Key Points
Therapeutic DCs stimulated via NK1R stimulate type 1–biased immunity.
Abstract
Substance-P and hemokinin-1 are proinflammatory neuropeptides with potential to promote type 1 immunity through agonistic binding to neurokinin-1 receptor (NK1R). Dendritic cells (DCs) are professional antigen-presenting cells that initiate and regulate the outcome of innate and adaptive immune responses. Immunostimulatory DCs are highly desired for the development of positive immunization techniques. DCs express functional NK1R; however, regardless of their potential DC-stimulatory function, the ability of NK1R agonists to promote immunostimulatory DCs remains unexplored. Here, we demonstrate that NK1R signaling activates therapeutic DCs capable of biasing type 1 immunity by inhibition of interleukin-10 (IL-10) synthesis and secretion, without affecting their low levels of IL-12 production. The potent type 1 effector immune response observed following cutaneous administration of NK1R-signaled DCs required their homing in skin-draining lymph nodes (sDLNs) where they induced inflammation and licensed endogenous-conventional sDLN-resident and -recruited inflammatory DCs to secrete IL-12. Our data demonstrate that NK1R signaling promotes immunostimulatory DCs, and provide relevant insight into the mechanisms used by neuromediators to regulate innate and adaptive immune responses.
Introduction
The immune and nervous systems are far more interconnected than originally thought. They sense and respond to danger, release shared mediators, and generate and store memory. Based on these similarities, the immune system has been considered “the sixth sense”.1-3 Our knowledge on neuroimmunology comes mainly from studies analyzing the effects of immune cells on the central nervous system, with limited information regarding the immune-regulatory mechanisms of the peripheral nervous system.4-6
As professional antigen (Ag)–presenting cells (APCs) of the immune system, dendritic cells (DCs) play crucial roles in induction of T-cell immunity and tolerance. Accordingly, the possibility of administering therapeutic DCs to regulate immunity in an Ag-specific manner has revolutionized the field of translational immunology over the past 2 decades.7 Numerous methodologies have been developed to generate DCs ex vivo that mimic the functions of tissue-resident or -recruited DCs, with the intent to activate or suppress specific immunity.7-11 In vitro–generated and/or –engineered therapeutic DCs are highly desired for positive vaccination against intracellular pathogens or tumors, and for negative immunization protocols to treat autoimmune disorders or transplant rejection, respectively.12,13
Considering the importance of neuropeptides in the regulation of immunity, the lack of information regarding their role on the biology of therapeutic DCs is surprising. Substance P (SP), a prototypical proinflammatory neuropeptide of the tachykinin family secreted by δ-fibers, is crucial for induction of the neuroinflammatory reflex and perpetuation of inflammation and autoimmunity.14-16 Likewise, the more recently described hemokinin-1 (HK-1) has strong proinflammatory effects.17 These tachykinins exert their effects by binding with high affinity to the neurokinin-1 receptor (NK1R), a 7 trans-membrane domain G-protein–coupled receptor expressed as either a full-length or a nonfunctional truncated isoform.18,19
In a previous publication, we demonstrated that skin Langerhans cells and bone marrow (BM)–derived DCs (BMDCs) express functional NK1R, and respond to NK1R agonists with increased cell survival and migration to skin-draining lymph nodes (sDLNs).20,21 These immunologic effects suggest that NK1R agonists could be used as adjuvants for enhancing the efficiency of DC vaccines. Here, we demonstrate that conditioning BMDCs with NK1R agonists, leads to their maturation and abrogation of interleukin-10 (IL-10) release without inducing IL-12p70 secretion. Inhibition of IL-10 occurs via blockade of the CREB1/TORC2 signaling pathway. Following subcutaneous administration as a cellular vaccine, Ag-loaded and NK1R-signaled BMDCs (Ag-NK1R-DCs) homed in sDLNs, promoting inflammation and potent CD4 T helper (Th1) and CD8 (Tc1)–mediated immunity. Generation of type 1 immunity in vivo required IL-12p70 from host lymph node (LN) DCs, including resident and recruited inflammatory DCs. These 2 DC subsets were activated in situ subsequently to the homing in the sDLNs of the injected BMDCs. Mechanistic studies demonstrated that the reduced levels of IL-10 produced by NK1R-DCs licensed IL-12 secretion by host DCs resulting in potent type 1 immunity.
Methods
Mice
Eight- to 12-week-old wild-type (WT) C57BL/6, C57BL/6-129S1-Il12atm1Jm/J (IL-12p35KO), -BFVB-Tg(Itgax-DTR/EGFP)57Lan/J (CD11c-DTR), -Tg(TcraTcrb)1100Mjb/J (OT-I), and CCR2KO mice were purchased from The Jackson Laboratory, housed in our pathogen-free facility, and used according to institutional animal care and use committee guidelines.
Generation and stimulation of BMDCs
BMDCs were generated as described21 and stimulated for 18 hours with: (1) the NK1R agonists SP, HK-1, (10−5 or 10−9M; Bachem), or [Sar9Met (O2)11]-SP (10−9M; Sigma-Aldrich), (2) lipopolysaccharide (LPS) (500 ng/mL; Sigma-Aldrich), or (3) agonist CD40 antibody (Ab) (clone HM40-3, 10 μg/mL; BD PharMingen), and pulsed or not with ovalbumin (OVA) + OVASIINFEKL peptide (1.0 mg/mL and 100 ng/mL, respectively) (Sigma-Aldrich) for 2 hours.
Analysis of NK1R-signaling pathways in BMDCs
For reporter assays, BMDCs were transfected by electroporation (1700 V, 20 msec, Neon device; Invitrogen) with pTAL-Luc (negative control), pNF-κB-Luc, pCREB-Luc, or pCMV-Luc (Mercury System Vectors; Clontech) and treated (18 hours, or not, control) with [Sar9Met (O2)11]-SP, LPS, or CD40 Ab. Luciferase was analyzed by luminometry as previously described.20,22 Quantitative reverse transcription–polymerase chain reaction (RT-PCR) was performed with commercially available primers specific for CREB1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (SABioscience).21 Relative quantitation of expression was determined by extrapolation to standard curve of complementary DNA from mouse brain. Data were normalized to the housekeeping gene GAPDH.
