Key Points
Mutations in GATA2 are a cause of human NK cell deficiency.
GATA2 is required for human NK cell maturation, specifically maintenance of the CD56bright subset.
Abstract
Mutations in the transcription factor GATA2 underlie the syndrome of monocytopenia and B- and natural killer (NK)-cell lymphopenia associated with opportunistic infections and cancers. In addition, patients have recurrent and severe viral infections. NK cells play a critical role in mediating antiviral immunity. Human NK cells are thought to mature in a linear fashion, with the CD56bright stage preceding terminal maturation to the CD56dim stage, considered the most enabled for cytotoxicity. Here we report an NK cell functional defect in GATA2-deficient patients and extend this genetic lesion to what is considered to be the original NK cell-deficient patient. In most cases, GATA2 deficiency is accompanied by a severe reduction in peripheral blood NK cells and marked functional impairment. The NK cells detected in peripheral blood of some GATA2-deficient patients are exclusively of the CD56dim subset, which is recapitulated on in vitro NK cell differentiation. In vivo, interferon α treatment increased NK cell number and partially restored function but did not correct the paucity of CD56bright cells. Thus, GATA2 is required for the maturation of human NK cells and the maintenance of the CD56bright pool in the periphery. Defects in GATA2 are a novel cause of profound NK cell dysfunction.
Introduction
Spontaneous or autosomal dominant mutations in GATA2 are the unifying cause of syndromes variously described as monocytopenia with mycobacterial disease; dendritic cell (DC), myeloid, and natural killer (NK) cell lymphopenia; lymphedema and myelodysplasia (Emberger); and familial myelodysplasia/leukemia.1-6 These syndromes are characterized by DC, monocyte, and B- and NK-cell lymphopenia and susceptibility to myelodysplasia and mycobacterial and severe papilloma virus infections.1-4,7 Reported patients with GATA2 deficiency have an absence or profound reduction in the numbers of NK cells, monocytes, and B cells. In addition, both dermal and circulating DCs are affected.2 GATA2 functions in the regulation of hematopoiesis and in particular is required for maintenance and survival of the hematopoietic stem cell pool.8-13 GATA2 also functions in the formation of early blood and lymphatic vessels.6,14 The role of GATA2 mutation in the manifestation of the disease is incompletely understood but likely complex and thought to be linked to the generation or maintenance of progenitors required for the affected cell subsets.13
Few clinical immunodeficiencies featuring the absence of mature circulating NK cells have been documented.15 The first and most paradigmatic case described an adolescent girl with severe varicella who lacked peripheral NK cells and NK cell cytotoxic function and subsequently developed cytomegalovirus and herpesvirus infections.16 She presented with lymphopenia that was unresolved over her 10-day hospital stay. During subsequent visits over the next 4 years, she consistently had lymphopenia and lacked CD56+CD16+ cells in peripheral blood. Her peripheral blood mononuclear cells (PBMCs) had no activity in cytotoxicity assays, either in the presence or absence of interleukin (IL)-2. Subsequently, similar cases of fatal susceptibility to viral, particularly varicella zoster virus, infections were reported in people also found to lack CD56+ PBMCs. These cases have highlighted the critical role of NK cells in antiviral immunity but have lacked genetic or mechanistic unification.17-19
Here we describe the NK cell deficiency found in GATA2-deficient patients. We also identified that the original case of NK cell deficiency described in 1989 was caused by GATA2 deficiency. Although GATA2-deficient patients have severely decreased NK cell numbers, many have a low frequency of NK cells with specific loss of the CD56bright NK cell subset, in which we show GATA2 is highly expressed. The NK cells that are present are exclusively of the CD56dim subset and express the associated cell surface markers. Despite this, NK cell function is severely impaired. Thus, we show that GATA2 is required for the presence of CD56bright NK cells and functional maturation of the CD56dim subset. This locates GATA2 at a key place in human NK-cell development, providing novel and important insight into NK-cell maturation by underscoring the importance of the CD56bright pool for generation of functional circulating CD56dim NK cells.
Materials and methods
Blood samples
PBMCs were isolated from whole blood of sequence-proven GATA2-deficient patients or healthy donors by density centrifugation over Ficoll-Paque Plus lymphocyte isolation medium (GE Healthcare, Pittsburgh, PA). For patient 1, DNA was isolated from stored IL-2–dependent T-cell lines. Sequencing was performed at the National Institutes of Health as described.3 All patients signed informed consent in accordance with the Declaration of Helsinki, or in some cases, the research was deemed exempt by the institutional review board. All samples were obtained with the approval of the institutional review board of the Children’s Hospital of Philadelphia, Baylor College of Medicine, UT Southwestern Medical Center, and the National Institutes of Health.
