Key Points
Clonal-like expansion of NK cells in response to CMV infection causes stable imprints in the human KIR repertoire.
Education by inhibitory KIRs promotes the expansion of NK cells, causing repertoire skewing and a bias for self-specific inhibitory KIRs.
Abstract
Human natural killer (NK) cells are functionally regulated by killer cell immunoglobulin-like receptors (KIRs) and their interactions with HLA class I molecules. As KIR expression in a given NK cell is genetically hard-wired, we hypothesized that KIR repertoire perturbations reflect expansions of unique NK-cell subsets and may be used to trace adaptation of the NK-cell compartment to virus infections. By determining the human “KIR-ome” at a single-cell level in more than 200 donors, we were able to analyze the magnitude of NK cell adaptation to virus infections in healthy individuals. Strikingly, infection with human cytomegalovirus (CMV), but not with other common herpesviruses, induced expansion and differentiation of KIR-expressing NK cells, visible as stable imprints in the repertoire. Education by inhibitory KIRs promoted the clonal-like expansion of NK cells, causing a bias for self-specific inhibitory KIRs. Furthermore, our data revealed a unique contribution of activating KIRs (KIR2DS4, KIR2DS2, or KIR3DS1), in addition to NKG2C, in the expansion of human NK cells. These results provide new insight into the diversity of KIR repertoire and its adaptation to virus infection, suggesting a role for both activating and inhibitory KIRs in immunity to CMV infection.
Introduction
Natural killer (NK) cells influence the outcome of human pregnancy and provide a first line of defense against several types of invading pathogens by mediating potent cytolytic effector functions and by the release of proinflammatory cytokines. The function of NK cells is regulated by a vast array of germline-encoded cell surface receptors that mediate signals for activation or inhibition.1 Many NK cell receptors are paired with activating and inhibitory counterparts, sharing the same ligand, albeit with different binding affinities.2 One such example of paired receptors are the lectin-like heterodimers CD94/NKG2C (activating) and CD94/NKG2A (inhibitory), both binding to the nonclassic HLA-E molecule in humans.3 Other examples are found among receptors within the killer cell immunoglobulin-like receptor (KIR) gene cluster, located within the leukocyte receptor complex on human chromosome 19. This gene cluster contains up to 14 KIR genes encoding receptors with activating (2DS1-5, 3DS1), inhibitory (2DL1-3, 2DL5, and 3DL1-3), or dual (2DL4) signaling potential.4,5 The KIR gene-cluster is divided into group A haplotypes, dominated by inhibitory KIRs, and group B haplotypes, containing a varying number of activating and inhibitory KIRs.6 KIR expression is highly variable among individuals and is determined by variation in KIR gene content, copy number, extensive polymorphisms in KIR genes, and probabilistic mechanisms involving epigenetic regulation of transcription.7
Among the inhibitory KIRs, 5 have well-defined specificities for distinct groups of HLA class I alleles.4 KIR2DL3 and KIR2DL1 bind to HLA-C1 and HLA-C2, respectively; KIR2DL2 binds to both HLA-C1 and HLA-C2; KIR3DL1 binds to HLA-Bw4; and KIR3DL2 displays peptide-dependent binding to HLA-A3/A11. Although inhibitory interactions between KIR and their cognate HLA class I ligands abrogate effector responses of NK cells, they are also, somewhat paradoxically, required for the functional education of NK cells in a process referred to as NK cell licensing.8 The strength of the inhibitory interactions between the receptors and their ligands determines the overall functional reactivity of the NK cell when faced with targets that lack the corresponding HLA class I ligand.
The biology and molecular specificities of the activating KIRs are less well defined, and most interactions with presumed HLA class I ligands are weak or nonexistent.9 Phylogenetic analysis and evolutionary reconstruction have suggested that activating KIRs have emerged rather recently, approximately 13.5 to 18 million years ago, from an ancestral inhibitory KIR.10 This event was followed by a human-specific expansion of the KIR B haplotypes as they underwent selection for resistance to infections and reproductive success.11 In this context, epidemiologic studies link activating KIR genes to resistance against numerous virus infections.12 For example, KIR3DS1 in conjunction with HLA-Bw4 with an isoleucine at position 80 is associated with slower progression of HIV infection to AIDS.13 In addition, donor KIR2DS1 protects against human cytomegalovirus (CMV) reactivation in settings of allogeneic hematopoietic stem cell transplantation.14
Although structurally different than KIRs, the lectin-like Ly49 family of molecules in the mouse serves a remarkably similar role to KIRs in humans. They are expressed stochastically on NK cells, interact with major histocompatibility complex (MHC) class I molecules, and comprise paired inhibitory and activating variants.15 Among the mouse Ly49 receptors, the activating receptor Ly49H binds specifically to the mouse CMV-encoded MHC class I homolog, m157.16 Ly49H+ NK cells undergo clonal-like expansion during challenge with mouse CMV and decline in numbers after the resolution of infection, leaving a population of NK cells with heightened responses to rechallenge by the same antigen.17 Additional activating Ly49 receptors contribute to the MHC class I-dependent recognition of CMV in some mouse strains.18 In humans, Guma and colleagues showed that CMV seropositivity is associated with elevated frequencies of NKG2C+ NK cells.19 Since then, CMV-associated expansion of NKG2C+ NK cells have been reported in various human disease settings including immunodeficiency,20 HIV infection,21 acute hantavirus infection,22 and after stem cell transplantation.23-25 It is tempting to speculate that activating KIRs could play a role similar to Ly49H, in mice, and NKG2C in humans, by promoting the expansion of NK cell subsets after the onset of CMV infection. However, studies of the biology of activating KIRs have been hampered by the extensive cross-reactivity of monoclonal antibodies between the structurally related activating and inhibitory KIRs.
Here, we provide a high-resolution analysis of the human “KIR-ome” at a single cell level in more than 200 individuals, revealing specific and stable imprints of past virus infection. The adaptation of human NK cells, detected as expansions of educated/licensed KIR-expressing NK-cell subpopulations, was unique to infection with CMV and was not caused by any other herpesvirus infections. Finally, our analysis revealed expansions of NK cells expressing KIR2DS2, KIR2DS4, or KIR3DS1, providing evidence for a role of activating KIRs, in addition to NKG2C, in driving the clonal-like expansion of NK cells in response to CMV.
