Key Points
Donor T-Rapa cells were composed of Th1 and Th2 effectors with a reproducible gene expression profile.
Preemptive T-Rapa donor lymphocyte infusion was safe and associated with donor engraftment without excessive GVHD.
Abstract
In experimental models, ex vivo induced T-cell rapamycin resistance occurred independent of T helper 1 (Th1)/T helper 2 (Th2) differentiation and yielded allogeneic CD4+ T cells of increased in vivo efficacy that facilitated engraftment and permitted graft-versus-tumor effects while minimizing graft-versus-host disease (GVHD). To translate these findings, we performed a phase 2 multicenter clinical trial of rapamycin-resistant donor CD4+ Th2/Th1 (T-Rapa) cells after allogeneic-matched sibling donor hematopoietic cell transplantation (HCT) for therapy of refractory hematologic malignancy. T-Rapa cell products, which expressed a balanced Th2/Th1 phenotype, were administered as a preemptive donor lymphocyte infusion at day 14 post-HCT. After T-Rapa cell infusion, mixed donor/host chimerism rapidly converted, and there was preferential immune reconstitution with donor CD4+ Th2 and Th1 cells relative to regulatory T cells and CD8+ T cells. The cumulative incidence probability of acute GVHD was 20% and 40% at days 100 and 180 post-HCT, respectively. There was no transplant-related mortality. Eighteen of 40 patients (45%) remain in sustained complete remission (range of follow-up: 42-84 months). These results demonstrate the safety of this low-intensity transplant approach and the feasibility of subsequent randomized studies to compare T-Rapa cell-based therapy with standard transplantation regimens. This trial was registered at www.cancer.gov/clinicaltrials as #NCT 00077480.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) using nonmyeloablative host conditioning1,2 has reduced transplant-related mortality3 but is associated with increased tumor progression4 and graft rejection5 and remains limited by graft-versus-host disease (GVHD).6 Competing immune T-cell reactions underlie these clinical events. Donor T-cell–mediated GVHD and host T-cell–mediated rejection are reciprocally related,7 whereas donor T-cell–mediated graft-versus-tumor (GVT) effects and GVHD are intertwined.8 New approaches to modulate allogeneic T-cell immunity are therefore required. Imbalance between T helper 1 (Th1), T helper 2 (Th2), and other CD4+ T-cell subsets predisposes to human disease,9 including GVHD, which is primarily Th1 driven.10 As such, we hypothesized that allograft augmentation with T cells of mixed Th2 and Th1 phenotype may beneficially balance immunity after allogeneic HCT.
In murine models, we have evaluated the novel ex vivo application of rapamycin to control the Th2/Th1 balance posttransplant as an alternative to in vivo rapamycin drug therapy, which in various models has been found to prevent graft rejection and GVHD but abrogate antitumor effects through inhibition of Th1-type cells and preservation of Th2-type cells,11,12 prevent GVHD through promotion of regulatory T (TREG) cells13 or modulation of host antigen-presenting cell,14 and improve antiviral immunity mediated by CD8+ T cells.15 The ex vivo approach that we developed allows one to dissect these seemingly disparate potential in vivo drug effects on a purified T-cell subset under defined polarizing cytokine microenvironments. In our studies, we found that ex vivo rapamycin increased the capacity of interleukin (IL) 4 polarized donor Th2 cells to promote a balanced pattern of Th2/Th1 immune reconstitution for promotion of GVT effects and alloengraftment with reduced GVHD.16-19 Ex vivo rapamycin creates a state of T-cell starvation that induces autophagy,20 thereby resulting in an antiapoptotic T-cell phenotype that dictates persistent T-cell engraftment in mouse-into-mouse18 or human-into-mouse21 transplantation models. Rapamycin-resistant Th2 cells inhibited GVHD by multiple mechanisms, including IL-4 and IL-10 secretion, consumption of IL-2 required for propagation of pathogenic effector T cells, and modulation of host antigen-presenting cell.17 Furthermore, delayed administration of rapamycin-resistant Th2 cells after an initial donor Th1-type response optimized the balance of GVT effects and GVHD,16 thereby indicating that a mixed pattern of Th2 and Th1 immune reconstitution was desirable in the setting of cancer therapy. And finally, rapamycin-resistant Th2 cells prevented graft rejection through host T-cell conversion to a Th2-type profile,19 thus illustrating that this novel donor T-cell population may have particular application in transplant settings associated with increased graft rejection, such as the use of low-intensity host conditioning.
Building on these data, we transitioned from a phase 1 clinical trial of IL-4 polarized donor CD4+ T cells not manufactured in rapamycin22 to the current trial that incorporated ex vivo rapamycin during IL-4 polarization to produce donor “T-Rapa” cells. To improve the safety of our transplantation method and to incorporate an engraftment end point into the clinical trial (conversion of mixed chimerism), we developed an outpatient treatment platform consisting of low-intensity host conditioning (75% reduction in chemotherapy intensity relative to our previous studies of reduced-intensity transplantation).22 And, in an attempt to tailor posttransplant immune suppression to favor the manufactured T-Rapa cells rather than the unmanipulated T cells contained in the T-cell–replete hematopoietic cell allograft, we administered double-agent GVHD prophylaxis (cyclosporine plus Sirolimus) in the early posttransplant period and subsequent single-agent cyclosporine prophylaxis after T-Rapa cell adoptive transfer at day 14 posttransplant. This latter aspect of the protocol design was informed by our observation that ex vivo manufactured rapamycin-resistant allogeneic murine T cells, in particular the Th1 subset, were susceptible to the in vivo immune suppressive effects of rapamycin drug therapy.23
Methods
Clinical trial design, implementation, and end points
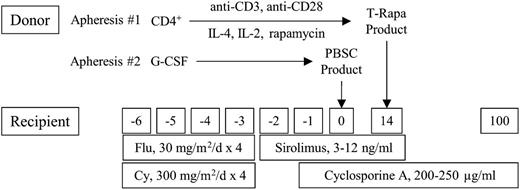
This phase 2 multi-institution protocol (Figure 1) was approved by the National Cancer Institute (NCI) and Hackensack University Medical Center (HUMC) institutional review boards and implemented according to an Investigational New Drug Application accepted by the Food and Drug Administration. Dates of transplant for this protocol ranged from December 13, 2005, to June 29, 2009; 35 patients were transplanted at the National Institutes of Health (NIH) Clinical Center, and 5 patients were transplanted at HUMC. Subjects provided written informed consent in accordance with the Declaration of Helsinki; enrollment was based on age (between 19 and 75), availability of 6/6 HLA-matched sibling donor, organ function, and hematologic malignancy diagnosis (acute and chronic myelogenous or lymphocytic leukemia, myelodysplastic syndrome, multiple myeloma, and Hodgkin and non-Hodgkin lymphoma [NHL]). Patients were eligible independent of their response to prior chemotherapy regimens; acute leukemia patients were eligible if blast frequency was <10%. Patients who had previously received autologous transplantation were eligible. Prior to transplant, patients received 1 to 3 cycles of etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, fludarabine, rituximab (EPOCH-FR) chemotherapy24 to achieve a predetermined level of host immune depletion (CD4 count ≤200/µL), to assess chemotherapy sensitivity, and to attempt to reduce disease burden. Fludarabine (30 mg/m2 per day) and cyclophosphamide (300 mg/m2 per day) were administered on days −6 to −3 prior to the granulocyte–colony-stimulating factor mobilized peripheral blood allograft; this dose of cyclophosphamide was 75% reduced relative to the regimen we previously evaluated.22 GVHD prophylaxis was cyclosporine (200-250 ng/mL; day −1 to +100, tapered at day +100 or earlier for progressive disease) and Sirolimus (3-12 ng/mL; day −2 to +14). T-Rapa cells were infused at day +14 post-HCT (2.5 × 107 cells per kg). The composite primary study objectives were to determine the safety and feasibility of preemptive T-Rapa cell donor lymphocyte infusion (DLI) and to characterize alloengraftment, antitumor effects, GVHD effects, and the Th2/Th1 balance post-HCT. The sample size of n = 40 was selected to give a reasonable estimation of the rate of acute GVHD: with this sample size, the maximum confidence interval width for the fraction of patients with grade 2 to 4 acute GVHD will not exceed ±15%. Organ toxicity was evaluated by NCI Common Toxicity Criteria (version 2.0); GVHD was evaluated using acute25 and chronic26 grading. Disease responses were evaluated by computed tomographic measurements and marrow examinations, with lymphoma responses measured by standard criteria27 ; progressive disease was treated with chemotherapy and/or unmanipulated DLI. Alloengraftment was monitored using variable N-terminal repeat polymerase chain reaction assays on total, CD3-enriched, or CD15-enriched cells.