Target cell lines
51Cr release assay
51Cr cytotoxicity assays were performed as described.20 Where indicated, assays were performed in the presence of 1000 U/mL IL-2 (Roche Diagnostics, Indianapolis, IN). For antibody-mediated cellular cytotoxicity assays, Raji targets were incubated with Rituximab. All assays were performed on fresh PBMCs except for the PBMCs of patient 8, which were done with cryopreserved cells thawed and rested 1 hour in RPMI 20% fetal calf serum. Lytic units 20, the lytic activity of NK cells required to kill 20% of targets (LU20), were calculated as previously described.20
Flow cytometry
Flow cytometry was performed on isolated PBMCs for the routine identification of NK cells and NK cell markers. NK cells were identified as being CD56+CD3−. The density of CD56 staining was used to identify CD56bright NK cells, and the demarcation between CD56bright and CD56dim populations was further confirmed using CD16 on NK cells to identify the CD56dimCD16+ population. For detailed NK-cell surface marker evaluation, percent positive was measured as CD56+CD3− cells expressing the marker of interest above that of an isotype control.22 Patients with NK-cell populations comprising <1% of the lymphocyte gate were excluded from detailed NK-cell surface marker analysis.
Western blotting
Purified B cells (CD19+), T cells (CD3+), monocytes (CD14+), and NK cells (CD56+CD3−) were isolated from peripheral blood by fluorescence-activated cell sorter (FACS) using a MoFlow cell sorter (Beckman Coulter, Indianapolis, IN). Western blot analysis was performed as described using GATA2 (ab22849; Abcam, Cambridge, MA) and actin (Sigma, St. Louis, MO) antibodies.20
In vitro NK-cell differentiation
Highly purified CD34+ hematopoietic precursors were isolated from peripheral blood by FACS sorting using a MoFlow (Beckman Coulter) and cultured in medium containing 5 ng/mL IL-3, 20 ng/mL IL-7, 20 ng/mL stem cell factor, 10 ng/mL Fms-related tyrosine kinase 3 ligand (Flt3L), and 10 ng/mL IL-15 (Peprotech, Rocky Hill, NJ) on irradiated EL08.1D2 stromal cells as described.21,23 After 30 days, cells were harvested and analyzed by FACS. Any remaining stromal cells were excluded from analysis based on forward/side scatter.
In vivo administration of IFNα
IFNα2b (106 U) was administered subcutaneously 3 times weekly.
Statistics
Data were compared using a Student 2-tailed t test or a 2-tailed Mann-Whitney U test (GraphPad 5.0 Software) where indicated.
Results
GATA2 is the cause of the hallmark case of NK-cell deficiency
The original case of classical NK cell deficiency was reported in 1989.16 In addition to her leukopenia and recurrent bacterial infections, the patient had disseminated varicella zoster virus, cytomegalovirus infections, and severe herpes simplex infection. She subsequently developed aplastic anemia and died during the process of hematopoietic stem cell transplantation (previously unreported). Because of her disease presentation and known NK-cell deficiency, we suspected that her condition was caused by a GATA2 mutation. This was confirmed by sequencing of DNA derived from a stored IL-2–dependent T-cell line from the patient (patient 1, Table 1) that identified a GATA2 frameshift mutation, c.1025_1026insGCCG; p.A342GfsX41, predicted to cause deletion of the second zinc finger in GATA2.
Description of GATA-deficient patient mutations and clinical phenotypes
| Patient ID and pedigree no. . | Mutation . | Clinical infectious phenotype . | Other clinical phenotype . |
|---|---|---|---|
| Patient 116 | c.1025_1026insGCCG; p.A342GfsX41 | Severe varicella zoster virus, cytomegalovirus, herpes simplex virus | Interstitial lung disease |
| Aplastic anemia | |||
| Patient 21 ,3 (12.I.1) | c.1083_1094del12; p.R361delRNAN | Disseminated Mycobacterium kansasii | |
| Severe perineal human papillomavirus | |||
| Hepatitis C virus with cirrhosis | |||
| Patient 31 (4.II.1) | c.1017+572C>T | Disseminated Mycobacterium avium | Common variable immunodeficiency |
| Disseminated histoplasmosis | Miscarriages | ||
| Basal and squamous cell skin carcinomas | |||
| Metastatic breast cancer | |||
| Pulmonary alveolar proteinosis | |||
| Patient 43 (17.I.1) | c.1061C>T; p.T354M | Disseminated Mycobacterium kansasii | |
| Severe human papillomavirus | |||