Methods
Human participants and cells
This study was conducted in accordance with the Declaration of Helsinki and was approved by the regional ethics committee in Stockholm, Sweden. A total of 204 healthy, randomly selected blood donors were included in the study. Donor characteristics are summarized in supplementary Table 1. Peripheral blood mononuclear cells were separated from buffy coats by density gravity centrifugation (Ficoll-Hypaque; GE Healthcare) and were either used directly after separation or cryopreserved in 10% dimethyl sulfoxide and 90% heat-inactivated fetal bovine serum for later use. A detailed account of donor exclusions on the basis of cross-reactive antibody-binding patterns and genetic deletions of NKG2C is provided as supplementary Information. Analyses of KIR repertoires in cord blood and longitudinal samples from children at ages 2 and 5 years were performed using frozen peripheral blood mononuclear cells. Untransfected 721.221 (221.wt), 721.221 cells transfected with a hybrid HLA-E containing the HLA-A2 signal sequence (221.AEH), K562, and RAJI cell lines were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 1 mM of L-glutamine, 100 μg/mL of penicillin, and 100 μg/mL of streptomycin (Invitrogen).
KIR and HLA typing
Genomic DNA was isolated from 200 μL of whole blood using a commercial kit (DNeasy, QIAGEN). KIR ligands were determined using the KIR HLA ligand kit (Olerup SSP; QIAGEN) for detection of the HLA-Bw4, HLA-C1, and HLA-C2 motifs. For analysis of HLA-A3/A11, and specific HLA-B alleles, complementary HLA genotyping was performed with the HLA-A and HLA-B low-resolution kit (Olerup SSP; QIAGEN). KIR genotyping was performed using a recently described high-throughput technology called quantitative KIR automated typing (qKAT).26 In brief, a set of 10 multiplex quantitative polymerase chain reaction assays was used to ascertain gene content and copy number for all 17 KIR genes and their major variants.
Virus serology
Details of the methods used to determine virus serologies for CMV, Epstein-Barr virus (EBV), varicella zoster virus (VZV), and herpes simplex virus (HSV) types 1 and 2 are provided in supplementary Methods.
Monoclonal antibodies and flow cytometry
NK cells were stained with the following antibodies: KIR2DL3-FITC, NKG2A-APC, KIR3DL1-AF700, CD57-Pacific Blue, CD14-Horizon-V500, CD19-Horizon-V500, KIR2DS4-Qdot.585, KIR3DL2-biotin (streptavidin-Qdot.605), KIR2DL1-Qdot.705, NKG2C-PE, CD56-ECD, CD3-PE.Cy5, KIR2DL2/S2/L3-PE.Cy5.5, KIR2DL1/S1-PE.Cy7, CD2-Pacific Blue, NKp30-AF647, CD161-APC, Siglec-9-PE, and CD7-AF647. Samples were acquired using an LSR-Fortessa 18-color flow cytometer (BD Sciences), and data were analyzed with FlowJo software version 9 (TreeStar). Further details, including the names of antibody clones and instrument set up, are provided in supplementary Methods.
Functional assays
Measurements of NK cell degranulation (CD107a) and intracellular cytokine production and proliferation (carboxyfluorescein diacetate succinimidyl ester [CFSE] dilution) were performed using standard protocols for flow cytometry. Details of coculture conditions, antibody-dependent cellular cytotoxicity (ADCC) assays, and stimulation conditions used in our study are provided in supplementary Methods.
Statistical analysis
For multiple group comparisons, 1-way analysis of variance or Kruskal-Wallis nonparametric tests were applied. For comparisons of independent groups, the Student t test or the Mann-Whitney U test was performed. For comparisons of matched groups, the paired Student t test or the Wilcoxon matched test was performed depending on the sample size. In Figure 2B, the Fisher exact test was used. In the relevant figures, n.s. indicates not significant; *** indicates P < .001; ** indicates P < .01; and * indicates P < .05. Analyses were performed using GraphPad software. For unbiased screening of potential subsets with unusually high KIR expression, statistical outliers were identified using the Chauvenet criterion. Further details of outlier identification of outliers are provided in supplementary Methods.
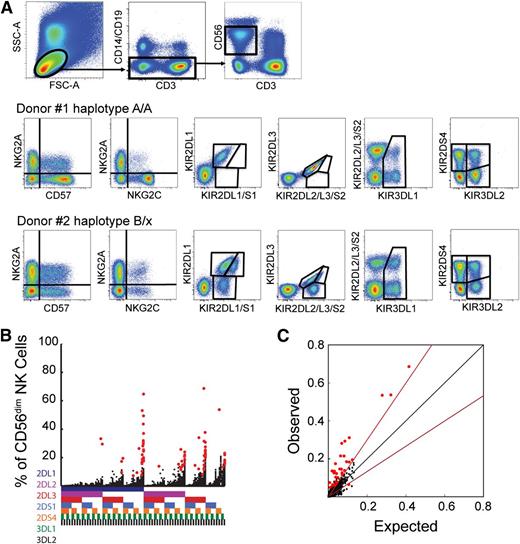
Characterization of human NK cell KIR repertoires. (A) 14-color flow cytometry panel for assessment of KIR2DL1, KIR2DL2/S2, KIR2DL3, KIR3DL1, KIR2DS1, KIR2DS4, and KIR3DL2 expression in CD56dim NK cell subsets expressing NKG2A, NKG2C, and/or CD57. Representative examples of stainings in KIR haplotype A homozygous and haplotype B/X donors are shown. (B) Frequency of CD56dim NK cells expressing the 7 analyzed KIRs and the 128 possible combinations thereof in 199 healthy donors. The presence of one KIR in a combination is represented by a color code below the graph: 2DL1 (dark blue), 2DL2/S2 (purple), 2DL3 (red), 2DS1 (light blue), 2DS4 (orange), 3DL1 (green), and 3DL2 (black). Red dots represent statistical outliers as defined by the Chauvenet criterion (see supplementary Information). (C) Observed frequencies of NK cells expressing 2 KIRs were compared with those expected from the product rule. The black line represents a perfect match between observed and expected values. The red line represents a 1.5-fold deviation from the product rule. Red dots represent statistical outliers as defined in (B).