For western blot analysis of TORC2, cytoplasmic and nuclear proteins were extracted from BMDCs using the NE-PER Nuclear Protein Extraction Kit (Thermo Scientific). Lysates were quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific). Seventy-five micrograms of total protein were reduced with 2× Laemmli sample buffer containing 100mM dithiothreitol (10 minutes, 70°C). Protein samples were then resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in a Kd precast gradient gel (Bio-Rad) and transferred onto low-fluorescence polyvinylidene difluoride membranes (Thermo Scientific). Blots were blocked with Odyssey Blocking buffer (Licor) (1 hour, room temperature [RT]) before probing with primary Ab (overnight, 4°C). Proteins were visualized with secondary Ab conjugated to IRDye (1 hour, RT), and scanned using Odyssey Imaging System (Licor). The following Abs were used: rabbit anti–β-actin, rabbit anti-histone 3 (Cell Signaling Technology), and rat anti-TORC2 (R&D Systems). Band densities were quantified using Image Studio Software (version 2; Licor).
Analysis of BMDC phenotype and cytokines
The BMDC phenotype was analyzed in untreated BMDCs or BMDCs cultured for 18 hours with the NK1R agonist [Sar9Met (O2)11]-SP (NK1R-DCs), or OVA + OVASIINFEKL for the last 2 hours (Ag-DCs), or with both treatments (Ag-NK1R-DCs). Next, BMDCs were stained with Alexa Fluor 488–CD11c Ab, and phycoerythrin (PE)–CD11a, -CD11b,-CD18,-CD40, -CD54, -CD80,-CD83, -CD86, -CD102, -CD209, -major histocompatibility complex (MHC) class II (IAb), -CCR7 Ab, or with immunoglobulin (Ig) isotype controls (BD PharMingen), and analyzed by flow cytometry.
For intracellular detection of cytokines in vivo, untreated DCs, Ag-DCs, or Ag-NK1R-DCs (transduced or not with recombinant adenovirus [RAd]–IL-10) were injected (2 × 106 per footpad) in WT or CCR2KO C57BL/6 mice. Intracellular cytokines were analyzed by flow cytometry as detailed.23 Briefly, cells were surface-labeled with PE-Cy7-CD11c (BD PharMingen), Pacific Blue–CD11b (Biolegend), Alexa Fluor 700–CD8α, and PerCP-Cy5.5-Ly6C Ab (eBioscience). After fixation/permeabilization, cells were labeled with PE-IL-12p40/70, tumor necrosis factor-α (TNF-α) (BD PharMingen), or -inducible nitric oxide synthase 2 (iNOS2) Ab (Santa Cruz Biotechnology) and analyzed by flow cytometry. For cytokine secretion, BMDCs were cultured with [Sar9Met (O2)11]-SP or LPS (18 hours). Secretion of IL-12p70 and IL-10 was assessed in DC culture supernatants by enzyme-linked immunosorbent assay (ELISA).20
In vitro Ag-presentation assays
Splenic OT-I-CD8 T cells were purified by negative selection (R&D Systems). Ag-DCs, NK1R-DCs, or Ag-NK1R-DCs generated form WT or CCR7KO C57BL/6 mice were cocultured with OT-I CD8 T cells (1:20 DC:T-cell ratio, for 3 days). Controls included cocultures of T cells with untreated DCs, or T cells alone. For some experiments, cultures were supplemented with neutralizing Ab against CD40 ligand (MR-1, 10 μg/mL; ISC BioExpress) or interferon-γ (IFN-γ) (20 μg/mL; BD PharMingen). Experiments analyzing T-cell proliferation were performed as described.20 Secretion of IFN-γ (BD PharMingen), IL-5, IL-13, and IL-12p70 (eBioscience) were analyzed by ELISA.20,21
Induction of OVA-specific T cells in vivo
Ag-DCs, NK1R-DCs, or Ag-NK1R-DCs generated from C57BL/6 mice were injected (2 × 106, footpad) in C57BL/6 mice (n = 6 per group). Controls included untreated mice and mice immunized with untreated DCs. Five days later, mice were killed, and IFN-γ, IL-5, and IL-13 secretion by OVA-specific CD4 T cells in response to ex vivo restimulation of splenocytes with OVA was quantified in culture supernatants by ELISA (BD PharMingen, eBioscience) as described.20 After skin immunization, Ag-specific in vivo killing assays and detection of cytokine secretion by specific CD8 T cells after ex vivo Ag restimulation was conducted as previously described.20
In vivo depletion of DCs
C57BL/6 CD11c-DTR mice were injected with diphtheria toxin (DT) (4 ng/g body weight) or phosphate-buffered saline (PBS) (control), 1 day before immunization (footpad) with Ag-DCs, NK1R-DCs, or Ag-NK1R-DCs. Ag-specific secretion of IFN-γ by splenic CD4 T cells was evaluated in enzyme-linked immunospot (ELISPOT) assays, and Ag-specific cytotoxic T lymphocyte (CTL) function was assessed by in vivo killing assays, as published.20
RAd transduction of BMDCs
BMDCs were infected with RAd–IL-10, or with RAd-Empty (multiplicity of infection = 100) as published.22 RAd transduction was assessed by ELISA (BD PharMingen) based on the amount of IL-10 secreted into culture supernatants over a 24-hour period from RAd-transduced BMDCs.
Microscopic analysis
Inguinal lymph nodes (LNs) from mice untreated or BMDC-immunized (footpad) were fixed in 4% formaldehyde, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS). For characterization of leukocyte subsets, LNs were snap-frozen20 sectioned and stained with Alexa Fluor 488–CD11c Ab, Alexa Fluor 647–CD11b Ab, or Alexa Fluor 647–CD3 Ab, and biotin-Ly6C followed by streptavidin-Cy3 (eBioscience, BD PharMingen, Jackson ImmunoResearch).
For delayed-type hypersensitivity (DTH) assays, skin samples were processed for H&E staining or labeled with biotin-CD3 Ab or biotin-F4/80 Ab, followed by streptavidin-peroxidase. Microscopic images were examined with an Axiostar plus microscope (Zeiss), equipped with epifluorescence and a digital camera (AxioCam Inc, Zeiss). Images were analyzed with AxioVision (Zeiss) and Adobe Photoshop CS4 software.
DTH assays
C57BL/6 mice (6 per group) were sensitized (footpad) or not with 1 dose (2 × 106) of Ag-DCs, or Ag-NK1R-DCs, infected or not with RAd–IL-10. After 7 days, mice were injected (intradermally) on the dorsal side of the ears with OVA (1 mg/50 μL per PBS). The severity of the DTH was assessed as previously described.20,21
Statistical analysis
Differences between more than 2 means ± 1 SD were compared with a 1-way analysis of variance followed by a Student Newman Keuls test. Comparisons between 2 different means ± 1 SD were performed by a Student t test. A P value < .05 was considered significant. Statistical analysis was performed using GraphPad Prism 4 software (GraphPad Software).