| Severe Trychophyton rubrum | |||
| Patient 51 ,25,27 (6.II.1) | c.1017+512del28 | Group C streptococcal osteomyelitis | |
| Severe perineal human papillomavirus | |||
| Patient 61 ,3 (15.II.1) | c.1186C>T; p.R398W | Disseminated mycobacterium avium complex | |
| Patient 725 (26.I.1) | c.302delG; p.G101AfsX16 | Disseminated cytomegalovirus | Acute myeloid leukemia |
| Cytomegalovirus gastroduodenitis | Chronic myelomonocytic leukemia | ||
| Severe perineal herpes simplex virus 2 | Myelodysplastic syndrome | ||
| Patient 8 | c.417dupT; p.V140CfsX44 | Severe human papillomavirus | Squamous cell carcinoma of the vulva |
| Patient ID and pedigree no. . | Mutation . | Clinical infectious phenotype . | Other clinical phenotype . |
|---|---|---|---|
| Patient 116 | c.1025_1026insGCCG; p.A342GfsX41 | Severe varicella zoster virus, cytomegalovirus, herpes simplex virus | Interstitial lung disease |
| Aplastic anemia | |||
| Patient 21 ,3 (12.I.1) | c.1083_1094del12; p.R361delRNAN | Disseminated Mycobacterium kansasii | |
| Severe perineal human papillomavirus | |||
| Hepatitis C virus with cirrhosis | |||
| Patient 31 (4.II.1) | c.1017+572C>T | Disseminated Mycobacterium avium | Common variable immunodeficiency |
| Disseminated histoplasmosis | Miscarriages | ||
| Basal and squamous cell skin carcinomas | |||
| Metastatic breast cancer | |||
| Pulmonary alveolar proteinosis | |||
| Patient 43 (17.I.1) | c.1061C>T; p.T354M | Disseminated Mycobacterium kansasii | |
| Severe human papillomavirus | |||
| Severe Trychophyton rubrum | |||
| Patient 51 ,25,27 (6.II.1) | c.1017+512del28 | Group C streptococcal osteomyelitis | |
| Severe perineal human papillomavirus | |||
| Patient 61 ,3 (15.II.1) | c.1186C>T; p.R398W | Disseminated mycobacterium avium complex | |
| Patient 725 (26.I.1) | c.302delG; p.G101AfsX16 | Disseminated cytomegalovirus | Acute myeloid leukemia |
| Cytomegalovirus gastroduodenitis | Chronic myelomonocytic leukemia | ||
| Severe perineal herpes simplex virus 2 | Myelodysplastic syndrome | ||
| Patient 8 | c.417dupT; p.V140CfsX44 | Severe human papillomavirus | Squamous cell carcinoma of the vulva |
GATA2 is required for NK cell–mediated cytotoxic activity
We evaluated 5 living patients with mutations affecting multiple regions of GATA2 (patients 2-6, Table 1). These patients showed various clinical signs of GATA2 deficiency, including disseminated nontuberculous mycobacterial infection, recurrent papilloma virus infection, and extensive cutaneous and genital herpesviral infections. Five of these patients have been previously described and all are known to have reduced numbers of NK cells.3,24,25
To assess the NK-cell deficiency in greater detail and its relation to the clinical phenotype of GATA2 deficiency, we tested NK-cell cytotoxic function using PBMCs isolated from whole blood of patients and gender-matched controls (Figure 1). All patients tested, with the exception of patient 4 who had some residual cytotoxic function, had a profound defect in NK cell–mediated cytotoxicity that was not significantly rescued by the addition of exogenous IL-2. In addition, antibody-mediated cellular cytotoxicity was also lacking in GATA2-deficient patients (supplemental Figure 1). Therefore, GATA2 integrity is required for the effective lysis of prototypical NK-cell targets by ex vivo human PBMCs. To account for the reduced numbers of NK cells within PBMCs, we calculated LU20 per NK cell (supplemental Figure 2). GATA2-deficient patients had decreased cytotoxicity per NK cell, suggesting a functional defect in addition to that caused by the reduction of NK cell number.
GATA2-deficient patients have defective NK cell cytotoxicity. NK cell functional activity was measured against susceptible K562 target cells in the presence of IL-2 where indicated (dashed line) using PBMCs isolated from whole blood. PBMCs from 5 patients (blue) were evaluated in combination with gender-matched controls (red).
GATA2-deficient patients have defective NK cell cytotoxicity. NK cell functional activity was measured against susceptible K562 target cells in the presence of IL-2 where indicated (dashed line) using PBMCs isolated from whole blood. PBMCs from 5 patients (blue) were evaluated in combination with gender-matched controls (red).