Phase 2 clinical trial design of ex vivo and in vivo Sirolimus for low-intensity allogeneic HCT. Donors underwent steady-state apheresis #1, whereby CD4+ T cells were purified by positive selection; costimulated with anti-CD3 and anti-CD28; and exposed to IL-4, IL-2, and rapamycin in culture for 12 days. The resultant “T-Rapa” cell product was cryopreserved and administered as a preemptive DLI at day 14 post-HCT. Donors underwent apheresis #2 after granulocyte–colony-stimulating factor (G-CSF) mobilization; the peripheral blood stem cell (PBSC) product was unmanipulated (T-cell replete). The recipient was treated for 4 consecutive days (days −6 through −3) with concomitant fludarabine (Flu, 30 mg/m2 per day) and cyclophosphamide (Cy, 300 mg/m2 per day). GVHD prophylaxis consisted of short-course Sirolimus (from day −2 until day +14 post-HCT) and cyclosporine A (from day −1 until day +100 post-HCT).
Phase 2 clinical trial design of ex vivo and in vivo Sirolimus for low-intensity allogeneic HCT. Donors underwent steady-state apheresis #1, whereby CD4+ T cells were purified by positive selection; costimulated with anti-CD3 and anti-CD28; and exposed to IL-4, IL-2, and rapamycin in culture for 12 days. The resultant “T-Rapa” cell product was cryopreserved and administered as a preemptive DLI at day 14 post-HCT. Donors underwent apheresis #2 after granulocyte–colony-stimulating factor (G-CSF) mobilization; the peripheral blood stem cell (PBSC) product was unmanipulated (T-cell replete). The recipient was treated for 4 consecutive days (days −6 through −3) with concomitant fludarabine (Flu, 30 mg/m2 per day) and cyclophosphamide (Cy, 300 mg/m2 per day). GVHD prophylaxis consisted of short-course Sirolimus (from day −2 until day +14 post-HCT) and cyclosporine A (from day −1 until day +100 post-HCT).
T-Rapa cell manufacturing
Donor lymphocytes were collected by a 10-L steady-state apheresis performed prior to stem cell mobilization. CD4 cells were positively selected (CliniMACS device; Miltenyi) and costimulated (tosylated magnetic beads [Dynal] conjugated with anti-CD3 [OKT3; Ortho] and anti-CD28 9.3 antibodies [3:1 bead/cell ratio]). Purified CD4+ T cells (900 × 106 cells at culture initiation) were propagated in polyolefin bags (Baxter) using X-VIVO 20 media (Lonza), 5% donor plasma, recombinant human IL-4 (1000 IU/mL; Schering), recombinant human IL-2 (20 IU/mL; Chiron), and Sirolimus oral solution (1 µM; Wyeth); cytokine- and rapamycin-replete media were added every 2 to 3 days to maintain cell concentrations at <1 × 106 cells per mL. After 12 days, beads were removed; T cells were washed to remove cytokines and Sirolimus, and then cryopreserved. All infused T-Rapa products met release criteria, which included the following: CD4 cell purity >70% (median CD4 purity was 99%), CD8 cell content <5% (median CD8 content was <0.1%), viability >70% (median viability was 95%), absence of bacterial and fungal growth, absence of endotoxin content by limulus assay, negative mycoplasma test, and <100 magnetic beads per 3 × 106 cells. T-Rapa products were manufactured centrally (NIH Clinical Center Department of Transfusion Medicine).
Gene expression profiling
Total RNA was extracted (mRNA Easy Kits; Qiagen) and quantified (ND-1000 Spectrophotometer; NanoDrop), and quality was verified (2100 Bioanalyser; Agilent). RNA was amplified, Cy5 labeled, and hybridized (Agilent chip) alongside Cy3-labeled Human Reference RNA (Stratagene). Microarrays were scanned, and images analyzed (Software 9.5.1.1; Agilent). Data were analyzed with BRB Array Tools (http://linus.nci.nih.gov/BRB-ArrayTools.html; 34 051 of 41 687 genes were evaluable; accession number GSE34911).
T-cell phenotype
Culture supernatants were evaluated for Th1 cytokines (interferon γ [IFN- γ], IL-2, and tumor necrosis factor α [TNF-α]), Th2 cytokines (IL-4, IL-5, IL-10, and IL-13), and IL-17 (Luminex; Bio-rad). For supernatant generation, T-Rapa products (culture day 12) were restimulated for 24 hours; to assess phenotype stability, T-Rapa products were costimulated, expanded without rapamycin or polarizing cytokines until culture day 18, and restimulated for supernatant generation. To evaluate post-HCT cytokine phenotype, peripheral blood lymphocytes were isolated (weeks 1, 2, 4, and 7-8) and costimulated; supernatants were evaluated for cytokine content (Luminex). For transcription factor detection, T cells were cultured (4 hours, no costimulation; with GolgiStop and GolgiPlug, BD), washed, surface stained (anti-CD4; anti-CD25), fixed and permeabilized (Fix/Perm buffer; eBioscience), and stained (anti–GATA-3; anti–T-bet; anti-FoxP3). Antibodies were purchased (BD, Biolegend, eBioscience); 6-color flow was performed (LSRII; BD). Post-HCT immune cell numbers were quantified.22
Statistical analyses
Gene expression was analyzed (Genomic Suite 6.4; Partek); induced genes were selected by t test (filters of P < .001, false discovery rate < .01). For hierarchical clustering, genes and samples were organized with Pearson correlation metric dissimilarity to measure distance on gene-averaged values. Enrichment of biological pathways for differentially expressed genes and Gene Ontology designations were determined using the Database for Annotation, Visualization and Integrated Discovery (DAVID) bioinformatics resource28 ; specific gene annotations were retrieved on Gene Cards (http://www.genecards.org/index.shtml). Changes in laboratory parameters between stated time points were analyzed using absolute or relative differences, depending on change distribution. Differences were tested to determine if they differed from zero. The GATA-3:T-bet ratio was tested to determine if it was different from one using Wilcoxon signed-rank analyses. P values are presented without multiple comparison adjustment, as this is an exploratory analysis. The cumulative incidence of acute GHVD (combining both classical acute and late acute forms, including liver transaminase elevation) and the cumulative incidence of chronic GVHD were determined using the method of Gooley,29 adjusting for the competing risk of death; the cumulative incidence of relapse was adjusted for the competing risk of death due to transplant-related mortality.
Results
Th2/Th1 phenotype of T-Rapa cells
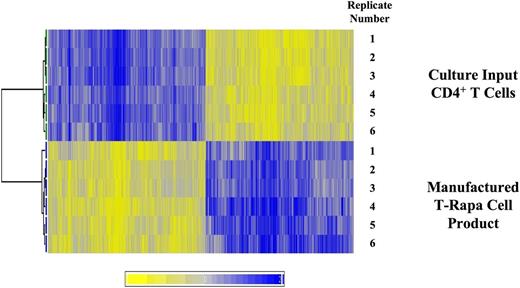
During the 12-day culture interval, median CD4+ T-cell number was 6.4-fold increased relative to day 0 culture input values (range, 4.5- to 19.1-fold increased). Relative to culture input CD4+ T cells, T-Rapa cells differentially expressed 18.1% (6147 of 34 051) of messenger RNA species, with similar numbers of genes upregulated (3185) or downregulated (2962) (illustrated in heat map, Figure 2). By gene ontology analysis, the 5 gene families most significantly upregulated in T-Rapa cells relative to day 0 culture input CD4+ T cells were cell cycle, DNA metabolism, stress response, glucose catabolism, and oxidative reduction; representative gene members in these families were upregulated 23- to 92-fold above values in the day 0 culture input cells (supplemental Table 1; see the Blood Web site). On the other hand, the 5 gene families most significantly downregulated in T-Rapa cells relative to the day 0 culture input CD4+ T cells were apoptosis, transcription, inflammation, cytokine production, and immune response; representative gene members in these families were downregulated 45- to 341-fold below values in the day 0 culture input cells (supplemental Table 1). In spite of this evidence that cytokine and immune response genes were dramatically downregulated in T-Rapa cell products, a limited number of Th2 and Th1 genes were significantly upregulated in T-Rapa cells: most notably, the Th2 cytokine IL-1330 and the Th1 cytokine IL-12Rβ231 were upregulated 21.5- and 18.4-fold, respectively. The gene expression pattern of T-Rapa cells was remarkably reproducible: in an evaluation of n = 21 T-Rapa cell clinical products, the interproduct gene expression variability by intraclass correlation coefficient analysis was 0.93.
