Key Points
A thymus with available stem-cell niches can support long-term renewal by resident hematopoietic progenitors.
Intrathymic administration of semiallogeneic BM progenitors results in long-term T-cell reconstitution in the absence of conditioning.
Abstract
Donor hematopoietic stem cells (HSCs) can correct T-cell deficiencies in patients with severe combined immunodeficiency by replacing resident thymus cells. However, as those progenitors that naturally migrate to the thymus are not capable of supporting long-term thymopoiesis, a successful transplant is thought to require the ongoing migration of donor progenitors. We previously showed that the forced intrathymic administration of histocompatible HSCs can sustain long-term thymopoiesis in ZAP-70-immunodeficient mice. However, it is not known whether T-cell reconstitution across histocompatibility barriers is modulated by intrathymic vs intravenous administration of HSCs. In the absence of conditioning, long-term thymopoiesis by semiallogeneic progenitors was detected in mice transplanted via the intrathymic, but not the intravenous, route. In intrathymic-transplanted mice, ongoing thymopoiesis was associated with a 10-fold higher level of early thymic progenitors (ETPs). The enhanced reconstitution capacity of these intrathymic-derived ETPs was corroborated by their significantly augmented myeloid lineage potential compared with endogenous ETPs. Notably, though, myeloablative conditioning resulted in a reduced expansion of intrathymic-administered donor ETPs. Thus, in the absence of conditioning, the forced thymic entry of HSCs results in a sustained T-cell development across histocompatibility barriers, highlighting the capacity of the thymus to support cells with long-term renewal potential.
Introduction
T-cell differentiation in the thymus arises from progenitor cells that are derived from bone marrow (BM) hematopoietic stem cells (HSCs). Under conditions in which patients undergo a transplant with donor HSCs administered by an intravenous route, T-cell generation requires that these cells, or their progeny, home to the thymus before differentiation. In humans, it is not clear whether intravenously injected HSCs can directly enter into the thymus or, alternatively, whether they directly home to the BM with only more committed common lymphocyte precursors entering into the thymus.
In mice, entry of progenitors into the thymus has been shown to be a major bottleneck in T-cell differentiation. For example, the murine thymus is not continually receptive to the import of hematopoietic progenitors, and during refractory periods, representing approximately 3 or 4 weeks in each cycle, donor progenitor cells efficiently differentiate into T cells only if they are directly injected into the thymus.1 It is important to note that in mice, HSCs themselves do not appear capable of seeding the thymus under physiologic conditions. Furthermore, the “thymic-settling progenitors” that naturally migrate to the thymus are not capable of supporting long-term thymopoiesis. Rather, they promote only a single wave of short-term thymopoiesis lasting 3 to 4 weeks.2
Previous studies have shown that transfer of thymocyte progenitors directly into the murine thymus results in only short-term thymocyte differentiation.2-4 On the basis of these experimental data, it was concluded that long-term thymocyte differentiation requires an ongoing migration of donor progenitors from the BM to the thymus, with new BM precursors replacing resident thymocytes.5 However, more recent studies, performed by our group and others, have found that in immunodeficient mice, under conditions in which competitive BM progenitors and/or early thymocyte progenitors are restricted, resulting in an available progenitor thymic “niche,” long-term thymus-autonomous T-cell differentiation can occur.6-8 This has important consequences for the outcome of transplantation for patients with genetic severe combined immunodeficiencies (SCIDs), wherein only transplanted donor hematopoietic progenitors can reconstitute the T-cell pool.
Patients with SCID experience opportunistic infections and die within the first years of life if not treated. HLA-identical hematopoietic stem cell transplantation is the treatment of choice, and overall survival duration has increased dramatically in recent years, reaching 90%.9-12 Under conditions in which HLA-identical donors are not available, patients with SCID are increasingly undergoing transplant with stem cells from HLA-haploidentical parents or from unrelated donors. However, it is important to note that significant complications, including graft failure, can occur. Furthermore, the kinetics of T-cell reconstitution are a critical factor because this process can require several months, a period during which morbidity and mortality risks are elevated.11,13-15
We have shown previously that the direct intrathymic injection of histocompatible wild-type (WT) progenitors into nonconditioned mice with a SCID phenotype, due to mutations in the ZAP-70 protein tyrosine kinase, results in a more rapid and diverse T-cell reconstitution than that detected after intravenous injection of the same progenitor population. Furthermore, we found that in the context of this immunodeficiency, wherein there is available “space” for a precursor niche, the forced intrathymic administration of hematopoietic progenitors can promote and sustain long-term thymopoiesis.6,16 Thus, under specific conditions, the thymic environment is capable of providing a niche for a progenitor cell(s) with the potential for long-term T-cell differentiation.