Characterization of human NK cell KIR repertoires. (A) 14-color flow cytometry panel for assessment of KIR2DL1, KIR2DL2/S2, KIR2DL3, KIR3DL1, KIR2DS1, KIR2DS4, and KIR3DL2 expression in CD56dim NK cell subsets expressing NKG2A, NKG2C, and/or CD57. Representative examples of stainings in KIR haplotype A homozygous and haplotype B/X donors are shown. (B) Frequency of CD56dim NK cells expressing the 7 analyzed KIRs and the 128 possible combinations thereof in 199 healthy donors. The presence of one KIR in a combination is represented by a color code below the graph: 2DL1 (dark blue), 2DL2/S2 (purple), 2DL3 (red), 2DS1 (light blue), 2DS4 (orange), 3DL1 (green), and 3DL2 (black). Red dots represent statistical outliers as defined by the Chauvenet criterion (see supplementary Information). (C) Observed frequencies of NK cells expressing 2 KIRs were compared with those expected from the product rule. The black line represents a perfect match between observed and expected values. The red line represents a 1.5-fold deviation from the product rule. Red dots represent statistical outliers as defined in (B).
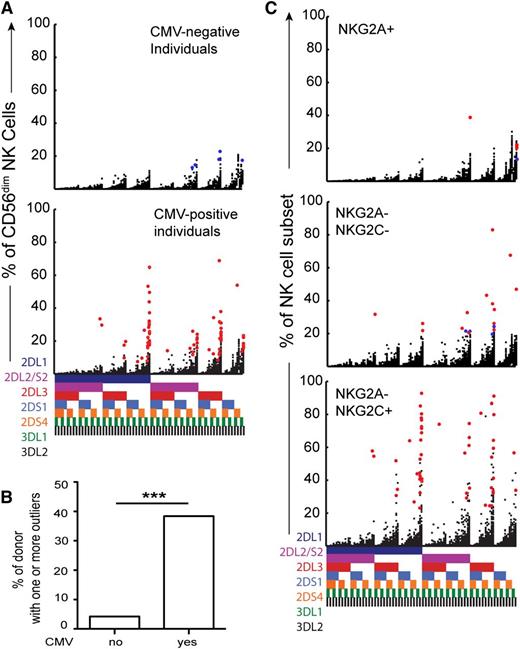
Dissecting the origin of the expanded KIR subsets. (A) Stratification of the cohort into CMV-seronegative (top, n = 48) and CMV-seropositive (bottom, n = 151) individuals. Red dots represent subsets with high relative frequencies (statistical outliers as defined in Figure 1B). (B) Shown are the frequencies of donors with 1 or more outliers according to CMV serostatus. (C) Representation of statistical outliers in CMV-seropositive (red dots) and CMV-seronegative (blue dots) donors originating from NKG2A+, NKG2C-NKG2A-, and NKG2C+ CD56dim NK cell subsets.
Dissecting the origin of the expanded KIR subsets. (A) Stratification of the cohort into CMV-seronegative (top, n = 48) and CMV-seropositive (bottom, n = 151) individuals. Red dots represent subsets with high relative frequencies (statistical outliers as defined in Figure 1B). (B) Shown are the frequencies of donors with 1 or more outliers according to CMV serostatus. (C) Representation of statistical outliers in CMV-seropositive (red dots) and CMV-seronegative (blue dots) donors originating from NKG2A+, NKG2C-NKG2A-, and NKG2C+ CD56dim NK cell subsets.
Results
Human CMV infection skews NK cell KIR repertoires
Initially, we determined KIR expression patterns on CD56dim NK cells in a cohort of 204 healthy donors by 14-color flow cytometry (Figure 1A). Among the 204 healthy individuals, 5 were excluded because KIR expression could not be resolved as a result of a known cross-reactivity of the anti-KIR2DS1/2DL1 mAb (clone EB6) (supplementary Information).27 An unbiased exploratory analysis of expression frequencies was used to determine the relative sizes of all 128 possible combinations of the 7 KIRs analyzed (Figure 1B). Among the 25 472 KIR expression frequencies assessed in the 199 donors, we identified 71 statistical outliers from a Gaussian distribution (detected in 60 of the donors), as determined by the Chauvenet criterion (Figure 1B, red dots). Expression of KIRs on the surface of NK cells is stochastic, and the coexpression of, for example, 2 KIRs can be calculated from their individual frequencies in accordance with the product rule, assuming random association of 2 independent events.28 However, KIR expression in the outliers deviated significantly from the product rule, suggesting that such subsets represented cells that had undergone a clonal-like expansion (Figure 1C).
As CMV is known to cause dynamic changes in the NK cell compartment of both mice and humans, we stratified the present cohort on the basis of seropositivity for CMV (Figure 2A). Outliers representing expansions of specific subsets were almost exclusively found in CMV-seropositive individuals (Figure 2A,B). Hence, 58 (38%) of the 151 CMV-seropositive donors displayed outliers in their KIR repertoires. In contrast, only 2 (4%) of 48 CMV-seronegative donors displayed such outliers. Because many of the CMV-seronegative donors had been exposed to other herpesviruses including EBV, HSV-1, HSV-2, and VZV (94%, 46%, 11%, and 100% seropositivity, respectively), the data suggested that these viruses do not cause major and/or sustained imprints in the human KIR repertoire. Supporting the involvement of NKG2C in responses to CMV,19 the majority of the observed expansions originated from the NKG2C+ NK cell subset (n = 48) (Figure 2C). Interestingly, however, we also found some outliers in the NKG2C-NKG2A- (n = 18) and, in rare cases, in the NKG2A+ subsets (n = 5) (Figure 2C).
Together, these results reveal the potential of high-resolution KIR phenotyping for tracing the adaptation of NK cells to virus infection. It is intriguing that in healthy individuals, the expansion of NK cell subpopulations with specific KIR expression patterns correlated exclusively with past CMV infection.
