immune cell recruitment during infection with a pathogenic human influenza virus.45 Thus, the role of endothelial S1P1 within chronically inflamed HIV-1+ lymph nodes merits further investigation.
How might reductions in S1P responsiveness take place? We show here that a number of immune activating signals (T-cell receptor engagement, exposure to type 1 interferon, to bacterial TLR ligands and to HIV-1) that are each known or proposed to drive immune activation in the setting of chronic HIV-1 infection tend to increase T-cell expression of CD69 and block S1P-induced Akt phosphorylation and actin polymerization. In mice, the double stranded viral RNA TLR-3 ligand poly I:C inhibits LN egress of T cells, regardless of their antigen reactivity.27 Thus, ongoing viral replication within these sites may induce a nonspecific inhibition of T-cell egress from the HIV-1 infected lymph node. When viral replication is controlled by antiretroviral therapies, there are dramatic reductions in a number of inflammatory mediators within lymphatic tissues.46 Here, we show that antiretroviral treatment is also associated with improvements in S1P responses in LN T cells.
There are limitations to this study. All control nodes were obtained during pelvic surgeries among women while nodes from all the HIV-1+ subjects were peripheral and these subjects included men and women who tended to be younger than the uninfected controls. We could not see an effect of age or gender on S1P1 responses (not shown). Because our uninfected control group was comprised of only lymph nodes from female donors, we were unable to assess an effect of gender on S1P responses. Nonetheless, the improvement in S1P responses after suppression of HIV-1 replication provides reasonable assurance that the defects we observed in untreated infection is largely either directly or indirectly related to viral replication and not so much to other differences in the populations studied.
The controlled movement of T cells into and out of lymphoid tissues is essential for maintaining proper immune homeostasis. Manipulation of this cellular process has profound immunomodulatory effects that have recently been applied in a clinical setting, as the S1P receptor pharmacological agonist, FTY720, is approved for the treatment of patients with multiple sclerosis and is associated with profound circulating lymphopenia, confirming that S1P receptor blockade interferes with systemic lymphocyte trafficking in humans.31 Our identification of cellular assays that reflect T-lymphocyte S1P1 bioactivity provides novel methods that now permit an exploration of this important system in human health and disease.
In HIV-1 infection, disruption of T-cell egress from lymph nodes could have profound implications for disease progression, as well as for the generation of immune responses to new and recall antigens.47,48 In mice chronically infected with lymphocytic choriomeningitis, prolonged lymph node egress shutdown through FTY720 treatment attenuated CD8 T-cell antiviral activity and led to higher levels of virus.49 Thus, improving T-cell responses to signals that control lymphocyte trafficking from the HIV-1 infected lymph node might improve both vaccine responses and disease outcome through ameliorating immune dysregulation in a key immunological organ.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jim Milligan at Merck Research Laboratories for an S1P1 C-terminal antibody, Markus Graeler at University of Hannover for S1P1-transfected cell lines, Jeffrey Lifson (National Cancer Institute, Frederick) for aldrithiol-2 inactivated HIV-1 viruses. The authors also thank the subjects who participated in this study and Suzanne Crowe (Burnett Institute, Melbourne), Volker Brinkmann (Novartis Pharmaceuticals), and members of the Cleveland Immunopathogenesis Consortium for their helpful discussions.
This work was supported by grants from the National Institutes of Health (AI 076174), the Case Western Reserve University Center for AIDS Research (AI 36219), and the Fasenmyer Foundation.
Authorship
Contribution: J.C.M., P.M., M.M., G.A.H., and J.A. performed the research; J.C.M., S.F.S., B.R., and M.M.L. designed the research and analyzed the data; C.V.H., G.H.M., M.K.J., E.K.H., and R.A.C. provided helpful guidance; J.C.M. and M.M.L. wrote the manuscript that was reviewed and approved by all authors; T.W.S., S.L., A.D.B., J.M.J., R.D., and J.H. provided lymph node specimens.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael M. Lederman, 2109 Adelbert Rd, Case Western Reserve University, Cleveland, OH 44106; e-mail: mxbl6@case.edu.