GATA2 mutation results in significantly reduced numbers of peripheral NK cells
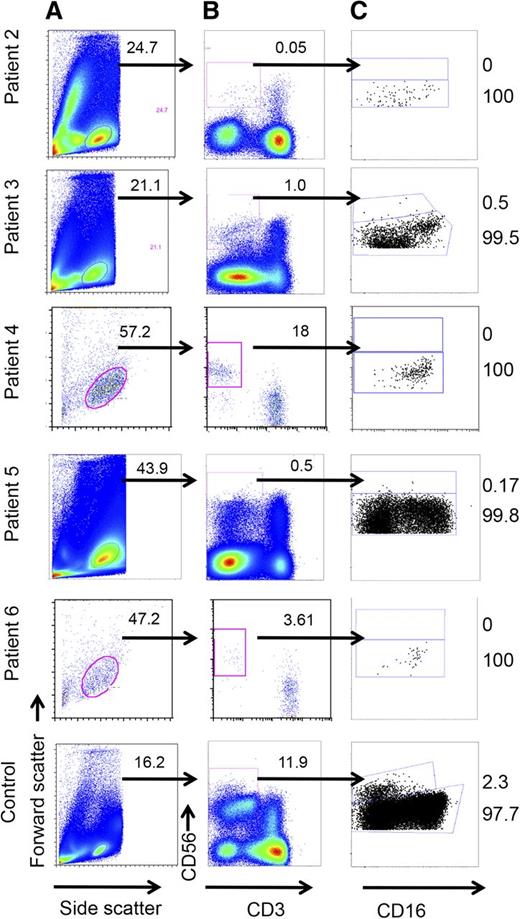
To further assess the effect of a GATA2 mutation on NK-cell phenotype and function, we evaluated the peripheral blood of GATA2-deficient patients and gender-matched controls by flow cytometry. Although patients had percentages of lymphocytes that fell within the normal range (Figure 2A), gating on CD56+CD3− cells showed a severe reduction in the number of NK cells in 4 of the 5 patients (Figure 2B). Corresponding healthy donor controls had NK cells comprising 8.26 ± 2.25% (mean ± standard deviation) of the lymphocyte gate; however, 4 GATA2-deficient patients had NK cells that fell below the range of controls tested (5.2–13.1%), with 3 patients having <1% NK cells. Patient 4 had a surprisingly large population of NK cells, comprising 17.9% of the lymphocyte population.
Reduced frequency of CD56+CD3− NK cells and enrichment of the CD56dim NK cell subset in GATA2 patients. Analysis of PBMCs from whole blood by FACS. Cells were (A) gated on lymphocytes, (B) further gated on CD56+CD3−, and (C) then analyzed for CD56 density with the aid of CD16 to identify CD56dim populations. (Bottom) One representative healthy donor control is shown.
Reduced frequency of CD56+CD3− NK cells and enrichment of the CD56dim NK cell subset in GATA2 patients. Analysis of PBMCs from whole blood by FACS. Cells were (A) gated on lymphocytes, (B) further gated on CD56+CD3−, and (C) then analyzed for CD56 density with the aid of CD16 to identify CD56dim populations. (Bottom) One representative healthy donor control is shown.
Mature NK cells can be divided into 2 subsets: CD56bright and CD56dim. Although the exact relationship between the subsets is unknown, it is thought that CD56bright NK cells are precursors of terminally mature CD56dim NK cells.26,26-28 In the peripheral blood of healthy donors, CD56bright NK cells comprise up to 20% of CD56+CD3− NK cells (mean, 7.8 ± 5.1%; n = 40; supplemental Figure 3). In GATA2-deficient patients, both those with NK cells within the normal range and those with reduced numbers showed an increase in the relative percentage of CD56dim NK cells and a marked paucity of CD56bright NK cells. Notably, all the patients tested had almost a complete absence (<1%) of CD56bright NK cells, and almost all their peripheral NK cells were within the CD56dim subset (Figure 2C). These included patient 4, with 0% of NK cells found in the CD56bright subset, despite a large population of CD56+CD3− NK cells (see above). Healthy control donors for each experiment showed numbers of CD56bright NK cells within the normal range, with a mean of 6.84% and a range of 2.3% to 12.1% (not shown).
NK cell surface marker expression is largely unaffected by GATA2 mutation
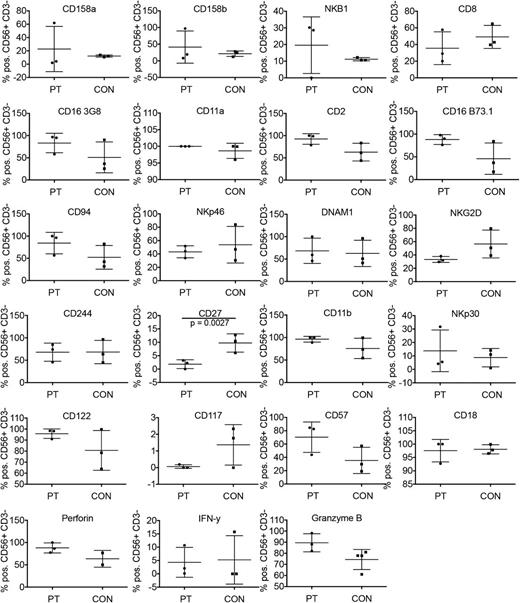
As the majority of patients tested had detectable numbers of NK cells in peripheral blood, we sought to determine whether these cells had a similar distribution of NK cell markers to healthy control donors. We evaluated 20 NK cell surface markers, as well as intracellular perforin, Granzyme B, and interferon (IFN)γ, in 5 GATA2-deficient patients and controls. NK cells from peripheral blood were identified by gating on lymphocytes and then further gating CD56+CD3− cells. The percentage of NK cells positive for the marker of interest was defined as being those with a mean fluorescence intensity of staining above that of the corresponding isotype control. Patients 2 and 5, who had <1% NK cells in peripheral blood, were excluded from phenotype analysis because there were too few cells to analyze. GATA2-deficient patients had similar expression of markers of interest to healthy control donors (Figure 3). Exceptions included those markers found predominantly on CD56bright NK cells, including CD27 (P = .0027 by Student two-tailed unpaired t test) and CD117, which were both lower on GATA2-deficient patients’ cells. Interestingly, NKG2D expression was also markedly lower, although its expression is not associated exclusively with 1 subset. To eliminate the bias of the abnormally large CD56dim population in GATA2-deficient patients, we also compared the CD56dim population of healthy donor controls to that of GATA2-deficient patients by gating on CD56dim CD56+CD3− NK cells and evaluating the same markers of interest (supplemental