However, it is important to note that these transplants were performed in a fully histocompatible context, rather than in the more challenging transplantation setting across histocompatibility barriers. In the histocompatible context, transplantation in patients with SCID is performed in the absence of a preconditioning regimen because host cells are not rejected by the patient. However, in mismatched or unrelated stem cell transplantation, debate still exists whether conditioning plays a positive or negative role. Patients with SCID have been successfully treated with unrelated and haploidentical stem cells in the absence of conditioning,12 but other studies have found that a myeloablative preparative regimen is associated with higher overall survival duration.17 Also, in the case of adenosine deaminase–deficient SCID, the absence of conditioning is associated with a high graft failure of haploidentical transplants.18
We therefore initiated studies to assess whether T-cell reconstitution across histocompatibility barriers would be modulated by the intrathymic administration of stem cells, in the absence or presence of a conditioning regimen. To this aim, we first assessed whether a myeloablative busulphan/cyclophosphamide–conditioning regimen alters the engraftment and differentiation of histocompatible donor progenitors after intravenous and intrathymic administration. As expected, preconditioning resulted in a significantly higher level of histocompatible progenitor cell engraftment, irrespective of whether cells were administered by an intravenous or intrathymic route. Furthermore, extensive B-cell differentiation was detected in both conditions; this was associated with a conditioning-associated vascular permeability of the thymus and the settling of transplanted BM precursors in the BM. In a myeloablative preconditioning regimen, the intravenous and intrathymic administration of semiallogeneic WT progenitor cells also resulted in donor-driven thymopoiesis. However, in the absence of conditioning, only intrathymic-transplanted mice showed evidence of long-term thymopoiesis by semiallogeneic progenitors. It is important to note that this thymopoiesis was associated with a sustained presence of c-Kithigh early thymic progenitors (ETPs), at percentages 10-fold higher than that of endogenous progenitors. Furthermore, the high progenitor capacity of ETPs differentiating from intrathymic-administered stem cells (referred to here as intrathymic ETPs) was demonstrated by their significantly augmented myeloid lineage potential. Thus, the forced thymic entry of progenitor cells can promote a sustained T-cell development across histocompatibility barriers.
Materials and methods
Mice and BM transplantation protocols
ZAP-70−/− mice (CD45.2+) were provided by A. Singer (National Institutes of Health, Bethesda, MD), and the strain was bred and maintained under pathogen-free conditions. Donor BM cells, harvested from the femurs and tibias of WT CD45.1+ C57Bl/6J mice or F1 CD45.1+CD45.2+ mice from C57Bl/6JxCBA/J crosses, were first incubated with a cocktail of specific antibodies (Abs) directed against lineage markers (CD4, CD8 [BioXcell, West Lebanon, NH], Ter119, B220, CD11b, and Gr-1 [Dynal, Compiegne, France], all rat α mouse hybridoma Abs) and then with α-rat IgG magnetic beads (Dynal) to deplete differentiated hematopoietic cells. ZAP-70−/− mice were either nonconditioned or preconditioned with busulphan (66 mg/kg total, given on days −4, −3) plus cyclophosphamide (200 mg/kg on day −2) before transplantation of 2 × 105 lineage-negative progenitor cells. Intravenous injections were carried out by injecting progenitor cells directly into the tail vein (100-μl volume). Intrathymic injections were performed as previously described.6 In brief, nonconditioned mice were anesthetized with isoflurane, and cells were directly injected into the thymus (20-μl total volume) by insertion of a 0.3-mL, 28-gauge, 8-mm insulin syringe through the skin into the thoracic cavity above the sternum. Preconditioned mice were anesthetized by injection with xylazine (10 mg/kg) and ketamine (100 mg/kg), thymi were exposed by thoracic surgery, and cells were injected into the thymus by insertion of a 0.3-mL, 28-gauge, 8-mm insulin syringe. All experiments were approved by the local animal facility institutional review board in accordance with national guidelines.