CMV induces expansion of NK cells expressing self-specific KIRs
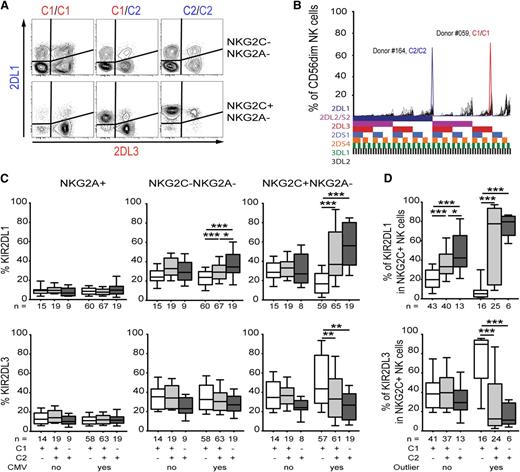
As most of the expanded subsets expressed at least 1 HLA-C-binding inhibitory KIR, namely KIR2DL1, KIR2DL2/S2, or KIR2DL3 (Figure 1B), we next examined whether cognate self-HLA class I molecules influenced the KIR expression patterns on the expanded cells. Indeed, expanded NKG2C+ NK cells in CMV-seropositive donors displayed near-clonal expression of KIR specific for self-HLA class I molecules (hereafter referred to as self-specific KIRs) (Figure 3A). Remarkably, in some donors, such expanded subpopulations represented more than 60% of the entire CD56dim NK cell compartment (Figure 3B). To analyze the effect of such NK cell expansions on the KIR repertoire, donors were stratified based on CMV serology in addition to their HLA class I background. CMV-seropositive individuals displayed a bias for self-specific KIRs that was most pronounced in the NKG2C+ NK cell subset (Figure 3C). Hence, HLA-C1/C1+ donors had high frequencies of NK cells expressing KIR2DL3 as their only inhibitory KIR, whereas HLA-C2/C2+ donors had high frequencies of NK cells expressing KIR2DL1 as their only inhibitory KIR. Consistent with these results, C1/C2 heterozygous donors presented with an intermediate profile of KIR2DL3 and KIR2DL1 expression (Figure 3C). Although some CMV-seropositive donors displayed an expansion of KIR2DL2/S2+ NK cells (Figure 2A), this could not be linked to the presence or absence of HLA-C1 or HLA-C2 (supplementary Figure 1). Among the outliers, we found only 1 that exclusively expressed KIR3DL1. This expansion occurred in an HLA-Bw4 donor, suggesting that similar skewing can be achieved for this educating KIR-HLA interaction. In contrast, CMV-seronegative donors exhibited essentially random KIR expression patterns without evidence of repertoire skewing, regardless of the NK cell subset analyzed (Figure 3C). Furthermore, independently of CMV serostatus, there was no bias for expression of self-specific KIRs in the NKG2A+ CD56dim NK cell compartment (Figure 3C, left panels).
CMV induces expansion of NK cells expressing a clonal pattern of self-specific KIRs. (A) Representative plot of KIR2DL1 and KIR2DL3 expression in NKG2C-NKG2A- (top) and NKG2C+ (bottom) CD56dim NK cell subsets. Three CMV-seropositive donors with different HLA genotypes are depicted. The color-coding pairs KIRs with their cognate HLA ligands. (B) Shown are 2 extreme examples of KIR repertoires in CMV-seropositive donors (C2/C2 blue and C1/C1 red) overlaid on the 48 KIR repertoires from CMV-seronegative individuals (n = 48). (C) The aggregated effect of the HLA-C genotype on KIR expression in all donors was examined by plotting frequencies of KIR2DL1 and KIR2DL3 in NKG2A+ (left), NKG2C-NKG2A- (middle), and NKG2C+ (right) NK cell subsets, respectively. Donors were stratified based on CMV serology and HLA background. (D) The effect of HLA-C genotype on self-specific KIR expression in CMV-seropositive individuals with and without evidence of NK cell expansion.
CMV induces expansion of NK cells expressing a clonal pattern of self-specific KIRs. (A) Representative plot of KIR2DL1 and KIR2DL3 expression in NKG2C-NKG2A- (top) and NKG2C+ (bottom) CD56dim NK cell subsets. Three CMV-seropositive donors with different HLA genotypes are depicted. The color-coding pairs KIRs with their cognate HLA ligands. (B) Shown are 2 extreme examples of KIR repertoires in CMV-seropositive donors (C2/C2 blue and C1/C1 red) overlaid on the 48 KIR repertoires from CMV-seronegative individuals (n = 48). (C) The aggregated effect of the HLA-C genotype on KIR expression in all donors was examined by plotting frequencies of KIR2DL1 and KIR2DL3 in NKG2A+ (left), NKG2C-NKG2A- (middle), and NKG2C+ (right) NK cell subsets, respectively. Donors were stratified based on CMV serology and HLA background. (D) The effect of HLA-C genotype on self-specific KIR expression in CMV-seropositive individuals with and without evidence of NK cell expansion.
To further delineate the contribution of NK cell expansion to the skewing of KIR repertoires, we stratified CMV-seropositive donors into those with and without evidence of NK cell expansion. This analysis revealed that the bias for self-specific KIRs was more pronounced in donors with NK cell expansion, supporting a causative effect of NK cell expansion on the observed KIR repertoire skewing associated with CMV infection (Figure 3D).
Education via self-specific KIRs promotes expansion of NKG2C+ NK cells
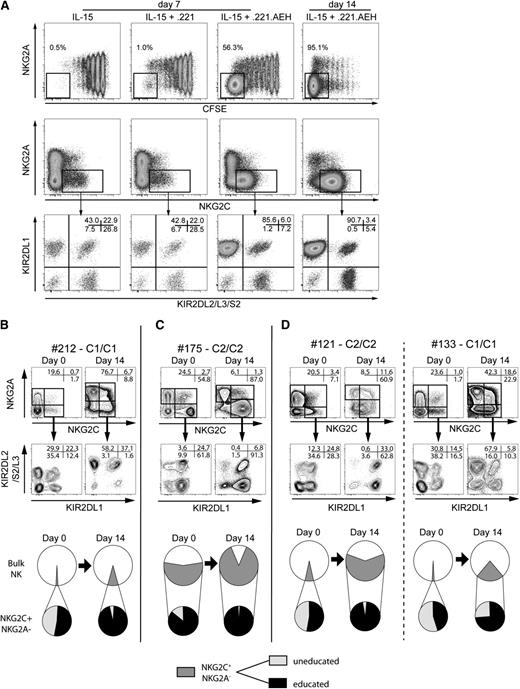
To gain insight into the mechanisms underlying the observed NK cell expansion and KIR repertoire skewing in CMV-seropositive donors, we used CFSE to label NK cells from CMV-seropositive donors without any visible preexisting expansion and cocultured these cells in the presence of IL-15 with HLA class I–negative 721.221 cells transfected with HLA-E (221.AEH). It is noteworthy that a majority of the NK cells that proliferated extensively in response to 221.AEH cells expressed NKG2C in combination with a (donor) self-specific KIR (Figure 4A). In contrast, no such polarized expansion of NK cells was seen after stimulation with wild-type 721.221 cells (221.wt).