Results
NK1R signaling promotes mature BMDCs unable to secrete IL-10
The finding that proinflammatory neuropeptides are released at high concentrations in inflammatory and autoimmune disorders prompted us to explore their potential use as adjuvants in the generation of immunostimulatory DCs for the purpose of future cell vaccine development.24,25 To assess whether signaling through NK1R increases BMDC maturation and T-cell stimulatory functions, BMDCs were left untreated (DC, control) or stimulated via NK1R (NK1R-DCs) with the synthetic and exclusive NK1R agonist [Sar9Met (O2)11]-SP (SarSP),22 or the naturally occurring NK1R agonists SP and HK-1 (the latter not shown). Untreated DCs or NK1R-DCs were next pulsed or not with OVA + OVASIINFEKL (Ag-DCs and Ag-NK1R-DCs, respectively). Regardless of the presence of Ag, signaling of BMDCs with NK1R agonists increased their expression of MHC class II (IAb), activation (CD83), and costimulatory (CD80, CD86, and CD40) molecules (Figure 1A-B). The secretion of the proinflammatory cytokines IL-1β, IL-6, TNF-α (not shown), or IL-12p70 was not altered (Figure 1C); however, NK1R-DCs did not secrete anti-inflammatory IL-10 (Figure 1C). Moreover, NK1R signaling inhibited IL-10 secretion by BMDCs cultured with LPS, included as positive stimulus to promote secretion of IL-10 and IL-12p70 simultaneously by DCs26,27 (Figure 1C). Thus, NK1R signaling induces maturation of BMDCs that lack the ability to secrete IL-10 without altering their secretion of proinflammatory cytokines.
NK1R-DCs are mature DCs that do not secrete IL-10. (A-B) Expression of Ag- presenting (MHC class II, IAb), activation (CD83), and costimulatory (CD80, CD86, and CD40) molecules by untreated DCs, Ag-DCs, NK1R-DCs, and Ag-NK1R-DCs, analyzed by flow cytometry. (A) Percentages of positive DCs, (B) intensity of expression of each marker, as MFI. Means ± 1 SD of 3 independent experiments are shown. (C) Secretion of IL-12p70 and IL-10 (analyzed by ELISA) by untreated DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs (the latter cultured with or without LPS). Means ± 1 SD from 3 independent experiments are displayed. MFI, mean fluorescence intensity.
NK1R-DCs are mature DCs that do not secrete IL-10. (A-B) Expression of Ag- presenting (MHC class II, IAb), activation (CD83), and costimulatory (CD80, CD86, and CD40) molecules by untreated DCs, Ag-DCs, NK1R-DCs, and Ag-NK1R-DCs, analyzed by flow cytometry. (A) Percentages of positive DCs, (B) intensity of expression of each marker, as MFI. Means ± 1 SD of 3 independent experiments are shown. (C) Secretion of IL-12p70 and IL-10 (analyzed by ELISA) by untreated DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs (the latter cultured with or without LPS). Means ± 1 SD from 3 independent experiments are displayed. MFI, mean fluorescence intensity.
NK1R-DCs promote cellular immunity that requires IL-12p70
Next, we tested whether NK1R agonists generate BMDCs that stimulate type 1–biased immunity in vivo. WT C57BL/6 mice were immunized (footpad) with untreated DCs (control), NK1R-DCs, Ag-DCs, or Ag-NK1R-DCs. Immunization with Ag-DCs elicited a mixed Th1/Th2 response, and weak Tc1/CTL immunity (Figure 2). In contrast, Ag-NK1R-DCs promoted robust Th1 (Figure 2A) and Tc1 (Figure 2B) responses, and significantly superior CD8 CTL function (Figure 2C).
NK1R signaling increases the capacity of BMDCs to elicit type 1 immunity. (A-B) Cytokine secretion (analyzed by ELISA) of splenic (A) CD4 or (B) CD8 T cells obtained from C57BL/6 mice 7 days after footpad immunization (1 dose) with untreated DCs, NK1R-DCs, Ag-DCs, or Ag-NK1R-DCs. (C) Analysis of Ag-specific CTL function by in vivo killing assays, 7 days after footpad immunization (1 dose) of C57BL/6 mice with untreated DCs, NK1R-DCs, Ag-DCs or Ag-NK1R-DCs. (A-B) Means ± 1 SD of triplicates from 1 of 3 experiments is shown. (C) Means ± 1 SD from 6 mice per experimental group are shown.
NK1R signaling increases the capacity of BMDCs to elicit type 1 immunity. (A-B) Cytokine secretion (analyzed by ELISA) of splenic (A) CD4 or (B) CD8 T cells obtained from C57BL/6 mice 7 days after footpad immunization (1 dose) with untreated DCs, NK1R-DCs, Ag-DCs, or Ag-NK1R-DCs. (C) Analysis of Ag-specific CTL function by in vivo killing assays, 7 days after footpad immunization (1 dose) of C57BL/6 mice with untreated DCs, NK1R-DCs, Ag-DCs or Ag-NK1R-DCs. (A-B) Means ± 1 SD of triplicates from 1 of 3 experiments is shown. (C) Means ± 1 SD from 6 mice per experimental group are shown.
Because our results indicate that in spite of producing negligible levels of IL-12p70, Ag-NK1R-DCs initiate type 1 immunity. We investigated whether this response required IL-12p70, and if so, which was the cellular source secreting IL-12p70. To address this question, we vaccinated (footpad) WT or IL-12p35KO C57BL/6 mice with untreated DCs (control), Ag-DCs, or Ag-NK1R-DCs generated from either WT or IL-12p35KO C57BL/6 mice. These experiments demonstrated that IFN-γ secretion by CD4 T cells and the CTL function mediated by CD8 T cells required IL-12p70 (Figure 3A-B). In contrast to an IL-12–independent mechanism, the type 1 responses observed required IL-12p70 and decreased significantly in host IL-12p35KO mice injected with WT DCs, demonstrating that IL-12p70 was produced mainly by host cells and not the injected DC vaccine (Figure 3A-B). The critical role of host-derived IL-12p70 in our model was further confirmed by comparing the Th1 responses elicited in WT mice immunized with WT or IL-12p35KO untreated DCs (control), Ag-DCs, or Ag-NK1R-DCs. WT and IL-12p35KO Ag-DCs elicited similar levels of IFN-γ secretion from Ag-specific CD4 T cells and these levels were equally increased by immunizations with WT- or IL-12p35KO Ag-NK1-DCs (Figure 3C).