Immunophenotyping and flow cytometry analyses
Cells, isolated from lymph nodes and thymi, were stained with the appropriate conjugated αCD3, αCD25, αCD45.1, αCD45.2, αCD62L, αCD4, αCD8, αCD44, αc-Kit, αSca-1, αlineage cocktail mouse mAbs (Becton Dickinson, San Diego, CA). Stained cells were analyzed by flow cytometry (FACSCanto; Becton Dickinson, San Jose, CA).
In vitro methylcellulose colony-forming assays
ETPs were isolated from the thymi of reconstituted mice, euthanized 16 weeks after transplantation, as well as from age-matched WT control mice. In brief, double-negative (DN) thymocytes were recovered by first depleting double-positive CD4+CD8+ (DP CD4+CD8+), single-positive CD4+ (SP4), SP CD8+ (SP8), and erythrocytes (TER-119+) using a cocktail of specific antibodies (CD4, CD8, and Ter119- all rat α mouse hybridoma Abs) followed by incubation with α-rat IgG magnetic beads (Dynal). DN thymocytes were then stained with αCD45.1, αCD25, αCD4, αCD8, αB220, αTER-119, αCD3, αc-Kit (clones 2B8 and ACK2), and αCD44 mAbs to sort CD44+/c-Kithigh ETPs on a FACSAria (Becton Dickinson, San Jose, CA). ETPs isolated from individual reconstituted and WT mice were cultured on MethoCult M3434 medium (Stemcell Technologies, Grenoble, France). Colonies were visualized and were counted 7 days after culture with an inverted bright-field microscope (Axiovert 200; Zeiss, Göttingen, Germany). Subsequently, colonies were picked and washed in phosphate-buffered saline supplemented with 2% fetal calf serum before staining with αc-Kit, αGr1, αCD11b, αCD25, and αTER-119 mAbs to monitor the identity of cells within colonies by flow cytometry (FACSCanto, Becton Dickinson).
Statistical analyses
Statistical significance was determined using an unpaired Student t test with a 2-tailed distribution unless otherwise indicated (Graph Pad Software, La Jolla, CA). Data were considered to be statistically different for P ≤ .05. All data are presented as means ± standard deviation (SD).
Results
Role of conditioning on the outcome of intravenous and intrathymic progenitor cell transplantation
Transplantation in ZAP-70−/− mice with fully histocompatible WT progenitor cells results in peripheral T-cell reconstitution, but ongoing thymopoiesis is restricted to conditions in which these cells are injected directly into the thymus.6,16 However, it is not known whether conditioning modulates the relative level of stem cell engraftment and sustained thymopoiesis after intravenous vs intrathymic administration of fully histocompatible grafts.
Mice were treated with different doses of busulphan and cyclophosphamide, a clinically relevant conditioning regimen, to determine an optimal sublethal conditioning protocol fostering donor HSC engraftment. Treatment with busulphan (33 mg/kg), given on days −4 and −3 (66 mg/kg total), in conjunction with cyclophosphamide (200 mg/kg) on day −2 resulted in an almost complete loss of thymocytes and peripheral lymphocytes at the time of transplantation (day 0). Hosts could compensate for these losses by days 7 to 10 (data not shown).
In the absence of pretransplant conditioning, donor thymocytes were detected at only minimal levels after the intravenous administration of WT progenitors (Figure 1).6 In contrast, under conditions in which WT precursor cells were “deposited” directly into the thymus, rather than into the peripheral circulation, their differentiation was maintained long-term, as reported previously (Figure 1A).6 Specifically, in the representative experiment shown in Figure 1, less than 1% donor cells were detected after conventional intravenous transplantation, whereas almost 50% of thymocytes were of donor origin after intrathymic transplantation. The presence of donor cells in intrathymic-transplanted mice was associated with ongoing thymopoiesis, as shown by the physiologic repartition of thymocytes between distinct developmental stages; 88% of donor cells possessed a DP (CD4+CD8+) phenotype (Figure 1A). In contrast, the few donor cells detected in intravenous-transplanted mice were of a SP phenotype, likely representing recirculating peripheral T cells.