T-Rapa cell products have relatively equal numbers of genes that are up- or downregulated in expression relative to culture input CD4+ T cells. T-Rapa cell products were manufactured ex vivo (n = 6) and compared with the purified CD4+ T cells used to initiate the cultures (n = 6). RNA was purified from each paired sample, with further analysis by gene expression microarray. The heat map illustrates that genes were consistently differentially expressed, with 18.1% of genes (6147 of 34 051) being differentially expressed in T-Rapa products relative to input CD4 cells (P < .001); the number of upregulated genes in T-Rapa cells was relatively equal to the number of downregulated genes.
T-Rapa cell products have relatively equal numbers of genes that are up- or downregulated in expression relative to culture input CD4+ T cells. T-Rapa cell products were manufactured ex vivo (n = 6) and compared with the purified CD4+ T cells used to initiate the cultures (n = 6). RNA was purified from each paired sample, with further analysis by gene expression microarray. The heat map illustrates that genes were consistently differentially expressed, with 18.1% of genes (6147 of 34 051) being differentially expressed in T-Rapa products relative to input CD4 cells (P < .001); the number of upregulated genes in T-Rapa cells was relatively equal to the number of downregulated genes.
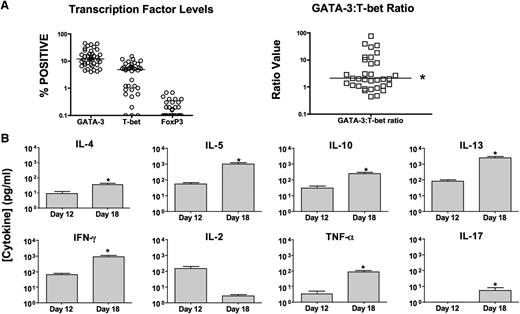
T-Rapa products had minimal contamination with CD4+Foxp3+ cells (<1%) and preferentially expressed GATA-3 (median CD4+GATA-3+, 11.8%) relative to T-bet (median CD4+T-bet+, 5.1%); the median intraproduct GATA-3/T-bet ratio was 2.1 (Figure 3A). T-Rapa products secreted low levels of Th2 cytokines, which increased after extended culture without polarizing cytokines and rapamycin (Figure 3B; median values; day 12 to day 18 of culture: IL-4 [1-11 pg/mL], IL-5 [21-363 pg/mL], IL-10 [10-159 pg/mL], and IL-13 [24-725 pg/mL]). T-Rapa products secreted low levels of IFN-γ and TNF-α, which increased after extended culture (IFN-γ [10-418 pg/mL]; TNF-α [1-41 pg/mL]). T-Rapa cell IL-2 secretion actually decreased after extended culture, whereas IL-17 secretion increased from undetectable levels to <10 pg/mL after extended culture.
T-Rapa cells express a balanced Th2/Th1 cytokine phenotype. (A) T-Rapa cell clinical products were analyzed by intracellular flow cytometry for expression of GATA-3 (Th2 cell transcription factor), T-bet (Th1 cell transcription factor), and FoxP3 (TREG cell transcription factor) (left). The intraproduct ratio of GATA-3:T-bet in T-Rapa cells was >1:1 (*P < .05). (B) T-Rapa cell clinical products were evaluated at the time of DLI (day 12 of culture) and after an additional culture interval intended to evaluate effector function and differentiation plasticity (day 18 of culture). At each time point, the T-Rapa cells were costimulated, and the resultant supernatants were tested for cytokine content by Luminex (mean ± standard error of the mean; n = 40; *, increase from day 12 to day 18, P < .05). Values are expressed as pg/mL (1 × 106 cells per mL per 24 hours).
T-Rapa cells express a balanced Th2/Th1 cytokine phenotype. (A) T-Rapa cell clinical products were analyzed by intracellular flow cytometry for expression of GATA-3 (Th2 cell transcription factor), T-bet (Th1 cell transcription factor), and FoxP3 (TREG cell transcription factor) (left). The intraproduct ratio of GATA-3:T-bet in T-Rapa cells was >1:1 (*P < .05). (B) T-Rapa cell clinical products were evaluated at the time of DLI (day 12 of culture) and after an additional culture interval intended to evaluate effector function and differentiation plasticity (day 18 of culture). At each time point, the T-Rapa cells were costimulated, and the resultant supernatants were tested for cytokine content by Luminex (mean ± standard error of the mean; n = 40; *, increase from day 12 to day 18, P < .05). Values are expressed as pg/mL (1 × 106 cells per mL per 24 hours).
Conversion of mixed chimerism after T-Rapa infusion
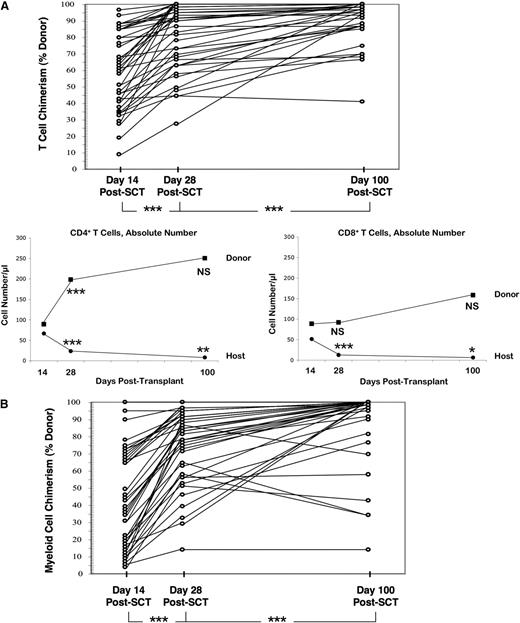
Although each patient engrafted with donor cells, the low-intensity conditioning yielded mixed donor/host T lymphoid chimerism at day 14 post-HCT (Figure 4A; median donor chimerism, 61%). After T-Rapa cell infusion at day 14 post-HCT, median values increased to 89% and 94% at days 28 and 100 post-HCT, respectively. Median estimated absolute number of donor CD4+ T cells increased from 89/µL (day +14) to 198/µL and 250/µL (days 28 and 100 post-HCT, respectively). Reciprocally, median estimated numbers of host CD4+ T cells decreased from 67/µL (day +14) to 24/µL and 8/µL (days 28 and 100 post-HCT, respectively). Median estimated numbers of donor CD8+ T cells did not increase significantly after T-Rapa cell infusion (values at days 14, 28, and 100 post-HCT: 89, 92, and 158/µL, respectively); in contrast, median estimated numbers of host CD8+ T cells decreased from 51/µL (day +14) to 13/µL and 6/µL (days 28 and 100 post-HCT, respectively). As detailed in supplemental Table 2, the T-Rapa cell products were composed primarily of central memory CD4+ T cells (mean value, 66.4%). Immune reconstitution post-HCT was characterized by relatively balanced numbers of naive, central memory, and effector memory cells in the CD4 compartment through day 180 post-HCT; by comparison, CD8 cell immune reconstitution was biased toward the effector memory subsets, including both CD45RA+ and CD45RA– populations (supplemental Table 2). At 1 year post-HCT, median values for CD4, CD8, and B-cell counts were 423, 297, and 151/μL, respectively (n = 22 evaluated); at 1 year post-HCT, median values for serum immunoglobulins IgG, IgM, and IgA were 502, 46, and 40 mg/dL, respectively (n = 14 evaluated). Donor myeloid chimerism was mixed at day 14 post-HCT (Figure 4B; median, 37%); after T-Rapa cell infusion, median values increased to 81% and 99% at days 28 and 100 post-HCT, respectively.