Figure 4). Although several markers showed minor differences in expression level when gated on total NK cells, including CD16 and perforin, gating of control donor cells on CD56dim NK cells confirmed that this was due in part to the absence of the CD56bright population in GATA2-deficient patients. Despite their functional defect in NK cell cytotoxicity, all GATA2-deficient patients had similar numbers of perforin-expressing cells as healthy donors when gated on both total and CD56dim NK cells (Figure 3; supplemental Figure 4). Similarly, Granzyme B expression was within the normal range in all patients evaluated (Figure 3), especially when patient cells were compared directly to the control CD56dim population (supplemental Figure 4). This, in combination with normal numbers of cells expressing other markers of NK cell maturation, including CD57 and KIRs, suggests that the CD56dim NK cells found in GATA2-deficient patients express most maturation markers found in healthy control donors.
Expression of NK cell surface markers. Analysis of NK cells from whole blood using the gating strategy described in Figure 2. Shown are percent positive of CD56+CD3− NK cells for each GATA2-deficient patient and corresponding healthy donor controls with each point representing the value from a single subject. The large horizontal bar denotes the mean and the vertical bar demonstrates the standard deviation. Excluded from analysis are those patients with <1% of NK cells in peripheral blood.
Expression of NK cell surface markers. Analysis of NK cells from whole blood using the gating strategy described in Figure 2. Shown are percent positive of CD56+CD3− NK cells for each GATA2-deficient patient and corresponding healthy donor controls with each point representing the value from a single subject. The large horizontal bar denotes the mean and the vertical bar demonstrates the standard deviation. Excluded from analysis are those patients with <1% of NK cells in peripheral blood.
GATA2 is expressed in CD56bright NK cells
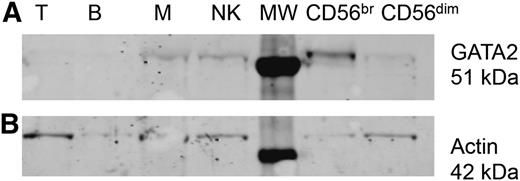
GATA2 is expressed early in hematopoietic development and plays a role in the maintenance of adult stem cells.13,29 We sought to identify the expression of GATA2 in mature lymphoid populations from blood. Various populations (CD56+CD3− NK cells, CD3+ T cells, CD19+ B cells, and CD14+ monocytes) were isolated from the peripheral blood of healthy control donors and subjected to western blot analysis to detect GATA2 protein. As seen in Figure 4, GATA2 expression was detected at low levels in CD14+ monocytes and CD56+CD3− NK cells but not CD19+ B cells or CD3+ T cells. Because GATA2-deficient patients have a specific deficit in CD56bright NK cells, we isolated CD56bright and CD56dim NK cells from healthy donors and evaluated these separately. Although the CD56+CD3− NK cell population appeared to express low levels of GATA2, this expression was almost exclusively restricted to the CD56bright subset. Densitometry analysis showed a 15-fold increase in GATA2 expression in CD56bright NK cells compared with CD56dim (normalized to actin loading control). As CD56bright NK cells constitute a relatively low percentage of the NK-cell compartment (20% of the donor shown), GATA2 expression in the population as a whole seems fairly low. However, when evaluated in isolation, CD56bright NK cells have significantly greater expression of GATA2 than CD56dim NK cells, thus supporting a particular role for GATA2 for the CD56bright NK cell subset.
Expression of GATA2 in mature NK cells. B cells, T cells, monocytes, and NK cells (all 106) or 5 × 105 CD56bright or CD56dim NK cells were isolated from peripheral blood of a healthy donor by FACS, lysed, and immunoblotted for (A) GATA2 and (B) actin as a loading control. Shown is 1 of 3 representative experiments. B, CD19+ B cells; M, CD14+ monocytes; MW, molecular weight protein ladder; NK, CD56+CD3− NK cells; T, CD3+ T cells.
Expression of GATA2 in mature NK cells. B cells, T cells, monocytes, and NK cells (all 106) or 5 × 105 CD56bright or CD56dim NK cells were isolated from peripheral blood of a healthy donor by FACS, lysed, and immunoblotted for (A) GATA2 and (B) actin as a loading control. Shown is 1 of 3 representative experiments. B, CD19+ B cells; M, CD14+ monocytes; MW, molecular weight protein ladder; NK, CD56+CD3− NK cells; T, CD3+ T cells.