Reconstitution of ZAP-70−/− mice after intravenous and intrathymic histocompatible progenitor cell transplantation in the absence and presence of conditioning. ZAP-70−/− mice were transplanted with histocompatible WT BM progenitors, administered by either intravenous or intrathymic routes, in nonconditioned or busulphan/cyclophosphamide preconditioned hosts. (A) The presence of CD45.1 donor cells in the thymi of reconstituted mice was assessed 25 weeks after transplantation. Representative dot plots showing the percentages of CD45.1+ cells are presented. Control stainings show CD45.1 expression in WT donor mice and ZAP-70−/− recipients. The percentages of thymocytes in the boxed quadrants are noted (upper panels). The CD4/CD8 phenotype of thymocytes in the boxed quadrants is presented in the lower panels. The percentages of DN, DP, and SP thymocytes are indicated. (B) The relative proportion of donor cells in lymph nodes of ZAP-70−/− mice transplanted by intravenous and intrathymic routes, in the absence or presence of conditioning (cond), was assessed. The presence of donor cells was monitored 25 weeks after transplantation with each point representing data from 1 mouse. The mean percentage of donor cells in each condition is indicated with a line. (C) The presence of donor CD3+ and CD19+ cells in lymph nodes of preconditioned ZAP-70−/− recipients transplanted by intravenous and intrathymic routes was monitored by flow cytometry. The percentages of CD3+ and CD19+ cells are indicated in each quadrant, and staining of WT donor and ZAP-70−/− recipients are presented (upper panels). The percentages of donor-derived CD19+ B cells were assessed as a function of CD45.1 expression, and data for representative mice in each group are shown.
Reconstitution of ZAP-70−/− mice after intravenous and intrathymic histocompatible progenitor cell transplantation in the absence and presence of conditioning. ZAP-70−/− mice were transplanted with histocompatible WT BM progenitors, administered by either intravenous or intrathymic routes, in nonconditioned or busulphan/cyclophosphamide preconditioned hosts. (A) The presence of CD45.1 donor cells in the thymi of reconstituted mice was assessed 25 weeks after transplantation. Representative dot plots showing the percentages of CD45.1+ cells are presented. Control stainings show CD45.1 expression in WT donor mice and ZAP-70−/− recipients. The percentages of thymocytes in the boxed quadrants are noted (upper panels). The CD4/CD8 phenotype of thymocytes in the boxed quadrants is presented in the lower panels. The percentages of DN, DP, and SP thymocytes are indicated. (B) The relative proportion of donor cells in lymph nodes of ZAP-70−/− mice transplanted by intravenous and intrathymic routes, in the absence or presence of conditioning (cond), was assessed. The presence of donor cells was monitored 25 weeks after transplantation with each point representing data from 1 mouse. The mean percentage of donor cells in each condition is indicated with a line. (C) The presence of donor CD3+ and CD19+ cells in lymph nodes of preconditioned ZAP-70−/− recipients transplanted by intravenous and intrathymic routes was monitored by flow cytometry. The percentages of CD3+ and CD19+ cells are indicated in each quadrant, and staining of WT donor and ZAP-70−/− recipients are presented (upper panels). The percentages of donor-derived CD19+ B cells were assessed as a function of CD45.1 expression, and data for representative mice in each group are shown.
In hosts preconditioned before progenitor transplantation, the situation differed markedly. Intrathymic as well as intravenous administration of progenitors resulted in physiologic levels of thymopoiesis at 25 weeks posttransplant. Donor cells accounted for more than 80% of all thymocytes following either mode of administration, and the vast majority of these cells were of a DP phenotype (Figure 1A). Thus, in conditioning, thymopoiesis originating from transplanted donor progenitors was maintained at very high levels, irrespective of the mode of progenitor cell administration.