Education promotes amplification and skewing of the KIR repertoire. (A) NK cells were labeled with CFSE and were cultured for 7 or 14 days with IL-15 alone or together with irradiated 221.wt or 221.AEH cells. At day 7 or day 14, cells were assessed for KIR and NKG2A/C expression. (B-D) Size of the NKG2C+ subset and KIR expression at day 0 and day 14 in coculture experiments with 221.AEH cells. Shown are representative examples (top) and pie charts (bottom) of NKG2C+ NK cell amplification and KIR skewing in (B) a CMV-seropositive donors without preexisting expansion, (C) a CMV-seropositive donor with preexisting expansion, and (D) 2 CMV-seronegative donors (C2/C2 left and C1/C1 right).
Education promotes amplification and skewing of the KIR repertoire. (A) NK cells were labeled with CFSE and were cultured for 7 or 14 days with IL-15 alone or together with irradiated 221.wt or 221.AEH cells. At day 7 or day 14, cells were assessed for KIR and NKG2A/C expression. (B-D) Size of the NKG2C+ subset and KIR expression at day 0 and day 14 in coculture experiments with 221.AEH cells. Shown are representative examples (top) and pie charts (bottom) of NKG2C+ NK cell amplification and KIR skewing in (B) a CMV-seropositive donors without preexisting expansion, (C) a CMV-seropositive donor with preexisting expansion, and (D) 2 CMV-seronegative donors (C2/C2 left and C1/C1 right).
To discern whether culture conditions gave rise to the amplification of a preexisting, albeit minimal, KIR skewing in CMV-seropositive donors with and without statistical outliers, we compared KIR expression on NKG2C+ NK cells at day 0 and at day 14 after coculture with 221.AEH cells. In donors without preexisting expansions, we noted a profound skewing toward self-specific KIRs in combination with a relative amplification of the NKG2C+ subset (Figure 4B). In donors with expansions, the preexisting skewing was further enhanced as the NKG2C+ NK cells expanded (Figure 4C). Importantly, skewing and subsequent amplification were also observed in cultures of NK cells from CMV-seronegative donors (Figure 4D).
Together, these data strongly suggest that KIR-mediated education promotes the expansion of NK cells stimulated via NKG2C.
Expanded NK cells display distinct phenotypic and functional properties
As mentioned above, the exploratory analysis of KIR repertoires in 204 healthy donors identified 18 and 5 statistically significant outliers in the NKG2A-NKG2C- and NKG2A+ compartments, respectively (Figure 2B). To examine the nature of these subsets in relationship to the relatively more frequent expanded NKG2C+ subpopulations, we performed a detailed evaluation of the phenotype and function of the NKG2C+ and NKG2C- outliers.
Among the 18 NKG2C- outliers, 8 displayed phenotypes similar to those of the expanded NKG2C+ subsets. They exhibited coordinated changes in the expression of several molecules, some of which have been associated previously with the differentiation of CD56dim NK cells (Figure 5A,B).29-31 All of these 8 expansions were derived from CMV-seropositive donors. The remaining ten NKG2C- outliers were derived from donors with contracted haplotypes expressing few KIR genes (on average 4.1 compared with 5.6 in the whole cohort) and exhibited less-differentiated phenotypes, suggesting that they had not undergone expansion (Figure 5B and supplementary Table 2). Of note, with fewer KIR genes expressed, the relative frequency of each subset becomes higher, potentially explaining why these subsets were falsely classified as outliers. Four of the false-negative outliers in this NK cell compartment were found in CMV-seronegative donors. With respect to the NKG2A+ outliers, only the one with the most striking expression frequency (Figure 2) had a differentiated phenotype. What is intriguing is that in this particular case, the expanded NKG2A+ subsets coexpressed NKG2C (supplementary Figure 2). Importantly, none of the donors harboring NKG2C- expansions displayed homozygous deletion of the NKG2C gene.32
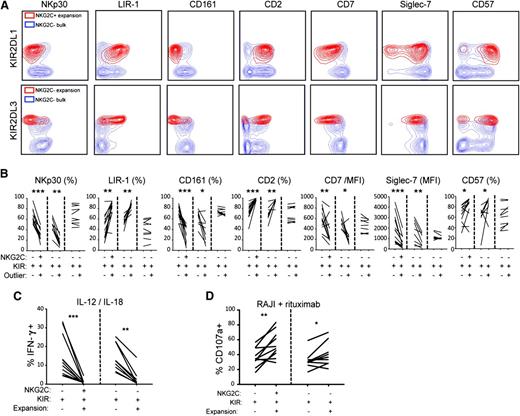
Expanded NK cells display distinct phenotypic and functional properties. (A) Representative staining of the indicated surface molecules on NK cell expansions in the NKG2C+ and NKG2C-NKG2A- NK cell subsets from CMV-seropositive donors. (B) Aggregated phenotypes of 10 representative NKG2C+ expansions compared with the 18 identified NKG2C-NKG2A- outliers with (n = 8) and without (n = 10) a differentiated phenotype. (C) NK cells were stimulated overnight with IL-12 and IL-18 in the presence of brefeldin A and were stained intracellularly for IFN-γ production. The figure shows IFN-γ production in NK cell expansions originating from NKG2C+ (n = 10) and NKG2C-NKG2A- (n = 8) compartments. (D) NK cells were stimulated for 2 hours with rituximab-coated Raji cells in ADCC experiments and were stained for CD107a to monitor degranulation. The figure shows CD107a expression in NK cell expansions originating from NKG2C+ (n = 10) and NKG2C-NKG2A- (n = 8) compartments. In (B-D), all comparisons were made with the relevant KIR+ NK cell subset.
Expanded NK cells display distinct phenotypic and functional properties. (A) Representative staining of the indicated surface molecules on NK cell expansions in the NKG2C+ and NKG2C-NKG2A- NK cell subsets from CMV-seropositive donors. (B) Aggregated phenotypes of 10 representative NKG2C+ expansions compared with the 18 identified NKG2C-NKG2A- outliers with (n = 8) and without (n = 10) a differentiated phenotype. (C) NK cells were stimulated overnight with IL-12 and IL-18 in the presence of brefeldin A and were stained intracellularly for IFN-γ production. The figure shows IFN-γ production in NK cell expansions originating from NKG2C+ (n = 10) and NKG2C-NKG2A- (n = 8) compartments. (D) NK cells were stimulated for 2 hours with rituximab-coated Raji cells in ADCC experiments and were stained for CD107a to monitor degranulation. The figure shows CD107a expression in NK cell expansions originating from NKG2C+ (n = 10) and NKG2C-NKG2A- (n = 8) compartments. In (B-D), all comparisons were made with the relevant KIR+ NK cell subset.