Type 1 immunity elicited by footpad immunization with NK1R-DCs requires IL-12p70 released by host DCs. (A) Secretion of IFN-γ (analyzed by ELISA) by splenic CD4 T cells from WT or IL-12p35KO C57BL/6 mice 7 days after footpad immunization (1 dose) with untreated DCs, Ag-DCs, or Ag-NK1R-DCs. (B) Percentage of Ag-specific CTL function (in vivo killing assays) in WT or IL-12p35KO C57BL/6 mice, 7 days following footpad immunization (1 dose) as described in panel A. (C) Secretion of IFN-γ (analyzed by ELISA) by splenic CD4 T cells isolated from WT mice, 7 days after footpad immunization (1 dose) with WT or IL-12p35KO untreated DCs, Ag-DCs, or Ag-NK1R-DCs. (D) Assessment of IFN-γ–secreting CD4 T cells (by ELISPOT) from spleens of CD11c-DTR mice, depleted or not of host DCs with DT injection 1 day before immunization, and analyzed 7 days following footpad immunization. (E) Percentage of Ag-specific CTL activity (determined using in vivo killing assays) in CD11c-DTR mice treated or not with DT 1 day before administration of untreated DCs, Ag-DCs, or Ag-NK1R-DCs, analyzed 7 days after vaccination. Means ± 1 SD from 5 mice per group are shown. NS, not significant difference.
Type 1 immunity elicited by footpad immunization with NK1R-DCs requires IL-12p70 released by host DCs. (A) Secretion of IFN-γ (analyzed by ELISA) by splenic CD4 T cells from WT or IL-12p35KO C57BL/6 mice 7 days after footpad immunization (1 dose) with untreated DCs, Ag-DCs, or Ag-NK1R-DCs. (B) Percentage of Ag-specific CTL function (in vivo killing assays) in WT or IL-12p35KO C57BL/6 mice, 7 days following footpad immunization (1 dose) as described in panel A. (C) Secretion of IFN-γ (analyzed by ELISA) by splenic CD4 T cells isolated from WT mice, 7 days after footpad immunization (1 dose) with WT or IL-12p35KO untreated DCs, Ag-DCs, or Ag-NK1R-DCs. (D) Assessment of IFN-γ–secreting CD4 T cells (by ELISPOT) from spleens of CD11c-DTR mice, depleted or not of host DCs with DT injection 1 day before immunization, and analyzed 7 days following footpad immunization. (E) Percentage of Ag-specific CTL activity (determined using in vivo killing assays) in CD11c-DTR mice treated or not with DT 1 day before administration of untreated DCs, Ag-DCs, or Ag-NK1R-DCs, analyzed 7 days after vaccination. Means ± 1 SD from 5 mice per group are shown. NS, not significant difference.
To investigate whether the source of IL-12p70 in our model was host DCs, endogenous CD11c+DCs were depleted in CD11c-DT receptor (DTR) transgenic C57BL/6 mice by DT injection 1 day prior immunization with untreated DCs, Ag-DCs, or Ag-NK1R-DCs. Depletion of endogenous DCs significantly decreased IFN-γ secretion by responder CD4 T cells (Figure 3D), and CTL function of CD8 T cells (Figure 3E). Together, our data indicate that type 1 immunity induced by Ag-NK1R-DCs requires IL-12p70 secreted by host DCs and not by the DC vaccine.
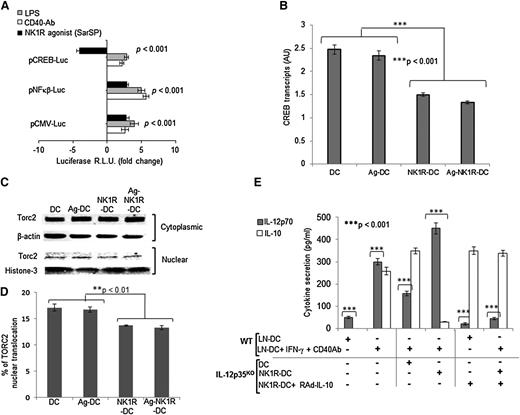
NK1R agonists decrease IL-10 transcription in BMDCs by inhibition of CREB1/TORC2 signaling
Our data suggest that generation of type 1 immunity by NK1R-DCs was caused, at least in part, by secretion of IL-12p70 released by host DCs following abrogation of IL-10 secretion by the DC vaccine. Therefore, we analyzed the signaling pathways that may be involved in these regulatory mechanisms. We used reporter assays to investigate the possible role of CREB1 in modulating Il-10 gene transcription in NK1R-stimulated BMDCs. Untreated or NK1R-DCs were transfected with plasmids encoding luciferase (Luc) under the control of a CREB1- or a nuclear factor κB (NFκB)–inducible promoter (pCREB1-Luc and pNFκB-Luc, respectively) (NFκB is a factor necessary for DC maturation which is inhibited by IL-10). Expression of reporter Luc in this approach requires activation and nuclear translocation of CREB-1 and NFκB, respectively. Positive controls included BMDCs stimulated with LPS or CD40 ligation, and BMDCs transfected with pCMV-Luc (the cytomegalovirus [CMV] promoter contains both CREB1 and NFκB enhancer elements).22 NK1R-DCs displayed reduced Luc expression if transfected with pCREB1-Luc, and significantly augmented Luc expression if transfected with plasmids containing NFκB or CMV promoters (Figure 4A). As expected, signaling DCs with LPS and via CD40 ligation induced high expression of Luc under control of all the promoters selected (Figure 4A). Reduction of CREB1 levels in NK1R-DCs was further investigated by quantitative RT-PCR, which confirmed that agonistic signaling of BMDCs via NK1R decreases significantly CREB1 transcripts (Figure 4B).
NK1R-DCs downregulate CREB1/TORC2 nuclear translocation resulting in decreased IL-10 and increased IL-12p70 secretion by LN-DCs. (A) Expression of luciferase (relative luminic units) by BMDCs transfected with plasmid DNA encoding the reporter protein under the transcriptional control of CREB1-, NFκB-binding enhancer elements, or the CMV promoter. BMDCs were left untreated (control) or cultured with the NK1R agonist SarSP, LPS, or agonist CD40 Ab. (B) Expression of CREB transcripts analyzed by quantitative RT-PCR in untreated-DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs. Means ± 1 SD of triplicates of 1 representative experiment of 2 are illustrated. (C-D) Negative regulation of TORC2 translocation in BMDCs nuclei by NK1R signaling. (C) Western blot analysis of TORC2 content in cytoplasmic and nuclear protein lysates prepared from untreated-DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs, resolved by SDS-PAGE, and probed for TORC2. (D) Percentages TORC2 translocation based on normalized density values of 3 independent experiments. (E) Secretion of IL-12p70 and IL-10 (by ELISA) in 24-hour supernatants of cocultures of (1) IL-12p35KO BMDCs, left untreated (DC), NK1R-DCs, the latter transduced or not with RAd–IL-10, and (2) DCs freshly-isolated from LNs (LN-DC) stimulated or not (control) with IFN-γ and agonist CD40 Ab. (A-E) Means ± 1 SD of triplicates from 1 representative experiment of 3 are shown.