T-Rapa cell infusion results in predominate donor CD4+ T-cell reconstitution. (A) Percent donor T lymphoid chimerism for each patient at days 14, 28, and 100 after allogeneic hematopoietic stem cell transplant (SCT) (top; ***, day 28 > day 14 and day 100 > day 28; P < .0001). Post-SCT numbers of donor vs host CD4+ or CD8+ T cells were estimated by multiplying CD4 and CD8 cell absolute numbers by percent CD3 chimerism values. The figure shows median estimated values for absolute numbers of donor and host CD4+ T cells (left) and CD8+ T cells (right) at days 14, 28, and 100 post-SCT (comparisons are day 28 vs day 14 and day 100 vs day 28; ***P < .001; **P < .01; *P < .05; between n = 23 and n = 33 evaluated for each paired analysis). (B) Percent donor myeloid chimerism for each patient at days 14, 28, and 100 post-SCT (***, day 28 > day 14 and day 100 > day 28; P < .0001). NS, not significant.
T-Rapa cell infusion results in predominate donor CD4+ T-cell reconstitution. (A) Percent donor T lymphoid chimerism for each patient at days 14, 28, and 100 after allogeneic hematopoietic stem cell transplant (SCT) (top; ***, day 28 > day 14 and day 100 > day 28; P < .0001). Post-SCT numbers of donor vs host CD4+ or CD8+ T cells were estimated by multiplying CD4 and CD8 cell absolute numbers by percent CD3 chimerism values. The figure shows median estimated values for absolute numbers of donor and host CD4+ T cells (left) and CD8+ T cells (right) at days 14, 28, and 100 post-SCT (comparisons are day 28 vs day 14 and day 100 vs day 28; ***P < .001; **P < .01; *P < .05; between n = 23 and n = 33 evaluated for each paired analysis). (B) Percent donor myeloid chimerism for each patient at days 14, 28, and 100 post-SCT (***, day 28 > day 14 and day 100 > day 28; P < .0001). NS, not significant.
T-Rapa recipients express a balanced Th2/Th1 profile
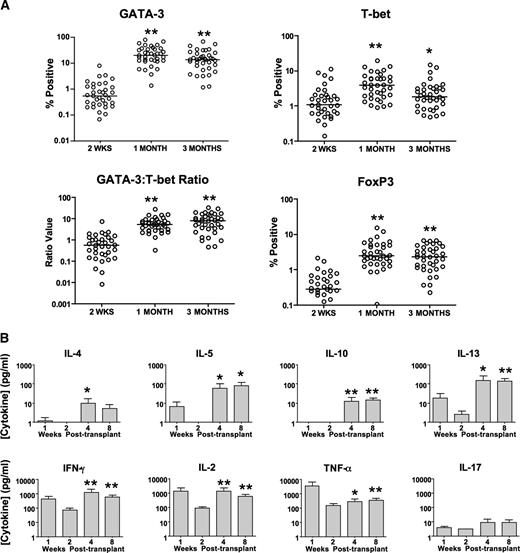
Low frequencies of Th2 and Th1 cells were detected at day 14 post-HCT just prior to T-Rapa cell infusion (Figure 5A, median values; CD4+GATA-3+, 0.6%; CD4+T-bet+, 1.1%). After T-Rapa cell infusion, median frequencies of CD4+GATA-3+ cells increased to 20.2% and 13.9% at 1 and 3 months post-HCT, respectively; median frequencies of CD4+T-bet+ cells modestly increased to 4.0% and 1.8% (1 and 3 months post-HCT). The median intrapatient GATA-3/T-bet ratio was 0.6 at day 14 post-HCT and increased to 5.4 and 8.1 at 1 and 3 months post-HCT, respectively. Median frequencies of CD4+FoxP3+ cells were low at day 14 post-HCT (0.3%) and increased at 1 and 3 months post-HCT (2.5% and 2.4%, respectively).
T-Rapa cell recipients have immune reconstitution of a mixed Th2 and Th1 cytokine phenotype. (A) Percentage of CD4+ T cells expressing GATA-3, T-bet, and FoxP3 as measured by intracellular flow cytometry at day +14 posttransplant (just before T-Rapa cell DLI) and at 1 month and 3 months post-HCT (comparisons are 1 month vs day 14 and 3 months vs day 14; **P < .001; *P < .05). Intrapatient ratio of GATA-3 to T-bet expressing CD4+ T cells is shown for each time point. (B) At 1, 2, 4, and 8 weeks post-HCT, peripheral blood mononuclear cells were costimulated, and the 24-hour supernatant was tested for cytokine content by Luminex (mean ± standard error of the mean; between n = 32 and n = 34 evaluated for each paired analysis; comparisons are week 4 vs week 2 and week 8 vs week 2; **P < .001; *P < .01).
T-Rapa cell recipients have immune reconstitution of a mixed Th2 and Th1 cytokine phenotype. (A) Percentage of CD4+ T cells expressing GATA-3, T-bet, and FoxP3 as measured by intracellular flow cytometry at day +14 posttransplant (just before T-Rapa cell DLI) and at 1 month and 3 months post-HCT (comparisons are 1 month vs day 14 and 3 months vs day 14; **P < .001; *P < .05). Intrapatient ratio of GATA-3 to T-bet expressing CD4+ T cells is shown for each time point. (B) At 1, 2, 4, and 8 weeks post-HCT, peripheral blood mononuclear cells were costimulated, and the 24-hour supernatant was tested for cytokine content by Luminex (mean ± standard error of the mean; between n = 32 and n = 34 evaluated for each paired analysis; comparisons are week 4 vs week 2 and week 8 vs week 2; **P < .001; *P < .01).
Post-HCT T cells secreted low levels of Th2 cytokines at days 7 and 14 post-HCT (Figure 5B; IL-4, IL-5, IL-10, and IL-13 values were typically <10 pg/mL); after T-Rapa cell infusion, these values were generally increased at 1 and 3 months post-HCT. By comparison, post-HCT T cells secreted higher levels of Th1 cytokines at days 7 and 14 post-HCT (IL-2, INF-γ, and TNF-α levels ranging from 100 to 10 000 pg/mL); after T-Rapa cell infusion, these values were either stable or increased at 1 and 3 months post-HCT. Post-HCT T-cell secretion of IL-17 was not detected prior to T-Rapa infusion and modestly increased at 1 and 3 months post-HCT. Just prior to T-Rapa infusion (at day 14 post-HCT), low frequencies of post-HCT CD4+ and CD8+ T cells secreted cytokines by cytokine capture flow cytometry analysis; by comparison, at day 28 post-HCT, increased frequencies of both CD4+ and CD8+ T cells secreted the type I cytokines IL-2 and IFN-γ and the type II cytokines IL-4 and IL-10 (supplemental Figure 1).
To evaluate antigen-specific T-cell responses post-HCT, transplant recipients were evaluated for immune responses against cytomegalovirus (CMV). In 3 of the 4 HLA-A02+ recipients who developed CMV viremia post-HCT, there was an increased frequency of CMV-specific T cells by flow cytometry analysis (supplemental Figure 2); by comparison, each of the 6 HLA-A02+ recipients who did not develop CMV viremia post-HCT had frequencies of CMV-specific T cells near background levels. In addition, in 13 transplant cases where the donor or host (or both) was CMV seropositive, we observed increased secretion of IFN-γ and IL-4 in response to overlapping CMV peptides at day 60 post-HCT (supplemental Table 3). Consistent with their minimally differentiated effector state, the T-Rapa cell products secreted minimal cytokines in response to either overlapping CMV peptides or a superantigen-like positive control stimulation. Finally, we evaluated the T-cell receptor V-β repertoire of both the T-Rapa cell products and day 60 post-HCT CD4+ T cells: the T-Rapa products had a diverse T-cell repertoire similar to normal donor CD4 cells, whereas the post-HCT CD4+ T cells tended to have a more skewed T-cell receptor repertoire (supplemental Figure 3).