NKG2C- subsets with a differentiated phenotype shared functional properties with expanded NKG2C+ subsets. Thus, both NKG2C+ and NKG2C- expanded subsets represented extremes of the previously characterized NK cell differentiation process,30,31 with poor ability to produce interferon γ (IFN-γ) after IL-12/IL-18 stimulation (Figure 5C), but stronger ADCC against rituximab (anti-CD20mAb)–coated Raji cells (Figure 5D) than unexpanded NK cell subsets. Hence, the expansion of specific subpopulations of NK cells after CMV infection is associated with NK cell differentiation and can occur independently of NKG2C.
A role for activating KIRs in driving the expansion of NKG2C- NK cell subsets
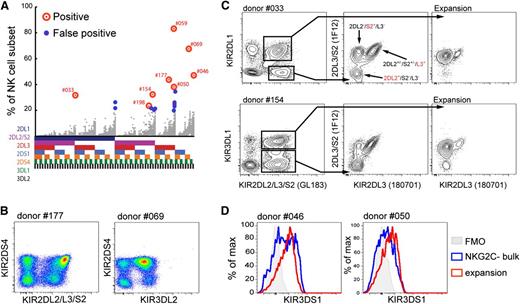
Next, we set out to identify the possible mechanisms behind the expansion of NKG2C- NK cells. All of the 8 expanded NK cell subsets within the NKG2C- compartment were from donors carrying group B KIR haplotypes (Figure 6A and supplementary Table 3). This indicated that activating KIRs might play a role in promoting the proliferation of these NK cell subsets. Among the 8 donors, 2 displayed expansion of KIR2DS4+ NK cells (Figure 6A,B and supplementary Table 3). As none of the expanded NKG2C- NK cell populations expressed KIR2DS1, the remaining 6 identified NKG2C- outliers could not be fully deciphered with the flow cytometry panel used in the exploratory KIR analysis. However, KIR genotyping of these donors suggested that the expanded populations could potentially express KIR2DS2 or KIR3DS1 (supplementary Table 3). Phenotypically, 3 of the 6 expansions were positive for GL183 (anti-KIR2DL2/DS2/2DL3) (Figure 6A and supplementary Table 3). To discriminate between KIR2DL2 and KIR2DS2, we combined the mABs GL183 and 180701 (KIR2DL3) with the recently described 1F12 antibody (KIR2DL3/S2) (Figure 6C).33 In all 3 donors (#033, #154, and #198), the expanded cells exclusively expressed KIR2DS2 (Figure 6C and supplementary Table 3). The 3 remaining undefined expanded populations were all KIR3DS1+ at the genetic level. By combining DX9 (KIR3DL1) and Z27 (KIR3DL1/DS1),34 we showed that all 3 expanded NKG2C- NK cell subsets (in donors #046, #050, and #059) were KIR3DS1+ (Figure 6D and supplementary Table 3). Hence, in all 8 expanded subpopulations, we could demonstrate the expression of KIR2DS2, KIR2DS4, or KIR3DS1. Furthermore, although 4 of the 8 expanded subpopulations coexpressed KIR2DL1, KIR2DL3, or KIR3DL1 in the presence of their cognate ligands, the remaining expanded subpopulations lacked self-specific inhibitory KIRs. Thus, unlike the scenario for NKG2C+ NK cell expansion, education through inhibitory receptors was not mandatory for the expansion of NK cells expressing activating KIR.
Expanding NKG2C-NKG2A- NK cells express activating KIRs. (A) Expansions in the NKG2C-NKG2A- compartment with a differentiated phenotype are marked with red circles. False-positive outliers with less differentiated phenotypes are marked with small blue circles. Gray dots represent the KIR repertoires in the 199 donors. (B) KIR expression patterns in representative CMV-seropositive donors with KIR2DS4+ expansion in the NKG2A-NKG2C- NK cell subset. (C) Identification of NK cell expansions in CMV-seropositive donors expressing KIR2DS2 using combinations of the indicated anti-KIR antibodies. Expansions (right) and their respective GL183+ internal controls (middle) were gated out, as depicted in the bivariate plots (left). (D) Representative CMV-seropositive donors with NKG2A-NKG2C- expansions expressing KIR3DS1. KIR3DS1-single positive cells are shown (Z27+DX9-) after exclusion of KIR3DL1+ (Z27+DX9+) cells.
Expanding NKG2C-NKG2A- NK cells express activating KIRs. (A) Expansions in the NKG2C-NKG2A- compartment with a differentiated phenotype are marked with red circles. False-positive outliers with less differentiated phenotypes are marked with small blue circles. Gray dots represent the KIR repertoires in the 199 donors. (B) KIR expression patterns in representative CMV-seropositive donors with KIR2DS4+ expansion in the NKG2A-NKG2C- NK cell subset. (C) Identification of NK cell expansions in CMV-seropositive donors expressing KIR2DS2 using combinations of the indicated anti-KIR antibodies. Expansions (right) and their respective GL183+ internal controls (middle) were gated out, as depicted in the bivariate plots (left). (D) Representative CMV-seropositive donors with NKG2A-NKG2C- expansions expressing KIR3DS1. KIR3DS1-single positive cells are shown (Z27+DX9-) after exclusion of KIR3DL1+ (Z27+DX9+) cells.
Together, these results suggest a role for activating KIRs in driving the expansion of specific NK cell subsets.