NK1R-DCs downregulate CREB1/TORC2 nuclear translocation resulting in decreased IL-10 and increased IL-12p70 secretion by LN-DCs. (A) Expression of luciferase (relative luminic units) by BMDCs transfected with plasmid DNA encoding the reporter protein under the transcriptional control of CREB1-, NFκB-binding enhancer elements, or the CMV promoter. BMDCs were left untreated (control) or cultured with the NK1R agonist SarSP, LPS, or agonist CD40 Ab. (B) Expression of CREB transcripts analyzed by quantitative RT-PCR in untreated-DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs. Means ± 1 SD of triplicates of 1 representative experiment of 2 are illustrated. (C-D) Negative regulation of TORC2 translocation in BMDCs nuclei by NK1R signaling. (C) Western blot analysis of TORC2 content in cytoplasmic and nuclear protein lysates prepared from untreated-DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs, resolved by SDS-PAGE, and probed for TORC2. (D) Percentages TORC2 translocation based on normalized density values of 3 independent experiments. (E) Secretion of IL-12p70 and IL-10 (by ELISA) in 24-hour supernatants of cocultures of (1) IL-12p35KO BMDCs, left untreated (DC), NK1R-DCs, the latter transduced or not with RAd–IL-10, and (2) DCs freshly-isolated from LNs (LN-DC) stimulated or not (control) with IFN-γ and agonist CD40 Ab. (A-E) Means ± 1 SD of triplicates from 1 representative experiment of 3 are shown.
In addition to CREB1, the recently described CREB1-specific coactivator-2 (TORC2) participates in the activation of the Il-10 promoter.26,27 Hence, we investigated whether in addition to CREB1 inhibition, NK1R-DCs have deficient activation and nuclear translocation of TORC2. We compared the content of TORC2 in the cytoplasm and nuclei of untreated DCs, Ag-DCs, NK1R-DCs, and Ag-NK1R-DCs. The percentage of TORC2 translocated was significantly lower in NK1R-DCs and Ag-NK1R-DCs compared with untreated or Ag-DCs (Figure 4C-D).
Together, these data demonstrate that NK1R signaling of BMDCs negatively regulates the CREB1/TORC2 pathway necessary for the activation of the Il-10 promoter. Under our conditions, secretion of IL-10 was recovered following transduction of BMDCs with RAd encoding IL-10 under the transcriptional control of the CMV promoter (not shown), demonstrating that NK1R stimulation affects CREB1/TORC2 signaling to inhibit secretion of IL-10 in BMDCs.
IL-10 inhibition in NK1R-DC vaccine results in increased IL-12 secretion by host DCs
Our findings suggest that reduced ability of NK1R-DCs to produce IL-10 may license IL-12p70 release by endogenous sDLN-DCs. We investigated this possibility by coculturing BMDCs left untreated (DC control) or NK1R-DCs with CD11c+ DCs isolated from sDLNs (1:1 ratio). sDLN-DCs were left untreated or incubated simultaneously with CD40 Ab and IFN-γ, the latter to stimulate IL-12p70 secretion. BMDCs were generated from IL-12p35KO mice to analyze exclusively the regulation of IL-12p70 in LN-DCs. NK1R-DCs led to significantly increased IL-12p70 secretion by sDLN vs control LN-DCs cultured with untreated DCs (Figure 4E).
If abrogation of IL-10 by NK1R-DCs was responsible for the increase in IL-12p70 secreted by host DCs, re-establishment of IL-10 release by NK1R-DCs would revert the effect. As shown in Figure 4E, infection of BMDCs with RAd-IL-10 overcame the effect of NK1R signaling on IL-10 secretion by BMDCs. Importantly, co-incubation of LN-DCs with NK1R-DCs expressing transgenic IL-10 (1:1 cell ratio) led to a significant decrease in IL-12p70 secretion by LN-DCs in response to CD40 ligation and IFN-γ stimulation (Figure 4E). Thus, NK1R signaling inhibits IL-10 production by BMDCs vaccines, resulting in enhanced secretion of IL-12p70 by host LN-DCs.
Immunity induced by NK1R-DCs requires their homing in sDLNs
There is increasing evidence that DCs generated ex vivo can be effectively used for positive vaccination to stimulate T-cell function through interactions with host DCs in lymphoid organs.28-30
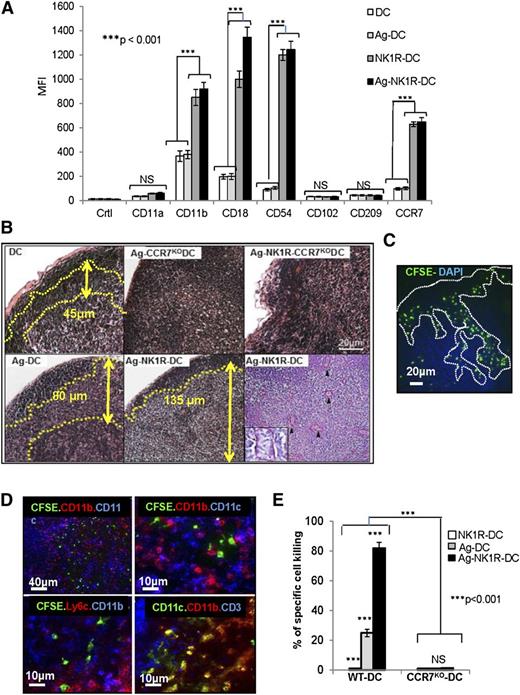
First, we investigated whether NK1R-DCs were better equipped than untreated DCs to interact with DCs and T cells in sDLNs by assessing the expression of DC-DC and DC–T-cell adhesion molecules including, CD11a, CD11b, CD18, CD54, CD102, and CD209. We also analyzed whether CCR7 was required for the injected DCs to home in sDLNs to exert their enhanced T-cell stimulatory functions in vivo. NK1R-DCs and Ag-NK1R-DCs significantly increased the levels of surface CCR7, CD11b, CD18, and CD54 compared with untreated DCs and Ag-DCs (Figure 5A). Expression of CD11a, CD102, and CD209 remained unchanged regardless of the DC treatment. These findings indicate that NK1R-DCs are better equipped to interact with other immune cells once they home in sDLNs.