In vitro differentiation of NK cells from CD34+ precursors
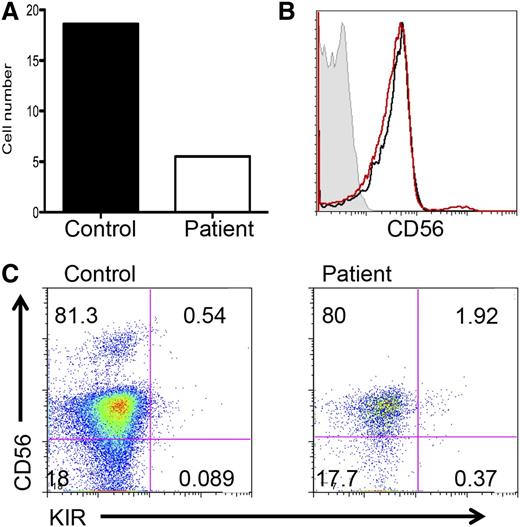
As reported previously, GATA2-deficient patients had seemingly normal numbers of CD34+ cells.2 To determine whether the impairment in NK-cell development was intrinsic to NK cells, we isolated CD34+ cells from the peripheral blood of patient 3 and a healthy age- and gender-matched control. We then cultured these cells in vitro on EL08.1D2 stromal cells in the presence of cytokines previously shown to induce NK-cell maturation.21 The donors had similar frequencies of CD34+ in peripheral blood, and 2000 cells per donor were cultured. As early as 10 days after the initiation of culture, patient cells appeared to show poor expansion. Whereas control cells became cytotoxic toward stromal cells at approximately day 21, patient cells appeared to fail to mediate this cytotoxicity, as assessed by the presence or absence of stromal cells in culture, and at this time point patient cells continued to show failure to thrive. After 4 weeks, the normal donor cells showed significant expansion compared with the patient cells (Figure 5A). Cells from both patient and control were CD56+CD3− by FACS and the normal donor's cells contained a CD56bright population comprising 2.3% of total NK cells (Figure 5B). Interestingly, NK cells generated from the patient fell exclusively within the CD56dim compartment, and a population of them was KIR+ (Figure 5B-C). Therefore, GATA2 is a cell intrinsic factor required for NK-cell maturation and generation or survival of CD56bright cells.
GATA2 is required for NK cell differentiation from CD34+ precursors. Highly purified CD34+ hematopoietic precursors from GATA2-deficient patient 3 or a healthy donor were isolated from peripheral blood by FACS and cultured on EL08.1D2 stromal cells in the presence of IL-3, IL-6, IL-7, stem cell factor, Flt3L, and IL-15. After 30 days, cells were isolated and (A) expansion was calculated based on cell number recovered for control (black) and patient (white). (B) Patient cells (black) and control cells (red) were analyzed for CD56 expression. Control cells were 2.3% CD56bright. Isotype control staining is shown in gray. (C) CD56 on (left) control cells or (right) patient cells was analyzed in combination with pan-KIR antibody (bottom).
GATA2 is required for NK cell differentiation from CD34+ precursors. Highly purified CD34+ hematopoietic precursors from GATA2-deficient patient 3 or a healthy donor were isolated from peripheral blood by FACS and cultured on EL08.1D2 stromal cells in the presence of IL-3, IL-6, IL-7, stem cell factor, Flt3L, and IL-15. After 30 days, cells were isolated and (A) expansion was calculated based on cell number recovered for control (black) and patient (white). (B) Patient cells (black) and control cells (red) were analyzed for CD56 expression. Control cells were 2.3% CD56bright. Isotype control staining is shown in gray. (C) CD56 on (left) control cells or (right) patient cells was analyzed in combination with pan-KIR antibody (bottom).