NK cell subset expansions are stable with time
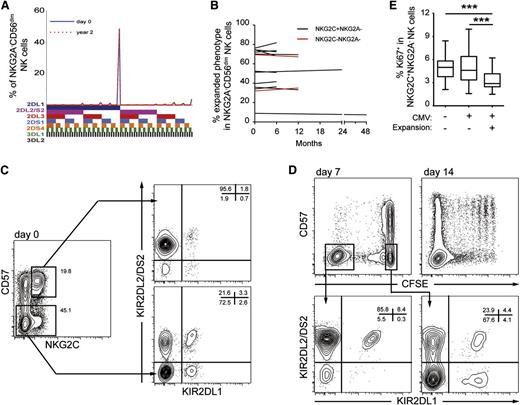
The exploratory analysis of KIR expression and the identification of outliers represent snapshots of the repertoires at any given time point. To examine possible dynamics with time, we monitored KIR expression patterns longitudinally for periods ranging from 6 months to 4 years. The expanded NK cell subsets remained stable both in composition and in size (Figure 7A,B). This contrasts with the dynamic expansion/contraction observed during acute CMV infection after transplantation.25,35 We noted a tendency for more dynamic behavior within the expansions present in a small but unique cohort of children sampled at birth (cord blood) and at 2 and 5 years old. During the first 2 years of life, children infected with CMV, but not uninfected children, displayed an expansion of NKG2C+ NK cells (supplementary Figure 3). In 2 of the 5 children with evidence of NK cell subset expansions at 2 years old, we noted a decline of the expanded phenotype at age 5 years. Despite a dynamic phase during acute CMV infection and possibly also during early life, the adaptation of the NK cell compartment to CMV results in remarkably stable imprints in healthy adult individuals.
Stability of NK cell expansions with time. (A) Example of KIR repertoire stability in an adult with a sampling interval of 2 years. Shown is the frequency of cells expressing the indicated combination of KIRs in the NKG2A- CD56dim NK cell compartment. (B) Summary showing the stability of NKG2C+ (black) and activating KIR+ (red) expansions in CMV-seropositive individuals at sampling intervals ranging from 6 months to 4 years. (C,D) NK cells were labeled with CFSE, stimulated in vitro with IL-15, and cocultured with 221.AEH cells. (C) Density plots show KIR expression patterns of the indicated NK cell subsets at day 0, before proliferation. (D) Density plots show the phenotype of proliferating (CFSE-negative) and nonproliferating cells (CFSE-positive) at days 7 and 14, and their KIR expression patterns at day 7 (bottom). Results are representative of 3 independent experiments. (E) Frequency of Ki67+ NKG2C+ NK cells in peripheral blood of healthy donors. Three groups of donors were analyzed: CMV-seronegative donors (n = 46), and CMV-seropositive donors with (n = 48) and without (n = 100) expansion in the NKG2C+ NK cell compartment. Bars in the box and a whisker plot represent the highest and the lowest interquartiles.
Stability of NK cell expansions with time. (A) Example of KIR repertoire stability in an adult with a sampling interval of 2 years. Shown is the frequency of cells expressing the indicated combination of KIRs in the NKG2A- CD56dim NK cell compartment. (B) Summary showing the stability of NKG2C+ (black) and activating KIR+ (red) expansions in CMV-seropositive individuals at sampling intervals ranging from 6 months to 4 years. (C,D) NK cells were labeled with CFSE, stimulated in vitro with IL-15, and cocultured with 221.AEH cells. (C) Density plots show KIR expression patterns of the indicated NK cell subsets at day 0, before proliferation. (D) Density plots show the phenotype of proliferating (CFSE-negative) and nonproliferating cells (CFSE-positive) at days 7 and 14, and their KIR expression patterns at day 7 (bottom). Results are representative of 3 independent experiments. (E) Frequency of Ki67+ NKG2C+ NK cells in peripheral blood of healthy donors. Three groups of donors were analyzed: CMV-seronegative donors (n = 46), and CMV-seropositive donors with (n = 48) and without (n = 100) expansion in the NKG2C+ NK cell compartment. Bars in the box and a whisker plot represent the highest and the lowest interquartiles.
Continuous replenishment of the clonal-like phenotype
Finally, to gain insight into the possible mechanisms behind the stability of the KIR repertoire, we examined whether the same specificity could be generated in CMV-seropositive donors with preexisting expansions. Indeed, in a C1/C1 donor, showing in vivo expansion of KIR2DL2+ NK cells (Figure 7C), expanded cell populations with a similar KIR phenotype could be generated in vitro after coculture with 221.AEH cells in the presence of IL-15 (Figure 7D). The cellular source of proliferating cells was the relatively less-differentiated CD57-NKG2C+ NK cell subset rather than the more-differentiated CD57+NKG2C+ NK cell subset. At steady state, the NKG2C+ NK cells displayed a slow turnover rate in vivo, as determined by low frequencies of Ki67-expressing cells (Figure 7E). Together, these results suggest that the stability of the expanded KIR phenotype may be achieved by continuous replenishment from more immature NK cells rather than by long-term survival or stem cell–like renewal of previously expanded cells.
Discussion
Our present study provides a comprehensive analysis of NK cell KIR repertoire diversity in healthy humans. Strikingly, adaptation of the NK cell compartment to CMV infection resulted in a profound and stable imprint in the KIR repertoires because of expansion of NK cells expressing self-specific inhibitory KIRs. In sharp contrast, KIR expression in CMV-seronegative donors was essentially random, arguing against intrinsic selection processes during NK cell development as well as imprinting by other common persistent virus infections. Furthermore, the presented platform for high-resolution KIR phenotyping allowed us to assess the expression of activating KIRs in donors carrying the KIR B haplotype, revealing the involvement of KIR2DS2, KIR2DS4, and KIR3DS1 in the adaptation of the NK cell compartment to CMV infection.