NK1R-signaled BMDCs are required to home in sDLNs to promote type 1 immunity. (A) Flow cytometric analysis of the expression of homotypic adhesion molecules and the chemokine receptor CCR7 by untreated-DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs. Mean ± 1 SD of MFI of 3 independent experiments are illustrated. (B) Microscopic analysis (H&E, PAS) of sDLN, 24 hours after subcutaneous immunization with WT or CCR7KO BMDCs untreated (DC), NK1R-DCs, or Ag-NK1R-DCs. Paracortical areas (dotted lines) are defined by the presence of PAS+ high endothelial veins (arrowheads, inset). H&E and PAS, ×200. Inset, ×500. (C) Identification by fluorescence microscopy of CFSE+ NK1R-DCs 24 hours after subcutaneous injection and homed in the subcapsular and paracortical areas of sDLN (dotted lines). Nuclei were stained blue with DAPI, ×200. (D) Colocalization of CFSE+ Ag-NK1R-DCs (injected subcutaneously, 24 hours prior), and endogenous CD11c+CD11b+, CD11c+CD11b−, and CD11c+Ly6C+ DCs in sDLNs (top and bottom left panels) and CD3+ T cells (bottom right panel). Fluorescence microscopy, ×100 and ×200. (E) Comparative analysis of CTL function in WT mice skin-vaccinated with WT or CCR7KO, NK1R-DCs, Ag-DCs, or Ag-NK1R-DCs. The Ag-specific CTL activity was measured 7 days after immunization by in vivo killing assays. Means ± 1 SD from 5 mice per group are shown. CFSE, carboxyfluorescein diacetate succinimidyl ester; DAPI, 4,6 diamidino-2-phenylindole.
NK1R-signaled BMDCs are required to home in sDLNs to promote type 1 immunity. (A) Flow cytometric analysis of the expression of homotypic adhesion molecules and the chemokine receptor CCR7 by untreated-DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs. Mean ± 1 SD of MFI of 3 independent experiments are illustrated. (B) Microscopic analysis (H&E, PAS) of sDLN, 24 hours after subcutaneous immunization with WT or CCR7KO BMDCs untreated (DC), NK1R-DCs, or Ag-NK1R-DCs. Paracortical areas (dotted lines) are defined by the presence of PAS+ high endothelial veins (arrowheads, inset). H&E and PAS, ×200. Inset, ×500. (C) Identification by fluorescence microscopy of CFSE+ NK1R-DCs 24 hours after subcutaneous injection and homed in the subcapsular and paracortical areas of sDLN (dotted lines). Nuclei were stained blue with DAPI, ×200. (D) Colocalization of CFSE+ Ag-NK1R-DCs (injected subcutaneously, 24 hours prior), and endogenous CD11c+CD11b+, CD11c+CD11b−, and CD11c+Ly6C+ DCs in sDLNs (top and bottom left panels) and CD3+ T cells (bottom right panel). Fluorescence microscopy, ×100 and ×200. (E) Comparative analysis of CTL function in WT mice skin-vaccinated with WT or CCR7KO, NK1R-DCs, Ag-DCs, or Ag-NK1R-DCs. The Ag-specific CTL activity was measured 7 days after immunization by in vivo killing assays. Means ± 1 SD from 5 mice per group are shown. CFSE, carboxyfluorescein diacetate succinimidyl ester; DAPI, 4,6 diamidino-2-phenylindole.
Administration of Ag-DCs was associated with inflammation of the sDLN demonstrated by expansion of the paracortical area containing endothelial venules. This effect was significantly accentuated following injection of Ag-NK1R-DCs (Figure 5B) capable of homing efficiently to sDLNs (Figure 5C). Conversely, injection of equally treated BMDCs obtained from CCR7KO mice did not cause histologic changes in sDLNs (Figure 5B). Further characterization of DC populations in sDLNs by immunofluorescence microscopy showed that the injected NK1R-DCs localized in proximity to host CD11c+CD11b+Ly6c+ DCs (recruited inflammatory DCs) and CD11c+CD11b−Ly6C− conventional DCs (Figure 5D). LN homing of Ag-NK1R-DCs was necessary for exerting their T-cell stimulatory function as demonstrated by CTL function abrogation following immunization of WT mice with Ag-NK1R-DCs generated from syngenic CCR7KO mice (Figure 5E). Importantly, NK1R-signaled CCR7KO BMDCs expressed high levels of surface CD86 and effectively stimulated OVA-specific (OT-I) CTL in vitro (not shown), suggesting that their defect in inducing specific type 1 T-cell responses in vivo was due to their inability to home in sDLNs. Therefore, NK1R-DCs are required to home in sDLNs where they likely interact with host DCs to elicit type 1 immunity.
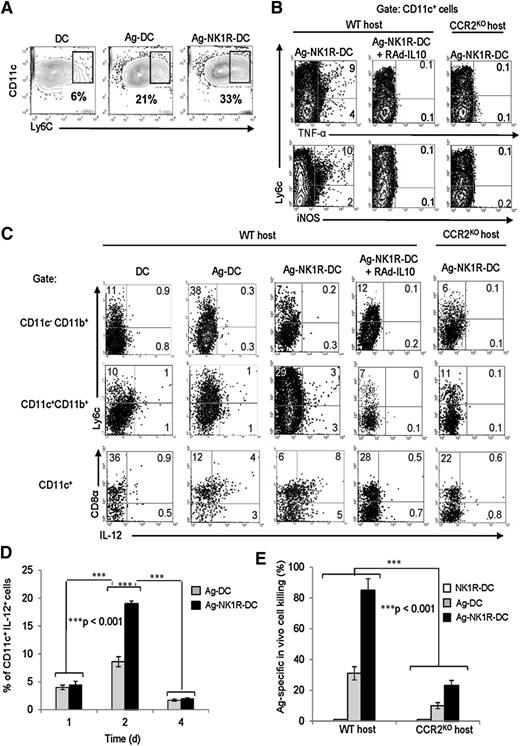
Immunity induced by NK1R-DCs requires LN recruitment of inflammatory DCs
Because administration of NK1R-DCs resulted in inflammation of those sDLNs where the exogenous DCs colocalized with host CD11c+CD11b+Ly6c+ DCs, we next investigated whether LN homing of the injected DCs results in recruitment of endogenous inflammatory DCs into the sDLNs. When compared with controls, the percentage of CD11c+Ly6C+TNF-α+iNOS+ inflammatory DCs in sDLN increased significantly after immunization with Ag-NK1R-DCs (Figure 6A-B). Under such conditions, inflammatory DCs and conventional CD8α+ DCs contained intracellular IL-12 (Figure 6C). By contrast, IL-12 was undetectable in sDLNs of mice vaccinated with Ag-NK1R-DCs secreting transgenic IL-10 (Figure 6C). Intracellular secretion of IL-12 was detected 1 day following administration of Ag-NK1R-DCs, peaked on day 2, and decreased thereafter (Figure 6D). These results provide evidence that downregulation of IL-10 by Ag-NK1R-DCs releases the inhibitory effect of IL-10 on IL-12 secretion by endogenous DCs in sDLNs.