Patient characteristics and post-HCT outcome
Median patient age was 51 years (range, 23-69; 17 females, 23 males; Tables 1 and 2). To assess risk of disease progression after reduced-intensity HCT,4 patients were classified as having low-risk (n = 9, 22.5%), standard-risk (n = 7, 17.5%), or high-risk (n = 24, 60%) diagnoses. Median number of prior regimens was 3 (range, 1-5). Twenty of 40 patients (50%) were refractory to their most recent prior chemotherapy, with 8 being primary refractory. Twenty-three of 40 patients (57.5%) were refractory to an outpatient chemotherapy regimen consisting of EPOCH-FR.24 Thirty-two of 40 patients (80%) proceeded to low-intensity transplant with measurable disease.
Transplantation outcome according to malignancy risk factors
| Risk level§ . | UPN . | Age/sex . | Malignancy characteristics* . | GVHD† . | Overall outcome‡ . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study entry . | Pre-HCT . | Acute . | Late acute . | Chronic . | ||||||||||||
| Dx . | # . | Res . | Res . | NED . | s . | g . | l . | Gr . | Tumor . | Survival . | Death . | |||||
| 1 | 1 | 65/M | MCL | 2 | 1° R | PR | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 211 (PD) |
| 2 | 48/F | MDS | 2 | S | CR | Y | 0 | 0 | 0 | 0 | tr | Y (o, s) | CR | + 2566 | n.a. | |
| 10 | 35/F | FCL | 5 | S | PR | N | 0 | 0 | 0 | 0 | 0 | Y (o, s, e, v) | CR | N | 955 (PE) | |
| 12 | 66/M | MF | 1 | R | SD | N | 0 | 0 | 0 | 0 | 0 | N | CR | + 1696 | n.a. | |
| 13 | 59/M | MCL | 4 | S | CR | Y | 0 | 0 | 0 | 0 | g | Y (s, e, l) | CR | N | 1053 (MI) | |
| 17 | 65/F | FCL | 3 | S | SD | N | 0 | 0 | 0 | 0 | tr, g | Y (o, s, e) | CR | + 1717 | n.a. | |
| 18 | 37/M | FCL | 3 | 1° R | PR | N | 0 | 0 | 0 | 0 | 0 | Y (o) | CR | + 1598 | n.a. | |
| 20 | 60/M | MCL | 3 | S | CR | Y | 0 | 0 | 0 | 0 | 0 | N | CR | + 1514 | n.a. | |
| 35 | 53/M | MCL | 2 | 1° R | SD | N | 0 | 0 | 0 | 0 | tr | N | CR | + 1312 | n.a. | |
| 2 | 5 | 39/F | CML | 1 | S | hCR | N | 0 | 0 | 0 | 0 | tr | Y (e) | mCR | + 2468 | n.a. |
| 8 | 55/M | CLL | 3 | S | PR | N | 3 | 0 | 0 | 2 | tr, s | N | PD | N | 772 (PD) | |
| 11 | 50/F | MM | 2 | R | SD | N | 0 | 0 | 0 | 0 | tr | Y (s) | SD | + 1710 | n.a. | |
| 22 | 63/M | CLL | 5 | R | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 911 (PD) | |
| 30 | 55/F | MDS | 1 | S | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | + 1340 | n.a. | |
| 32 | 66/F | MM | 1 | 1° R | SD | N | 3 | 1 | 0 | 2 | tr | Y (s, o, e) | CR | + 1356 | n.a. | |
| 33 | 63/M | CLL | 2 | S | SD | N | 0 | 0 | 0 | 0 | 0 | Y (o, s) | CR | + 1326 | n.a. | |
| Risk level§ . | UPN . | Age/sex . | Malignancy characteristics* . | GVHD† . | Overall outcome‡ . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study entry . | Pre-HCT . | Acute . | Late acute . | Chronic . | ||||||||||||
| Dx . | # . | Res . | Res . | NED . | s . | g . | l . | Gr . | Tumor . | Survival . | Death . | |||||
| 1 | 1 | 65/M | MCL | 2 | 1° R | PR | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 211 (PD) |
| 2 | 48/F | MDS | 2 | S | CR | Y | 0 | 0 | 0 | 0 | tr | Y (o, s) | CR | + 2566 | n.a. | |
| 10 | 35/F | FCL | 5 | S | PR | N | 0 | 0 | 0 | 0 | 0 | Y (o, s, e, v) | CR | N | 955 (PE) | |
| 12 | 66/M | MF | 1 | R | SD | N | 0 | 0 | 0 | 0 | 0 | N | CR | + 1696 | n.a. | |
| 13 | 59/M | MCL | 4 | S | CR | Y | 0 | 0 | 0 | 0 | g | Y (s, e, l) | CR | N | 1053 (MI) | |
| 17 | 65/F | FCL | 3 | S | SD | N | 0 | 0 | 0 | 0 | tr, g | Y (o, s, e) | CR | + 1717 | n.a. | |
| 18 | 37/M | FCL | 3 | 1° R | PR | N | 0 | 0 | 0 | 0 | 0 | Y (o) | CR | + 1598 | n.a. | |
| 20 | 60/M | MCL | 3 | S | CR | Y | 0 | 0 | 0 | 0 | 0 | N | CR | + 1514 | n.a. | |
| 35 | 53/M | MCL | 2 | 1° R | SD | N | 0 | 0 | 0 | 0 | tr | N | CR | + 1312 | n.a. | |
| 2 | 5 | 39/F | CML | 1 | S | hCR | N | 0 | 0 | 0 | 0 | tr | Y (e) | mCR | + 2468 | n.a. |
| 8 | 55/M | CLL | 3 | S | PR | N | 3 | 0 | 0 | 2 | tr, s | N | PD | N | 772 (PD) | |
| 11 | 50/F | MM | 2 | R | SD | N | 0 | 0 | 0 | 0 | tr | Y (s) | SD | + 1710 | n.a. | |
| 22 | 63/M | CLL | 5 | R | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 911 (PD) | |
| 30 | 55/F | MDS | 1 | S | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | + 1340 | n.a. | |
| 32 | 66/F | MM | 1 | 1° R | SD | N | 3 | 1 | 0 | 2 | tr | Y (s, o, e) | CR | + 1356 | n.a. | |
| 33 | 63/M | CLL | 2 | S | SD | N | 0 | 0 | 0 | 0 | 0 | Y (o, s) | CR | + 1326 | n.a. | |
CLL, chronic lymphocytic leukemia; Dx, diagnoses; F, female; FCL, follicular non-Hodgkin lymphoma; M, male; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MF, myelofibrosis; MM, multiple myeloma; N, no; n.a., not applicable; NED, no evidence of disease status; UPN, unique patient number; Y, yes.
#, number of prior regimens. Res, disease response: 1° R, primary refractory; S, sensitive to last regimen; and R, refractory to last regimen. Pre-HCT, indicates disease status at time of low-intensity transplant: Res, response to EPOCH-F(R): PR, partial response; CR, complete response; SD, stable disease; and hCR, hematologic CR.
Acute GVHD score of skin (s), gut (g), and liver (l); Gr, overall grade (0-4). Late acute GVHD, manifested as elevated liver transaminase levels (tr), skin (s), or gut involvement (g). Chronic GVHD sites of involvement: oral (o), skin (s), eye (e), vaginal (v), and lung (l).
Overall outcome for last tumor staging: PD, progressive disease; CR, complete remission; mCR, molecular CR; and SD, stable disease. Ongoing survival, in days post-HCT (+). Death, day post-HCT; cause due to PD, presumed pulmonary embolus (PE), or myocardial infarction (MI).
Risk of progressive disease post-HCT: level 1, low risk; level 2, standard risk.