Recent insights into NK cell biology clearly demonstrate that the phenotype and function of NK cells are more dynamic than previously appreciated, challenging the conceptual border between innate and adaptive immunity.36 Hence, NK cells differentiate throughout their lifespan, tune their responsiveness by interactions with HLA class I molecules, and undergo specific expansion after virus infection, features commonly attributed to adaptive immune behavior.37 One objective of our study was to explore whether dynamic changes in randomly generated KIR repertoires could be used to trace adaptations to virus infections in humans. Indeed, our current unbiased exploratory analysis revealed the existence of expanded and highly differentiated NK cell subsets visible as stable alterations in overall KIR expression profiles at steady state. Notably, such expansions were observed almost exclusively in CMV-seropositive donors. In fact, all 5 statistical outliers found in the 2 CMV-seronegative donors (Figure 2A) had less differentiated phenotypes and were among the false-positive outliers in the NKG2A-NKG2C- (n = 4) and NKG2A+ (n = 1) NK cell subsets, as revealed by combining KIR repertoire analysis with phenotypic assessment of differentiation status. Hence, none of the 48 CMV-seronegative individuals displayed evidence of NK cell expansion, although many of them were seropositive for HSV-1, HSV-2, VZV, and EBV; and, most likely, to human herpesvirus-6/-7 (HHV-6/-7), given the high prevalence of HHV-6/7 in the Swedish population. This outcome raises the intriguing question of whether CMV is the only virus that triggers NK cell expansion in humans. Although the mechanisms that promote expansion and functional reprogramming of human NK cells are not fully understood, it is tempting to speculate that stable alterations in KIR repertoires require low-grade virus replication and/or transcription of some early virus proteins leading to a long-term influence on newly developing NK cells. Among the many mechanisms that CMV uses to escape the immune system, one of the best described is the interference with MHC class I antigen presentation.38 However, HLA-E is resistant to such effects because of CMV gpUL40–mediated stabilization of the complex.39 In this context, Gumá and collaborators showed that CMV-infected fibroblasts triggered the expansion of NKG2C+ NK cells in vitro.40 Similar results were obtained by coculturing NK cells with HLA class I–negative 721.221 cells transfected with HLA-E.41 Extending these results, we show that NK cell education (ie, expression of a self-specific KIR) promotes the efficient expansion of NKG2C+ NK cells in vitro. These findings are in line with a recent study showing expansion of NK cells expressing self-specific KIRs after coculture with CMV-infected fibroblasts.42 Importantly, our results demonstrate that educated subsets can be amplified, leading to global KIR repertoire skewing in both CMV-seropositive and CMV-seronegative individuals. In donors with preexisting expansion, recapitulation of the KIR phenotype in vitro was achieved by a marked proliferation of less-differentiated CD57- NK cells, rather than by expansion of the preexisting differentiated CD57+ NK cell subset. These results are compatible with a continuous replenishment of educated NKG2C+ NK cells and could explain the stability of the expanded phenotype during several years. This interpretation is supported by a recent study, in which persistence of NKG2C+ NK cells was dependent on CMV antigens in the recipient.24 Importantly, this proposed mechanism does not exclude the possibility that other viruses cause temporary dynamic changes in the repertoire, which disappear when the infection resolves.
Skewing of KIR repertoires toward self-specific KIRs has previously been observed in patients infected with hantavirus,22 chikungunya virus,43 chronic HBV/HCV,44 and HIV.45 It is possible that acute or chronic coinfection with other viruses causes reactivation of CMV at levels below detection with conventional technologies, but still sufficient to prime newly emerging NK cells. Such a mechanism is compatible with the more transient (<6 months) skewing of the NK cell repertoire observed during acute hantavirus infection.22
Epidemiologic studies have suggested that KIR B haplotypes and activating KIRs are associated with a reduced risk for CMV reactivation after allogeneic stem cell transplantation.14 Interestingly, Gallez-Hawkins and collaborators noted increased transcripts of KIR2DS2 and KIR2DS4 on CMV reactivation after allogeneic hematopoietic stem cell transplantation.46 A striking observation in our study was the NKG2C-independent expansion of NK cells expressing activating KIR in CMV-seropositive donors. Of the 8 observed expansions, 2 were KIR2DS4+, 3 were KIR3DS1+, and 3 were KIR2DS2+. None of these activating KIRs have any known natural ligands,5 although KIR2DS4 binds weakly to HLA-A*1102 and a limited number of HLA-C1/-C2 alleles,9 and 3DS1*014 (a rare allele) binds weakly to HLA-Bw4.47 Indeed, the expansion of KIR3DS1+ and KIR2DS4+ NK cells was observed in the absence of their presumed weak HLA class I ligands, suggesting that virally encoded or other non-HLA ligands may be involved. Although the molecular mechanism behind expansion of NK cell subsets with activating KIRs remains elusive, our findings provide indirect evidence for their involvement in dynamic NK cell responses to CMV infection. Whether activating KIRs also contribute to the expansion of NKG2C+ NK cells in haplotype B donors remains unclear. Although we did find rare examples of expanded subsets coexpressing NKG2C and activating KIR, the frequency of such coexpression could not be inferred from our exploratory analysis. However, because we observed similar frequencies of NKG2C+ expansions in group A homozygous donors, we could at least exclude that activating KIRs were necessary for the expansion of NKG2C+ NK cells.
A fundamental question that has gained much attention is whether NK cells are selected based on interactions with their cognate MHC class I molecules during development. The accumulation of NK cells expressing self-specific Ly49 molecules observed in mice48 and, similarly, KIRs in some cohorts of healthy adult donors, support the existence of such repertoire selection processes.49,50 However, there is no evidence for negative selection or deletion of NK cells expressing a combination of receptors that could be harmful or useless.28 Furthermore, no bias for expression of self-specific KIRs could be detected in another cohort of healthy donors,51 or in neonates.52 Potentially explaining the discrepant results in earlier studies, our analysis, including 48 CMV-seronegative donors, shows that NK cells display a random repertoire without any significant influence from cognate HLA class I molecules. However, because education promotes NKG2C-driven proliferation after infection with CMV, the randomly formed KIR repertoires are profoundly imprinted by the virus infection resulting in biased expression of self-specific inhibitory KIRs in the affected individuals.
In summary, our analysis provides new insights into the diversity and dynamics of the human “KIR-ome.” Adaptation of KIR repertoires to CMV infection with expansion and differentiation of highly functional educated NK cells may have downstream consequences for the individual’s ability to resist subsequent infections and tumors. Finally, the discovery that NK cells with a given specificity can be rapidly generated in vitro open up new avenues for adoptive NK cell-based therapy guided by the HLA class I genotype of the patient.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Swedish Research Council, the Swedish Children’s Cancer Society, the Swedish Cancer Society, the Royal Swedish Academy of Sciences, the Tobias Foundation, the Karolinska Institutet, the Wenner-Gren Foundation, Oslo University Hospital, the MRC, and the Wellcome Trust.
Authorship
Contribution: V.B. designed and performed research, analyzed data, and wrote the manuscript. L.L.L., M.A.I., and J.T. performed research and analyzed data. J.-A.M. analyzed data. E.S.-E., A.T.B., and M.S. performed research. C.R. and E.S.-E. provided reagents and clinical samples, respectively. J.T., P.L., D.A.P., J.M., and H.-G.L. contributed to the writing of the manuscript. K.-J.M. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karl-Johan Malmberg, Center for Infectious Medicine, F59, Karolinska University Hospital, Huddinge, Stockholm, 14186 Sweden; e-mail: kalle.malmberg@ki.se; and Vivien Béziat, Center for Infectious Medicine, F59, Karolinska University Hospital, Huddinge, Stockholm, 14186 Sweden; e-mail: vivien.beziat@ki.se.