Given the significant thymopoiesis detected in conditioned hosts, it was of interest to study the mature lymphocytes in the periphery. As expected from the levels of detected thymocyte differentiation, the percentages of donor lymphocytes were significantly increased when transplants were performed in preconditioned hosts, irrespective of whether progenitors were injected by intravenous or intrathymic routes (60%-90% vs 20%-40% in nonconditioned hosts; Figure 1B). Furthermore, the relative percentages of donor CD3+ T cells and CD19+ B cells detected in lymph nodes of preconditioned mice transplanted by intravenous and intrathymic routes were strikingly similar to those detected in WT animals (Figure 1C). This result was not expected, as no donor B cells are detected when progenitors are administered via the intrathymic route into nonconditioned hosts.6 Although the percentage of donor B cells within the entire B-cell pool was lower in intrathymic- than in intravenous-transplanted preconditioned mice, the percentages of donor cells in the former remained significant (66% in the representative mouse, as shown in Figure 1C). Therefore, in the busulphan/cyclophosphamide preconditioning regimen, T-cell reconstitution by WT histocompatible progenitors is extremely efficient. Furthermore, reconstitution is not augmented by an intrathymic transplant strategy.
Long-term thymopoiesis by semiallogeneic progenitors is dependent on pretransplant conditioning or intrathymic transplantation
As discussed above, patients with SCID lacking histocompatible donors rely on progenitor cell transplantation functioning across histocompatible barriers. Therefore, we assessed the relative efficacy of thymopoiesis and T-cell reconstitution after intravenous and intrathymic administration of semiallogeneic progenitors in preconditioned ZAP-70−/− mice. To this end, ZAP-70−/− mice, which are of the C57Bl/6J background, were injected with lineage-negative progenitor cells isolated from the F1 progeny of C57Bl/6J X CBA/J crosses. At long-term time points after transplantation (up to 28 weeks), high numbers of semiallogeneic donor thymocytes were detected in both conditions (60%-90% of total thymocytes, Figure 2A). Also, thymopoiesis was functional as demonstrated by a repartition of thymocyte subsets, which closely resembled that of WT mice. As shown in figure 2B, more than 80% of donor thymocytes were of a DP phenotype with a physiologic repartition of mature CD4 and CD8 SP thymocytes.
Myeloablative conditioning promotes reconstitution by semiallogeneic BM progenitors after intravenous or intrathymic administration. ZAP-70−/− mice were preconditioned with busulphan and cyclophosphamide before transplantation with semiallogeneic lineage-negative BM progenitor cells (2 × 105) isolated from C57Bl/6JxCBA/J WT mice. Progenitors were administered by either intravenous or intrathymic routes and were sacrificed 28 weeks posttransplantation. (A) Bar graph quantification of the percentages of CD45.1+ donor cells detected in the thymi of intravenous- and intrathymic-reconstituted ZAP-70−/− mice 28 weeks posttransplantation (mean ± SD). (B) Representative dot plots showing the CD4/CD8 phenotypes of donor cells in the thymi of intravenous- and intrathymic-reconstituted ZAP-70−/− mice compared with donor and recipient mice. (C) Bar graph quantification of the percentages of CD45.1+ donor cells in the lymph nodes of intravenous- and intrathymic-reconstituted mice (mean ± SD; * P < .05). (D) Dot plots showing the CD3/CD19 phenotypes of donor cells in the lymph nodes of intravenous- and intrathymic-reconstituted mice compared with donor and recipient controls (upper panels). The proportion of donor-derived B cells in the LN of intravenous- and intrathymic-reconstituted mice was monitored as a function of CD45.1 expression (lower panels). The percentages of CD45.1+ cells are indicated, and data are representative of 3 independent experiments.
Myeloablative conditioning promotes reconstitution by semiallogeneic BM progenitors after intravenous or intrathymic administration. ZAP-70−/− mice were preconditioned with busulphan and cyclophosphamide before transplantation with semiallogeneic lineage-negative BM progenitor cells (2 × 105) isolated from C57Bl/6JxCBA/J WT mice. Progenitors were administered by either intravenous or intrathymic routes and were sacrificed 28 weeks posttransplantation. (A) Bar graph quantification of the percentages of CD45.1+ donor cells detected in the thymi of intravenous- and intrathymic-reconstituted ZAP-70−/− mice 28 weeks posttransplantation (mean ± SD). (B) Representative dot plots showing the CD4/CD8 phenotypes of donor cells in the thymi of intravenous- and intrathymic-reconstituted ZAP-70−/− mice compared with donor and recipient mice. (C) Bar graph quantification of the percentages of CD45.1+ donor cells in the lymph nodes of intravenous- and intrathymic-reconstituted mice (mean ± SD; * P < .05). (D) Dot plots showing the CD3/CD19 phenotypes of donor cells in the lymph nodes of intravenous- and intrathymic-reconstituted mice compared with donor and recipient controls (upper panels). The proportion of donor-derived B cells in the LN of intravenous- and intrathymic-reconstituted mice was monitored as a function of CD45.1 expression (lower panels). The percentages of CD45.1+ cells are indicated, and data are representative of 3 independent experiments.