Transplantation outcome according to malignancy risk factors
| Risk level§ . | UPN . | Age/sex . | Malignancy characteristics* . | GVHD† . | Overall outcome‡ . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study entry . | Pre-HCT . | Acute . | Late acute . | Chronic . | ||||||||||||
| Dx . | # . | Res . | Res . | NED . | s . | g . | l . | Gr . | Tumor . | Survival . | Death . | |||||
| 3 | 3 | 44/F | AML | 4 | S | hCR | N | 0 | 0 | 0 | 0 | tr | Y (o, s, v) | PD | N | 748 (PD) |
| 4 | 47/M | AML | 2 | S | PD | N | 2 | 1 | 0 | 2 | (n.e.) | (n.e.) | PD | N | 119 (PD) | |
| 6 | 67/M | DLC | 5 | R | PD | N | 1 | 0 | 0 | 1 | (n.e.) | (n.e.) | PD | N | 91 (PD) | |
| 7 | 24/M | AML | 3 | S | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 194 (PD) | |
| 9 | 32/M | HD | 5 | R | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 1004 (PD) | |
| 14 | 52/F | A-TCL | 4 | 1° R | PD | N | 0 | 0 | 0 | 0 | tr | Y (o, s, v, e) | CR | + 1669 | n.a. | |
| 15 | 30/M | DLC-EBV | 2 | 1° R | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 328 (PD) | |
| 16 | 59/M | AML | 3 | S | hCR | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 386 (PD) | |
| 19 | 42/F | HD | 3 | 1° R | SD | N | 0 | 0 | 0 | 0 | 0 | Y (s) | CR | + 1563 | n.a. | |
| 21 | 44/F | DLC (trFL) | 4 | S | SD | N | 0 | 0 | 0 | 0 | tr | Y (s) | CR | + 1514 | n.a. | |
| 23 | 60/F | DLC | 5 | R | PD | N | 0 | 0 | 0 | 0 | tr, g, s | N | PD | N | 192 (PD) | |
| 24 | 43/F | DLC | 5 | R | PD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 170 (PD) | |
| 25 | 33/M | NHL-GZ | 3 | R | PD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 268 (PD) | |
| 26 | 23/M | HD | 4 | R | SD | N | 0 | 0 | 0 | 0 | 0 | Y (s) | PD | N | 1024 (PD) | |
| 27 | 39/F | CML | 4 | R | hCR | N | 0 | 0 | 0 | 0 | tr | N | mCR | + 1457 | n.a. | |
| 28 | 66/M | DLC (trCLL) | 3 | R | SD | N | 0 | 2 | 0 | 3 | (n.e.) | (n.e.) | PD | N | 92 (PD) | |
| 29 | 47/M | A-TCL | 5 | S | CR | Y | 1 | 0 | 0 | 1 | 0 | Y (s, l) | CR | + 1402 | n.a. | |
| 31 | 27/F | HD | 2 | S | SD | N | 0 | 0 | 0 | 0 | g | Y (s, o) | CR | + 1355 | n.a. | |
| 34 | 45/M | NHL-GZ | 4 | S | CR | Y | 0 | 0 | 0 | 0 | 0 | N | CR | + 1325 | n.a. | |
| 36 | 49/F | DLC | 2 | S | PR | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 234 (PD) | |
| 37 | 52/M | DLC (trFL) | 4 | S | CR | Y | 0 | 0 | 0 | 0 | 0 | N | CR | + 1299 | n.a. | |
| 38 | 58/M | NHL-pDC | 1 | 1° R | CR | Y | 0 | 0 | 0 | 0 | 0 | Y (o, s, e) | PD | N | 525 (PD) | |
| 39 | 55/F | DLC (trFL) | 3 | S | CR | Y | 0 | 0 | 0 | 0 | 0 | N | CR | + 1285 | n.a. | |
| 40 | 69/M | CML | 4 | R | SD | N | 0 | 0 | 0 | 0 | 0 | N | SD | + 1272 | n.a. | |
| Risk level§ . | UPN . | Age/sex . | Malignancy characteristics* . | GVHD† . | Overall outcome‡ . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study entry . | Pre-HCT . | Acute . | Late acute . | Chronic . | ||||||||||||
| Dx . | # . | Res . | Res . | NED . | s . | g . | l . | Gr . | Tumor . | Survival . | Death . | |||||
| 3 | 3 | 44/F | AML | 4 | S | hCR | N | 0 | 0 | 0 | 0 | tr | Y (o, s, v) | PD | N | 748 (PD) |
| 4 | 47/M | AML | 2 | S | PD | N | 2 | 1 | 0 | 2 | (n.e.) | (n.e.) | PD | N | 119 (PD) | |
| 6 | 67/M | DLC | 5 | R | PD | N | 1 | 0 | 0 | 1 | (n.e.) | (n.e.) | PD | N | 91 (PD) | |
| 7 | 24/M | AML | 3 | S | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 194 (PD) | |
| 9 | 32/M | HD | 5 | R | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 1004 (PD) | |
| 14 | 52/F | A-TCL | 4 | 1° R | PD | N | 0 | 0 | 0 | 0 | tr | Y (o, s, v, e) | CR | + 1669 | n.a. | |
| 15 | 30/M | DLC-EBV | 2 | 1° R | SD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 328 (PD) | |
| 16 | 59/M | AML | 3 | S | hCR | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 386 (PD) | |
| 19 | 42/F | HD | 3 | 1° R | SD | N | 0 | 0 | 0 | 0 | 0 | Y (s) | CR | + 1563 | n.a. | |
| 21 | 44/F | DLC (trFL) | 4 | S | SD | N | 0 | 0 | 0 | 0 | tr | Y (s) | CR | + 1514 | n.a. | |
| 23 | 60/F | DLC | 5 | R | PD | N | 0 | 0 | 0 | 0 | tr, g, s | N | PD | N | 192 (PD) | |
| 24 | 43/F | DLC | 5 | R | PD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 170 (PD) | |
| 25 | 33/M | NHL-GZ | 3 | R | PD | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 268 (PD) | |
| 26 | 23/M | HD | 4 | R | SD | N | 0 | 0 | 0 | 0 | 0 | Y (s) | PD | N | 1024 (PD) | |
| 27 | 39/F | CML | 4 | R | hCR | N | 0 | 0 | 0 | 0 | tr | N | mCR | + 1457 | n.a. | |
| 28 | 66/M | DLC (trCLL) | 3 | R | SD | N | 0 | 2 | 0 | 3 | (n.e.) | (n.e.) | PD | N | 92 (PD) | |
| 29 | 47/M | A-TCL | 5 | S | CR | Y | 1 | 0 | 0 | 1 | 0 | Y (s, l) | CR | + 1402 | n.a. | |
| 31 | 27/F | HD | 2 | S | SD | N | 0 | 0 | 0 | 0 | g | Y (s, o) | CR | + 1355 | n.a. | |
| 34 | 45/M | NHL-GZ | 4 | S | CR | Y | 0 | 0 | 0 | 0 | 0 | N | CR | + 1325 | n.a. | |
| 36 | 49/F | DLC | 2 | S | PR | N | 0 | 0 | 0 | 0 | 0 | N | PD | N | 234 (PD) | |
| 37 | 52/M | DLC (trFL) | 4 | S | CR | Y | 0 | 0 | 0 | 0 | 0 | N | CR | + 1299 | n.a. | |
| 38 | 58/M | NHL-pDC | 1 | 1° R | CR | Y | 0 | 0 | 0 | 0 | 0 | Y (o, s, e) | PD | N | 525 (PD) | |
| 39 | 55/F | DLC (trFL) | 3 | S | CR | Y | 0 | 0 | 0 | 0 | 0 | N | CR | + 1285 | n.a. | |
| 40 | 69/M | CML | 4 | R | SD | N | 0 | 0 | 0 | 0 | 0 | N | SD | + 1272 | n.a. | |
A-TCL, anaplastic T-cell NHL; CLL, chronic lymphocytic leukemia; DLC, diffuse large cell; DLC-EBV, Epstein-Barr virus–driven DLC; DLC (trCLL), CLL transformed to DLC; DLC (trFL), transformed follicular NHL to DLC; Dx, diagnoses; F, female; HD, Hodgkin disease; M, male; N, no; n.a., not applicable; n.e., not evaluable; NED, no evidence of disease status; NHL-GZ, gray-zone NHL; NHL-pDC, plasmablastic dendritic cell NHL; UPN, unique patient number; Y, yes.
#, number of prior regimens. Res, disease response to last prior regimen: S, sensitive; R, refractory; 1° R, primary refractory. Pre-HCT, indicates disease status at time of low-intensity transplant: Res, response to EPOCH-F(R): PR, partial response; CR, complete response; SD, stable disease; hCR, hematologic CR; and PD, progressive disease.
Acute GVHD score of skin (s), gut (g), and liver (l); Gr, overall grade (0-4). Late acute GVHD, manifested as elevated liver transaminase levels (tr), skin (s), or gut involvement (g). Chronic GVHD sites of involvement: oral (o), skin (s), eye (e), vaginal (v), and lung (l).