In vivo IFNα restores NK cell number and/or function but does not correct the deficit of CD56bright NK cells
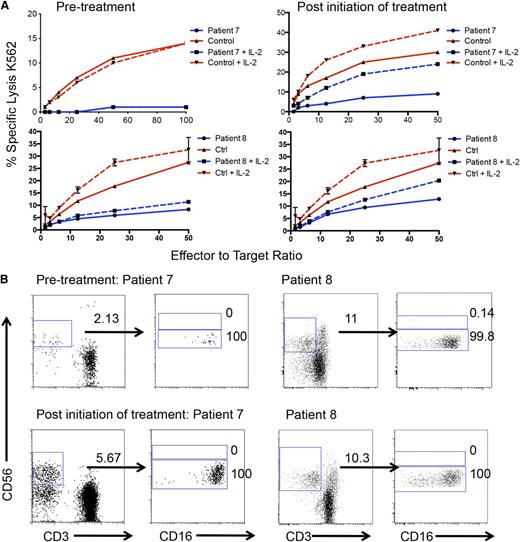
A seventh patient was identified who developed acute myeloid leukemia at age 15 years that was successfully treated (patient 7, Table 1). Approximately 5 years later, she developed severe cytomegalovirus viremia and cytomegalovirus gastroduodenitis. Nine months later, the patient developed severe, treatment-refractory perineal herpes simplex virus 2 infection. Evaluation at this time included a bone marrow biopsy that revealed myelodysplastic syndrome. In addition, an eighth patient was identified with severe and recalcitrant human papillomavirus and absolute monocytopenia in peripheral blood (patient 8, Table 1). Both patients’ cells displayed poor NK-cell cytotoxic function in the presence and absence of IL-2 (Figure 6A, left). Whereas patient 7 had low numbers of NK cells in peripheral blood, particularly CD56bright cells, patient 8 had NK cells that fell within the normal range. Strikingly, however, patient 8 had an almost absolute lack of CD56bright NK cells despite the large population of CD56+CD3− NK cells (Figure 6B). In an attempt to restore antiviral immunity in view of refractory infection, these patients were treated with 106 U of IFNα2b subcutaneously 3 times weekly. After 24 weeks of treatment, patient 7 had an increased number of CD56+CD3− cells in peripheral blood and an accompanying increase in NK-cell cytotoxic function both in the presence and absence of IL-2 (Figure 6A, top). Interestingly, despite a greater than twofold increase in the number of NK cells, all these cells remained within the CD56dim subset (Figure 6B, top). Evaluation of patient 7’s NK cell surface markers as in Figure 3 showed minor differences in the phenotype of expanded CD56dim cells, including perforin and CD57 (supplemental Figure 5). After 12 weeks of treatment, patient 8 did not have an increase in the number of NK cells in peripheral blood, and these all remained within the CD56dim subset (Figure 6B, bottom). However, some NK-cell function was restored, which could be further increased in vitro in response to IL-2 (Figure 6A, bottom). Therefore, IFNα2b stimulation can aid in the expansion of GATA2-deficient NK cells and partially restore some NK cell function but does not entirely restore the defect in NK-cell development and homeostasis seen in these patients.
In vivo–administered IFNα increases NK cell numbers and functionality but does not restore CD56bright insufficiency. PBMCs were isolated from whole blood of patients 7 and 8 before and after initiation of IFNα2b treatment. (A) Patient (blue) or control (red) NK cell cytotoxicity was assayed against susceptible K562 target cells (left) before and (right) after treatment in the presence (dashed) or absence of IL-2 (solid). (B) PBMCs from whole blood were isolated and analyzed by FACS as in Figure 2 (top) before and (bottom) after treatment.
In vivo–administered IFNα increases NK cell numbers and functionality but does not restore CD56bright insufficiency. PBMCs were isolated from whole blood of patients 7 and 8 before and after initiation of IFNα2b treatment. (A) Patient (blue) or control (red) NK cell cytotoxicity was assayed against susceptible K562 target cells (left) before and (right) after treatment in the presence (dashed) or absence of IL-2 (solid). (B) PBMCs from whole blood were isolated and analyzed by FACS as in Figure 2 (top) before and (bottom) after treatment.
Discussion
NK cell deficiency is considered a hallmark of GATA2 deficiency, and many clinical manifestations of the disease may be attributable to the absence of circulating NK cells.3 Multiple GATA2 mutations have been described, including frameshift and missense variants. Many of these mutations are predicted to be null mutations resulting in GATA2 haploinsufficiency,25 whereas others are reported to exert dominant negative effects.5 Herein we show that, whereas GATA2-deficient patients have low numbers of circulating NK cells, these are found entirely within the CD56dim subset and are not functional with regard to cytotoxicity. Therefore, GATA2 plays a critical role in the generation and/or maintenance specifically of the CD56bright pool of NK cells and also appears required, either directly or indirectly, for CD56dim NK-cell function.