The active donor cell thymopoiesis detected after intravenous and intrathymic transplantation of haploidentical progenitors was associated with an extremely high percentage of mature donor cells in the periphery (80%-95% of all lymph node [LN] cells, Figure 2C). Furthermore, as observed in preconditioned mice transplanted with histocompatible progenitors, donor B cells were detected after both intravenous and intrathymic transplantation of semiallogeneic progenitors (Figure 2D). However, within the CD19+ B-cell population, a significantly greater percentage of cells were of donor origin in intravenous- compared with intrathymic-transplanted mice (90.7% ± 2.6% vs 58.3% ± 13.1% in the 2 conditions, respectively; Figure 2D). This would be expected to occur based on an enhanced homing of progenitors to the BM, the site of B-cell differentiation, after intravenous compared with intrathymic transplantation.
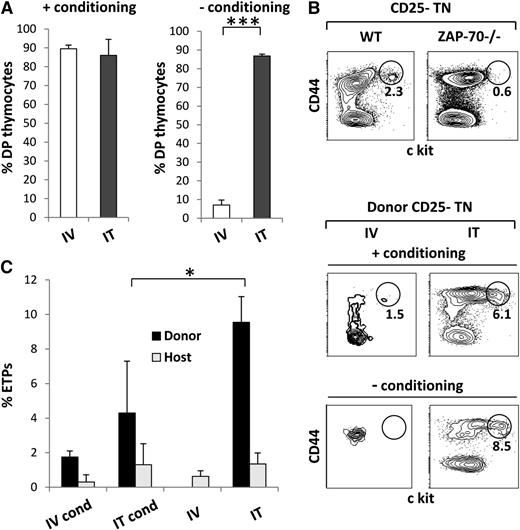
The data presented above demonstrate an efficient long-term thymopoiesis with associated T-cell reconstitution after either intravenous or intrathymic administration of histocompatible and haploidentical progenitors. However, it is important to note that the previous experiments were performed under conditions in which the ZAP-70−/− mice were preconditioned before transplantation. Also, in some SCID contexts, conditioning is associated with increased risk.9-11 Therefore, it was important to assess the outcome of semiallogeneic transplantation in the absence of conditioning and to determine whether progenitors could engraft in this context. As shown in Figure 3, semiallogeneic donor cells did not contribute to long-term thymopoiesis after intravenous administration. However, and in marked contrast, significant levels of donor semiallogeneic thymocytes were detected in mice transplanted by the intrathymic route. Furthermore, in intrathymic-transplanted mice, these cells represented an ongoing thymopoiesis as demonstrated by the finding that more than 80% were DP thymocytes (Figure 3B). The very few donor thymocytes detected in intravenous-transplanted mice likely represented recirculating T cells because they were either SP CD4+ or CD8+ cells (Figure 3B).
In the absence of conditioning, thymopoiesis by transplanted semiallogeneic BM progenitors is only sustained after intrathymic administration. ZAP-70−/− mice were transplanted with 2 × 105 semiallogeneic BM progenitors isolated from C57Bl/6JxCBA/J WT mice, in the absence of any preconditioning. Progenitors were administered by either intravenous or intrathymic routes. (A) Bar graph quantification of the percentages of CD45.1+ donor cells detected in the thymi of intravenous- and intrathymic-transplanted mice 28 weeks posttransplantation (mean ± SD). (B) The phenotype of donor thymocytes generated after intravenous and intrathymic transplantation of semiallogeneic progenitors was assessed by CD4/CD8 staining. Representative dot plots of transplanted mice, gated on donor thymocytes, as well as donor and recipient controls are shown. (C) Bar graph quantification of the percentages of donor cells (CD45.1+) in the LN of intravenous- and intrathymic-transplanted mice (mean ± SD; *P < .05). (D) The phenotype of donor cells in the periphery of transplanted mice was assessed by CD3/CD19 staining of lymph nodes. Dot plots show the phenotype of gated donor cells compared with WT and ZAP-70−/− controls. Data are representative of 3 independent experiments.