Overall outcome for last tumor staging: PD, progressive disease; CR, complete remission; mCR, molecular CR; and SD, stable disease. Ongoing survival, in days post-HCT (+). Death, day post-HCT; cause due to PD.
Risk of progressive disease post-HCT: level 3, high risk.
There were no deaths directly related to transplantation. There were no infusional toxicities or serious adverse events attributable to T-Rapa cell therapy. There were no cases of veno-occlusive disease, engraftment syndrome, or transplant-associated microangiopathy. CMV viremia by DNA–polymerase chain reaction test was detected in 3 of 21 (14.3%) CMV-negative recipients and 6 of 19 (31.6%) CMV-positive recipients. One of 40 recipients (5%) developed CMV infection (gastritis); 1 patient developed disseminated adenoviral infection after intensive therapy of progressive acute myelogenous leukemia (AML). Sixteen of 40 patients (40%) had bacteremia (n = 18 episodes) or candidemia (n = 1) attributable to neutropenia and/or line infection; there were no cases of bacterial or fungal pneumonia, 1 case of Mycobacterium avium complex pneumonia, and 1 case of fungal infection (Paecilonyces variotti). Late, nonrelapse mortality was due to postsurgical pulmonary embolus (n = 1) and myocardial infarction (n = 1).
Classical acute GVHD (grades 2-4 through day 100 post-HCT) was observed in 4 of 40 patients (10%; each case steroid responsive); in 3 cases, acute GVHD was preceded by early immune suppression taper and/or unmanipulated DLI for therapy of early malignant disease progression. Late acute GVHD, which occurred in 14 of 37 patients (37.8%), consisted primarily of liver transaminitis without other organ involvement (9 of 14 patients); 4 cases of late acute gut GVHD occurred (each case steroid responsive). After combining all cases of classically defined acute GVHD and all cases of late acute GVHD (including liver transaminase elevation, which occurred prior to day 100 post-HCT in some patients), there was a cumulative incidence probability of acute GVHD of 20% at day 100 post-HCT and 40% at day 180 post-HCT. Seventeen of 37 evaluable patients (45.9%) had classical chronic GVHD, with global severity scores of mild (n = 9), moderate (n = 6), or severe (n = 2); the median number of organs involved was 2 (range, 1-4), with tissue distribution of skin (n = 15), oral (n = 10), ocular (n = 7), vulvo-vaginal (n = 3), and lung (n = 2); the cumulative incidence probability of chronic GVHD at 2 years post-HCT was 42.5%. Ten of 40 patients (25%) developed overlap GVHD (classical chronic in combination with classical or late acute); 16 of 40 patients (40%) did not develop any form of GVHD.
Each patient with low-risk diagnoses achieved complete remission, although 1 patient died of isolated central nervous system disease; 6 of 9 low-risk diagnosis recipients (66.6%) are in sustained complete remission (median follow-up, 1647 days post-HCT; range, 1312-2566). Three of 7 patients (42.9%) with standard-risk diagnoses are in sustained complete remission at days 1326, 1356, and 2468 posttransplant. Nine of 24 recipients (37.5%) with high-risk diagnoses are in sustained complete remission (median follow-up, 1402 days post-HCT; range, 1285-1669). Posttransplant chemotherapy and/or unmanipulated DLI contributed to sustained complete remission in 5 of 18 (27.8%) patients; a total of 22 patients received chemotherapy and/or DLI for management of progressive disease. The cumulative probability of disease progression was 32.5% at 6 months post-HCT, 50% at 12 months post-HCT, 57.5% at 24 months post-HCT, and 57.5% at 36 months post-HCT. In total, 18 of 40 patients (45%) remain in sustained complete remission (range of follow-up: 42-84 months).
Discussion
Reduced-intensity allogeneic HCT yields mixed donor/host chimerism, reduces morbidity and mortality after transplant, and places the therapeutic emphasis on donor immunity rather than host conditioning. However, progress in the field has been generally restricted by an inability of current approaches to (1) preferentially favor donor immunity rather than residual host immunity and (2) balance donor T-cell effects once they predominate. As a result, transplant outcome can be variably limited by graft rejection, persistent mixed chimerism with associated reduced GVT effects, and GVHD. These obstacles would be anticipated to be particularly relevant with the low intensity of chemotherapy that we used in this study, which is 75% lower in alkylator dose than the reduced-intensity regimen that we previously evaluated.22 Here, we describe a new transplant platform that begins to overcome these obstacles through a unique allograft augmentation strategy that uses ex vivo rapamycin to manufacture a novel donor T-cell product that promotes a balanced Th2/Th1 immune reconstitution.
Ex vivo application of rapamycin to the T-cell manufacturing process yielded CD4+ effector T cells that expressed a mixed Th2/Th1 phenotype. Microarray analysis of such T-Rapa cells revealed a broad-based gene expression identity that was remarkably reproducible during clinical trial implementation; such reproducibility was achieved with a manufacturing method that incorporated clinical product cryopreservation and shipment to a multicenter site, thereby demonstrating that subsequent, definitive trials using T-Rapa cells will be feasible. Further studies will be required to evaluate if this gene expression identity dictates functional characteristics of the T-Rapa cell product; in subsequent clinical trials, it may be advantageous to use the gene expression identity as a release criterion for the T-Rapa cell product. Consistent with our results in experimental models,16,18,21 the clinical T-Rapa cell products had downregulation of apoptosis genes and were minimally differentiated on the basis of global downregulation of inflammation, cytokine production, and immune response genes. T-Rapa products were minimally contaminated with FoxP3+ cells and thus distinct from TREG cell products evaluated for GVHD prevention in the cord blood transplantation setting.32 At the time of infusion, T-Rapa cell products secreted low levels of cytokines and had a diverse T-cell receptor repertoire; this observation further supports our conclusion that T-Rapa cells represent minimally differentiated effectors, which, in experimental models,33 mediate increased therapeutic effects. T-Rapa cell products secreted high levels of cytokines ex vivo after removal of rapamycin (thus demonstrating their effector potential) and maintained a mixed Th2/Th1 profile ex vivo without exogenous cytokine addition and in the absence of rapamycin (thus illustrating their limited polarization plasticity34 ). The T-Rapa cell products were therefore composed of minimally differentiated Th2/Th1 effector CD4+ T cells rather than an anergic T-cell population35 or a TREG-enriched population36 observed in other experimental systems that also evaluated ex vivo rapamycin.
The clinical treatment platform we evaluated can be considered a low-intensity regimen as it can be administered in the outpatient setting and universally resulted in mixed donor/host T-cell chimerism, with a median of only 61% donor chimerism at day 14 post-HCT. This result, which stands in contrast to results using a reduced-intensity regimen that we previously evaluated,22,37 is likely due to 2 aspects of the current platform: (1) a 75% reduction in cyclophosphamide intensity during host conditioning; and (2) a requirement that the host CD4 count be <200 cells per µL prior to transplantation rather than the more stringent value that we previously used (<50 cells per µL). Preemptive DLI with T-Rapa cells in this setting was associated with the conversion of mixed chimerism toward predominant donor chimerism; no graft rejection occurred in T-Rapa recipients, which included many patients with <50% donor T-cell chimerism at day 14 post-HCT (n = 14). Previously, this level of mixed chimerism at the day 14 post-HCT time point was associated with increased graft rejection.5 It is difficult to compare engraftment results across clinical trials because of differences in patient selection and pretransplant treatment history; nonetheless, the current results indicate that the T-Rapa cell DLI did not promote donor-host tolerance or impair donor T-cell responses posttransplant. Although the T-Rapa cell DLI likely contributed to the rather rapid increase in donor chimerism, definitive clinical trials evaluating this transplant regimen without a DLI or with a control DLI consisting of unmanipulated donor T cells would be required to better address this question. Of note, previous investigations have evaluated CD4-enriched DLI, including in the following settings: for decreasing chimerism after T-cell–depleted transplantation38 ; for treatment of posttransplant relapse39 ; or, when used in combination with IL-2 therapy, for the treatment of chronic GVHD.40
In contrast to autologous adoptive T-cell therapy approaches that maximize host immune depletion through high-dose conditioning and immediate T-cell transfer,41 T-Rapa cells were administered remote from conditioning, in a state of host immunity that was relatively T-cell replete, and during calcineurin inhibitor therapy. Nonetheless, the CD4-purified donor T-Rapa cells appeared to break immune tolerance and predominate in vivo, as indicated by the following: (1) a rapid post-HCT increase in donor CD4+ T cells; (2) restricted expansion of donor CD8+ T cells; (3) concomitant reductions in host CD4+ and CD8+ T cells; (4) balanced CD4+ Th2/Th1 immune reconstitution with modest reconstitution of TREG cells; and (5) conversion of early mixed myeloid chimerism through an apparent graft-versus-myeloid lineage effect. The donor CD4 predominance observed after T-Rapa cell DLI suggests that the infused product expanded in vivo; however, the T-Rapa cells were not labeled for cell tracking, and as such, it is possible that CD4 cells contained in the mobilized allograft may have also contributed to the observed pattern of immune reconstitution. Indeed, our finding that both CD4+ and CD8+ T cells had balanced secretion of Th1 and Th2 cytokines post-HCT suggests that the CD4-purified T-Rapa cell DLI may have resulted in the in vivo modulation of T cells emanating from the mobilized allograft. Nonetheless, the preferential expansion of donor CD4 cells rather than donor CD8 cells after T-Rapa cell DLI stands in marked contrast to other clinical results in myeloablative or nonmyeloablative transplantation, in which a predominant CD8 cell reconstitution has been observed.42 Taken together, these results suggest that T-Rapa cells may represent a particularly potent effector T-cell population, as an array of biological effects were observed after adoptive transfer in the face of systemic immune suppression.