It is unclear what role GATA2 is playing in NK-cell development and function. Gata2 knockout mice die at embryonic day 10 to 11 with severe hematopoietic defects.8 Murine Gata2-null embryonic stem cells have poor survival and insensitivity to stem cell factor because of low levels of CD117 (c-kit ligand) on the cell surface.9 Haploinsufficiency of Gata2 in adult mice results in a diminished frequency of Lin−c-Kit+Sca-1+CD34− progenitors and impaired ability to generate selective lineages, combined with a decrease in cell cycling and increased apoptosis in the stem cell pool.13 In addition, independent of its role in stem cell maintenance, Gata2 regulates the self-renewal of the granulocyte macrophage precursor compartment through interactions with Hes-1.30 Therefore, GATA2 is required for the generation of the developing hematopoietic compartment, maintenance of the stem cell pool in adults, and the development of specific lineages. In GATA2-deficient patients, although overall numbers of CD34+ cells are unaffected in peripheral blood, flow cytometric analysis of bone marrow from 3 affected individuals showed an absence of both the CD34+ myeloid lymphoid precursor and granulocyte macrophage precursor, as well as the absence of the more restricted CD38+CD10+ B/NK-cell precursor.2 Therefore, GATA2 may be involved in the maintenance of primitive NK-cell precursors; however, our identification of high expression of GATA2 specifically in CD56bright NK cells suggests a role for GATA2 in the homeostasis of this subset. In vitro differentiation experiments suggest that patient cells show poor survival as early as 10 days after culture of CD34+ cells. Although not investigated here, this may correspond with the initiation of expression of CD5621 ; however further experiments will be required to determine the kinetics of GATA2 expression and its function during development. Some insight might be inferred from the different patients we studied. In particular, only 2 of our patients had missense mutations, and one of these (patient 4) was the only patient with residual NK-cell function and CD56bright NK cells. Overall, the findings suggest an unexpected specific role for this widely expressed hematopoietic transcription factor in the maintenance of a specific NK-cell developmental intermediate.
The origin of the CD56dim NK cells found in these patients is unknown. The presence of these CD56dim cells in the absence of CD56bright cells suggests that they may arise from an independent precursor; however, their markedly reduced numbers and lack of functionality, even when expanded, supports the dependence of CD56dim development on the CD56bright subset. We feel that, in light of substantive data suggesting a linear relationship between the subsets,26-28 our data do not necessarily support a model of independent precursors. Rather, we feel that the defect selectively affects the survival and homeostasis of the CD56bright subset. GATA2 regulates the antiapoptotic protein Bcl-xL in adult stem cells.13 Interestingly, IL-15 prevents apoptosis of the CD56dim subset through regulation of Bcl-xL, suggesting that this factor may be important for NK cell survival but differentially regulated in the different subsets.31 Another possibility is that GATA2 regulates cell surface markers required for NK-cell development, such as CD117.
There were few differences noted in mature NK cell surface markers between GATA2-deficient patients and controls. One exception was CD27, which was low/absent in all patients tested, including after initiation of IFNα2b treatment. However, recent studies of patients lacking CD27 expression suggest that it is not required for NK-cell development, because these patients have normal NK-cell numbers and only mildly diminished function.32,33 It was not possible to analyze CD117 expression on NK cells from these patients, because it is also expressed exclusively on the CD56bright NK-cell population. More thorough analysis of CD117 expression at earlier developmental stages is required to determine whether GATA2 is necessary for its expression and function in NK-cell development and whether this is a contributing factor to the NK-cell deficit and corresponding disease in these patients.
NKG2D was also expressed in a smaller percentage of patient NK cells. As NKG2D is not known to be required for human NK-cell development, it is unclear whether this contributes to the NK-cell deficiency in these patients. However, it is conceivable that this may contribute to the functional defect in some manner. Finally, it would be of interest to analyze the secondary lymphoid tissue of patients with GATA2 mutations to determine whether CD56bright NK cells are present, because CD56bright NK cells comprise the majority of NK cells found within some of these tissues.27
Alternatively, the observed defect in NK-cell number and function in GATA2-deficient patients may be caused by extrinsic defects in other populations, such as DCs and macrophages. NK cells and DCs colocalize within both inflamed and uninflamed human lymph nodes, and IL-15 presentation by DCs stimulates NK-cell proliferation.34 However, as NK cells derived from CD34+ precursors also failed to develop the CD56bright subset, we believe that GATA2 mutation results in intrinsic NK-cell defect. In addition, we showed that GATA2 is highly expressed in the CD56bright subset, suggesting it may be playing an important functional role in this population. However, further study will help to determine the relative contributions of affected populations to clinical manifestations.
In summary, we identified a GATA2 mutation as the cause of the originally reported and most often cited case of NK-cell deficiency. In addition, we showed a specific role for GATA2 in NK-cell development and homeostasis, thus identifying it as a critical regulator of NK-cell maturation and GATA2 mutation as a cause of classical NK-cell deficiency.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Disease grant R01 067946 (J.S.O.) and the Division of Intramural Research, National Institute of Allergy and Infectious Disease, National Institutes of Health.
Authorship
Contribution: E.M.M. performed experiments, analyzed data, and wrote the paper; A.P.H. performed and analyzed sequencing of the GATA2-deficient patients; L.M.-S. and G.M. performed experiments and analyzed data; J.B.R. analyzed data; L.D. provided samples from patients with GATA2 deficiency and clinical treatment of patient 7; J.I.C., C.S.Z., C.S., E.P.F., J.C.B., and G.U. provided samples from and clinical details regarding patients with GATA2 deficiency; J.L.S. and C.A.B. evaluated the initial case of NK cell deficiency (patient 1) and provided samples from this patient; and S.M.H and J.S.O. initiated and directed the research, designed the experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jordan S. Orange, 1102 Bates St, FC330, Houston, TX 77030; e-mail: orange@bcm.edu.