In sDLN, secretion of IL-12 is limited to endogenous DCs and induction of CTL function requires recruitment of inflammatory DCs. (A) Percentages of CD11c+Ly6C+ inflammatory DCs (flow cytometry) in sDLN after skin immunization with untreated DCs, Ag-DCs, or Ag-NK1R-DCs. (B) Further characterization (flow cytometry) of CD11c+Ly6C+ DCs coexpressing TNF-α or iNOS in sDLNs, 2 days after administration of WT Ag-NK1R-DCs, transduced or not with RAd–IL-10, to WT or CCR2KO mice. (C) Identification (flow cytometry) in sDLNs of the DC subsets secreting IL-12p40/70, 2 days after skin immunization of WT or CCR2KO mice with WT untreated-DCs, Ag-DCs, or Ag-NK1R-DCs, the latter transduced or not with RAd–IL-10. (D) Flow cytometric analysis of IL-12p70 kinetics in sDLNs following subcutaneous administration of Ag-DCs or Ag-NK1R-DCs. (E) Comparative analysis of the CTL function (in vivo killing assays), 7 days after skin vaccination of WT or CCR2KO mice with WT, Ag-DCs, NK1R-DCs, or Ag-NK1R-DCs. (A-C) Results from 1 representative of 3 independent experiments are shown. (D) Means ± 1 SD of 5 mice per experimental group are shown.
In sDLN, secretion of IL-12 is limited to endogenous DCs and induction of CTL function requires recruitment of inflammatory DCs. (A) Percentages of CD11c+Ly6C+ inflammatory DCs (flow cytometry) in sDLN after skin immunization with untreated DCs, Ag-DCs, or Ag-NK1R-DCs. (B) Further characterization (flow cytometry) of CD11c+Ly6C+ DCs coexpressing TNF-α or iNOS in sDLNs, 2 days after administration of WT Ag-NK1R-DCs, transduced or not with RAd–IL-10, to WT or CCR2KO mice. (C) Identification (flow cytometry) in sDLNs of the DC subsets secreting IL-12p40/70, 2 days after skin immunization of WT or CCR2KO mice with WT untreated-DCs, Ag-DCs, or Ag-NK1R-DCs, the latter transduced or not with RAd–IL-10. (D) Flow cytometric analysis of IL-12p70 kinetics in sDLNs following subcutaneous administration of Ag-DCs or Ag-NK1R-DCs. (E) Comparative analysis of the CTL function (in vivo killing assays), 7 days after skin vaccination of WT or CCR2KO mice with WT, Ag-DCs, NK1R-DCs, or Ag-NK1R-DCs. (A-C) Results from 1 representative of 3 independent experiments are shown. (D) Means ± 1 SD of 5 mice per experimental group are shown.
To confirm that inflammatory DCs are critical for the promotion of type 1 immunity in our model, we injected CCR2KO mice subcutaneously with Ag-NK1R-DCs. CCR2KO mice are unable to release inflammatory CCR2+ monocytes (precursors of inflammatory DCs)30 from the BM into circulation. Under these conditions, we did not detect increase in the percentages of inflammatory DCs or intracellular IL-12 in DCs in the sDLN (Figure 6C). Importantly, CTL function in CCR2KO mice vaccinated with Ag-NK1R-DCs was significantly diminished compared with that observed in WT animals (Figure 6E). These findings demonstrate that recruitment of inflammatory DCs to sDLN is required for the induction of the IL-12–dependent type 1 immunity that follows vaccination with NK1R-DCs.
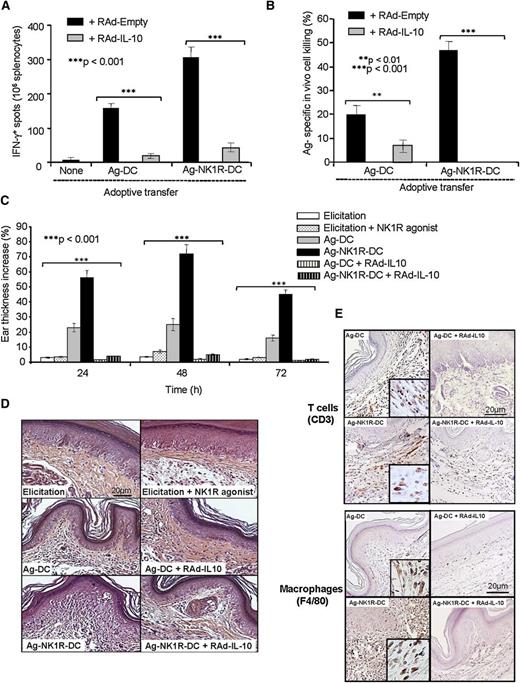
NK1R-DCs promote type 1 effector immunity that is prevented by IL-10
Thus far, our data have demonstrated that NK1R signaling decreases IL-10 secretion in BMDCs, and that skin vaccination with these cells is associated with the recruitment of inflammatory DCs and augmented production of IL-12 by host DCs in sDLNs. The link between the inability of NK1R-DCs to synthesize and/or release IL-10 and the enhanced type 1 effector immunity was further investigated in vivo in DTH assays. As expected, skin vaccination with Ag-NK1R-DCs induced potent type 1 cellular immunity (Figure 7A-B) composed of T cells and macrophages capable of homing to the skin after Ag rechallenge (Figure 7C-E). The capacity of Ag-NK1R-DCs to promote type 1 effector immunity was ablated following administration of Ag-NK1R-DCs secreting transgenic IL-10 (Figure 7A-E). Therefore, IL-10 deficiency in Ag-NK1R-DCs is critical in promote robust type 1 effector immunity in vivo.
The ability of NK1R-signaled BMDCs to elicit robust type 1 immunity is abrogated by transgenic IL-10 secretion. (A) Quantification of splenic IFN-γ–secreting CD4 T cells (ELISPOT) in mouse immunized subcutaneously with Ag-DCs or Ag-NK1R-DC, transduced with RAd–IL-10 or with control RAd-Empty. Means ± 1 SD of 3 independent experiments are shown. (B) Ag-specific CTL function (in vivo killing assay), analyzed 7 days after subcutaneous immunization with Ag-DCs or Ag-NK1R-DCs, transfected with RAd–IL-10 or control RAd-Empty. Means ± 1 SD from 5 mice per group are shown. (C) Percentage of ear thickness increase (DTH assay) assessed in mice sensitized with 1 dose of Ag-DCs or Ag-NK1R-DCs, transduced or not with RAd–IL-10. Mean ± 1 SD from 5 mice per group are shown. (D) Histologic analysis of the leukocyte infiltration in the ear skin from the area of DTH elicitation. H&E, ×200. (E) Detection of the T-cell and macrophage infiltrates in the ear skin from the area of DTH elicitation. Peroxidase, ×200. Insets, ×500.