Only 4 of 40 patients (10%) developed classical acute grade 2 to 4 GVHD through day 100 post-HCT, which compares favorably with our first-generation clinical trial of ex vivo expanded CD4 cells where we observed a rate of 64% (18 of 28 cases).22 The low rate of classical acute GVHD that we observed is similar to results obtained using host conditioning with total lymphoid irradiation,43 which also promoted Th2 cytokines. We did observe a significant incidence of late acute GVHD, in particular liver transaminase elevation (which occurred in several patients before day 100 post-HCT), which resulted in a cumulative overall incidence probability of all forms of acute GVHD of 20% and 40% at days 100 and 180 post-HCT, respectively. Several factors may have influenced the incidence, type, and severity of acute GVHD in our study, including balanced CD4+ Th2/Th1 immune reconstitution posttransplant with restricted donor CD8+ T-cell expansion posttransplant, low-level persistent mixed T-cell chimerism,44 low-intensity conditioning,45 and Sirolimus prophylaxis.46 With respect to this latter point, in a recent study performed by our collaborators,47 the rate of acute grade 2 to 4 GVHD in recipients of a matched related donor transplant after reduced-intensity conditioning and GVHD prophylaxis consisting of cyclosporine plus a short course of peritransplant Sirolimus without T-Rapa cell DLI was 27% (6 of 22 cases). Classical chronic GVHD was primarily of mild-to-moderate global severity. A substantial proportion of patients did not develop acute or chronic GVHD. As such, the overall GVHD profile of the current platform was not excessive, particularly considering that ex vivo activated donor T cells were administered in the context of a T-cell–replete peripheral blood allograft.
This study provided a rigorous clinical test for immune GVT effects because of the low intensity of host conditioning and because of the patient population, which had relatively low percentages of patients having a low-risk malignancy diagnosis4 (22.5%), receiving transplantation in complete remission (18%), and having chemotherapy-sensitive disease (50%). By comparison, previous studies using other low-intensity conditioning methods had higher frequencies of patients transplanted in remission and having low-risk diagnoses.4,48 In our trial, sustained complete remissions were achieved in some patients with primary refractory disease and diagnoses such as refractory chronic myelogenous leukemia (CML) that are difficult to eradicate with reduced-intensity transplantation.49 As such, the current transplant platform appears to be suitable in terms of antitumor potency for the majority of patients typically considered for reduced-intensity transplantation. However, T-Rapa cell infusion did not promote sufficient GVT effects in patients with refractory diffuse large cell NHL or multiply relapsed AML; ongoing efforts seek to overcome this GVT limitation through infusion of T-Rapa cells with an increased Th1 phenotype21 or enhanced tumor specificity through incorporation of chimeric antigen receptors.50
In summary, in this first human clinical trial of ex vivo manufactured allogeneic T-Rapa cells, we have demonstrated that the combined use of ex vivo and in vivo Sirolimus can be combined to provide a new platform for the safe implementation of low-intensity allogeneic HCT. These phase 2 clinical trial results suggest that the CD4-purified T-Rapa cells mediated distinct effects in vivo when administered as a preemptive DLI, in particular the rapid conversion of mixed chimerism toward full donor chimerism that was predominated by CD4+ T cells of a balanced Th2/Th1 cytokine phenotype. Preemptive T-Rapa cell infusion after low-intensity allogeneic HCT therefore represents a suitable platform for further studies. Our demonstration that the T-Rapa cell product can be safely administered in a multicenter manner indicates that it will be feasible to perform subsequent randomized studies comparing T-Rapa cell-based therapy to other types of DLI or other transplant regimens.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE34911).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank numerous individuals who made outstanding contributions to this work, including Bazetta Blacklock-Schuver and Sheila Phang for their creative and efficient efforts in patient recruitment and care coordination; Roger Kurlander, NIH Department of Laboratory Medicine, for his collaboration and professionalism in chimerism analysis; Maryalice Stetler-Stevenson and Constance Yuan for their care in providing clinical flow cytometry analyses; Jeanne Odom, Paula Layton, and Brenna Hansen for excellence in research nursing; Vicki Fellowes, for her irreplaceable efforts toward the development and implementation of T-Rapa cell manufacturing; Xiao-Yi Yan, Sarfraz Memon, Shoba Amarnath, Tania Felizardo, Jason Foley, and Yelena Kogan for their efforts with respect to laboratory studies and immunology end points; Elizabeth J. Read, for her involvement in cell manufacturing at the NIH during initial aspects of study implementation; Daniele Avila, Amanda Urban, Jennifer Mann, and Tiffani Taylor for their excellence and dedication in the care of protocol patients; Suzanne Murphy for expert assistance with the protocol and Investigational New Drug Application; the NIH Clinical Center and Department of Nursing; the Medical Oncology Fellows at the NCI; and numerous individuals at HUMC, including Tatyana Feldman, Anthony Mato, Carol Carini, and Andrea Ortega.
This work was supported by the Intramural Research Program, NCI Center for Cancer Research; and was also supported by the NCI Center for Treatment and Evaluation Program, in particular Dr Howard Streicher, for provision of IL-4.
Authorship
Contribution: D.H.F. designed the trial, provided patient care, performed data interpretation, and wrote the manuscript; M.E.M. performed laboratory end point experiments and data interpretation and assisted in writing manuscript; S.M.S. performed data analysis and interpretation; D.C.H. provided patient care and performed data interpretation; D.S. and H.M.K. provided clinical product manufacturing and data interpretation; F.T.H. performed laboratory end point experiments and data analysis and assisted in writing the manuscript; L.C. and M.S. performed laboratory end point experiments; S.F.L. participated in protocol design and transfusion medicine support; J.M. provided patient care and participated in protocol design; J.C.G.-B. and C.S. provided patient care and performed data analysis; N.M.H. and D.D.H. provided patient care; S.Z.P. provided patient care and performed data analysis; S.R., A.G., and M.D. participated in protocol design and provided patient care; R.K. participated in protocol design; A.P. provided patient care and research support; B.L.L. and C.H.J. participated in protocol design and manufacturing of clinical cellular product; R.E.G. participated in protocol design, provided patient care, and assisted in writing the manuscript; and M.R.B. participated in protocol design, provided patient care, performed data analysis, and assisted in writing the manuscript.
Conflict-of-interest disclosure: D.H.F., R.E.G., B.L.L., and C.H.J. are listed as coinventors on a related patent, Rapamycin-Resistant T Cells and Therapeutic Uses Thereof (US Patent 7,718,196; May 18, 2010). The remaining authors declare no competing financial interests.
Correspondence: Daniel H. Fowler, 10 Center Dr, Building 10, CRC, 3-3330, Bethesda, MD 20892; e-mail: dhfowler@helix.nih.gov.