The ability of NK1R-signaled BMDCs to elicit robust type 1 immunity is abrogated by transgenic IL-10 secretion. (A) Quantification of splenic IFN-γ–secreting CD4 T cells (ELISPOT) in mouse immunized subcutaneously with Ag-DCs or Ag-NK1R-DC, transduced with RAd–IL-10 or with control RAd-Empty. Means ± 1 SD of 3 independent experiments are shown. (B) Ag-specific CTL function (in vivo killing assay), analyzed 7 days after subcutaneous immunization with Ag-DCs or Ag-NK1R-DCs, transfected with RAd–IL-10 or control RAd-Empty. Means ± 1 SD from 5 mice per group are shown. (C) Percentage of ear thickness increase (DTH assay) assessed in mice sensitized with 1 dose of Ag-DCs or Ag-NK1R-DCs, transduced or not with RAd–IL-10. Mean ± 1 SD from 5 mice per group are shown. (D) Histologic analysis of the leukocyte infiltration in the ear skin from the area of DTH elicitation. H&E, ×200. (E) Detection of the T-cell and macrophage infiltrates in the ear skin from the area of DTH elicitation. Peroxidase, ×200. Insets, ×500.
Discussion
Signaling via NK1R, a shared receptor of the proinflammatory tachykinins SP and HK-1, triggers neurogenic inflammation and influences immunity outcome.31-33 Nevertheless, the impact of NK1R signaling on DCs, key regulators of the immune system, remains poorly investigated. In the present study, we analyzed the effects and mechanisms of NK1R signaling of ex vivo–generated DCs, the gold standard for DC-based vaccines.7,8,12 Our results demonstrate that NK1R stimulation promotes DC maturation, with increased expression of Ag-presenting, costimulatory, and adhesion molecules. These changes contributed to the DC ability to migrate efficiently from injection sites to the paracortical area of sDLNs and promote potent type 1 immunity. Importantly, NK1R-signaled DCs showed decreased synthesis and secretion of IL-10 that regulates negatively IL-12. Consequently, when NK1R-DCs were injected as a cell vaccine, they homed in sDLNs in close proximity to T cells and licensed host DCs to secrete IL-12, resulting in robust type 1 immunity.
NK1R signaling by SP has been associated with the onset of autoimmune disorders, including psoriasis, rheumatoid arthritis, and inflammatory bowel disease, and the severity of the symptoms in such experimental models is exacerbated in IL-10KO mice.34-36 Indeed, IL-10 is one of the most potent anti-inflammatory cytokines that contributes to maintain peripheral tolerance and tissue homeostasis in the steady state.37-39 Following Ag inoculation and elicitation of acute inflammation, compensatory release of IL-10 limits excessive tissue damage resulting from soluble mediators and immune effector cells. Accordingly, IL-10 secretion is stimulated in DCs and macrophages by a range of Toll-like receptor ligands. Moreover, Th1, Th2, and Th17 cells release IL-10 to prevent irreversible tissue destruction, chronic inflammation, and autoimmunity.37-39
In our model, NK1R agonists blocked the CREB1/TORC2 pathway in BMDCs, decreasing Il-10 gene transcription and abrogating IL-10 secretion. Moreover, this IL-10 inhibitory effect of NK1R agonists persisted following the induction of high levels of IL-10 by LPS.26,40 According to its proinflammatory potential, NK1R signaling of BMDCs reduced the transcriptional function of CREB1 while increasing nuclear translocation of NF-κB, which explains why NK1R agonists promote the differentiation of proinflammatory APCs.26,40,41
Over the past 2 decades, several laboratories have emphasized the need to generate optimally stimulated DCs ex vivo capable of secreting high levels of IL-12p70 to promote type 1–biased immunity for the purpose of positive vaccinations.7,8,42,43 To be effective, these therapeutic DC1 (administered in peripheral tissues) should retain their capability to secrete IL-12p70 when they encounter Ag-specific T cells in secondary lymphoid organs.44 The main drawback of current DC1 vaccine formulations is that IL-12p70 secretion is frequently exhausted by the time the injected DC1 reach the sDLN.45,46 To overcome this limitation, laboratories have used the rather risky approach of injecting therapeutic DC1 directly in the LN.47 Beyond technical problems with the methods of administration of cellular vaccines, recent evidence indicates that the biology of therapeutic DCs is far more complex than originally expected, and that the exogenous DCs mediate their stimulatory or regulatory effects on T cells indirectly, via interaction with endogenous DCs in secondary lymphoid organs.23,28,29 Our findings indicate that contrary to the accepted paradigm, elicitation of type 1–biased immunity following vaccination with Ag-loaded NK1R-signaled BMDCs depends on IL-12p70 produced by endogenous DCs. These DCs include LN-resident conventional and inflammatory DCs, the latter recruited from circulating CCR2+ precursors.30,48-50
Our data demonstrate that ex vivo stimulation of DCs via NK1R significantly enhances their potency to promote type 1 immunity when administered as cell-based vaccines. Effective activation of type 1 immunity requires homing of IL-10–deficient NK1R-signaled DCs in sDLNs with consequent release of IL-12p70 by the host DCs. Our results shed light into the mechanisms by which proinflammatory tachykinins acting via NK1R regulate DC functions. They provide insight into the neuroimmune regulation of DCs in view of future development of novel DC-based immunotherapies that may prove competent in promoting durable and protective type 1 immunity in vivo.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Paul Robbins (University of Pittsburgh) for providing the RAd.
This work was supported by National Institutes of Health (NIH) grants AI077511 (A.T.L.) and NIH P50 CA121973 (A.T.L., W. J. Storkus, and L.D.F.), and the T.E. Starzl Postdoctoral Fellowship in Transplantation Biology (D.M.R.-C.).
Authorship
Contribution: B.M.J. and T.L.S. performed most of the experimental work; D.M.R.-C. performed experiments and analysis of CREB-TORC2 signaling pathways; G.E. conducted RT-PCR studies and genetic analysis; O.A.T. and W. J. Shufesky helped with techniques; A.R.M., L.D.F., and W. J. Storkus collaborated on intellectual aspects and experimental designs; B.M.J., T.L.S., A.T.L., and A.E.M. conceived the study and experimental design, and wrote the manuscript; and A.T.L. supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.M.J. is Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
Correspondence: Adriana T. Larregina, Department of Dermatology, University of Pittsburgh, Suite 3880, Presby South Tower, 200 Lothrop St, Pittsburgh, PA 15213; e-mail: adrianal@pitt.edu.
References
Author notes
B.M.J. and T.L.S. contributed equally to this study.