In the absence of conditioning, thymopoiesis by transplanted semiallogeneic BM progenitors is only sustained after intrathymic administration. ZAP-70−/− mice were transplanted with 2 × 105 semiallogeneic BM progenitors isolated from C57Bl/6JxCBA/J WT mice, in the absence of any preconditioning. Progenitors were administered by either intravenous or intrathymic routes. (A) Bar graph quantification of the percentages of CD45.1+ donor cells detected in the thymi of intravenous- and intrathymic-transplanted mice 28 weeks posttransplantation (mean ± SD). (B) The phenotype of donor thymocytes generated after intravenous and intrathymic transplantation of semiallogeneic progenitors was assessed by CD4/CD8 staining. Representative dot plots of transplanted mice, gated on donor thymocytes, as well as donor and recipient controls are shown. (C) Bar graph quantification of the percentages of donor cells (CD45.1+) in the LN of intravenous- and intrathymic-transplanted mice (mean ± SD; *P < .05). (D) The phenotype of donor cells in the periphery of transplanted mice was assessed by CD3/CD19 staining of lymph nodes. Dot plots show the phenotype of gated donor cells compared with WT and ZAP-70−/− controls. Data are representative of 3 independent experiments.
Semiallogeneic thymocytes differentiating in intrathymic-transplanted mice resulted in a high level of T-cell reconstitution, with 40% of all lymph node cells representing donor T cells (Figure 3C). It is important to note, however, that intravenous-injected semiallogeneic progenitors were capable of promoting at least a single wave of thymopoiesis, even in the absence of conditioning, as 20% of lymphocytes, all with a T-cell phenotype, were of donor origin (Figure 3C-D). In contrast with the data emerging from preconditioned hosts, no donor B cells were detected in mice undergoing intravenous or intrathymic transplantation (Figure 3D). The absence of donor B cells from mice that were not preconditioned would be expected in the context of ZAP-70−/− hosts that maintain normal B-cell differentiation and homeostasis. The ensemble of these data indicates that intrathymic transplantation is superior to the conventional intravenous route in the absence of conditioning, resulting in long-term engraftment of progenitors across histocompatible barriers.
Long-term persistence of semiallogeneic donor-derived ETPs does not require BM engraftment of c-Kit+ progenitor cells
Under conditions in which ZAP-70−/− hosts were preconditioned, short-lived DP thymocytes were detected long-term (25-28 weeks), irrespective of whether haploidentical progenitors were transplanted by an intravenous or intrathymic route (Figure 4A). In contrast, in the absence of conditioning, these semiallogeneic DP thymocytes were only detected after intrathymic transplantation. We previously found that long-term thymopoiesis by histocompatible donor progenitors was associated with the occupancy of an available stromal niche.6 In the absence of BM engraftment, we hypothesize that it is the forced entry of a WT BM precursor into a thymic niche, not accessible to circulating host precursors, that allows for long-term development of thymocytes.
Long-term persistence of semiallogeneic donor-derived ETPs is detected only after intrathymic transplantation of BM progenitors. (A) Bar graph quantification of the percentages of donor-derived DP cells (CD4+CD8+) detected in the thymi of mice 28 weeks after transplantation of intravenous- or intrathymic-administered semiallogeneic progenitors, in the absence or presence of a preconditioning regimen. Data are presented as mean ± SD (***P < .0001). (B) Representative dot plots showing the percentages of ETPs in the thymi of C57Bl/6 (WT) and ZAP-70−/− mice. ETPs were assessed by CD44/c-Kit staining on gated donor-derived CD25-TN thymocytes. The percentages (n = 6) as well as absolute numbers (n = 8) of ETPs were determined, and mean ± SD are presented (P = .003 for percentages and P = .006 for absolute numbers). (C) Representative dot plots showing the percentages of ETPs detected in the thymi of mice transplanted by intravenous or intrathymic administration, in the absence or presence of prior conditioning. Bar graph quantification of the percentages of donor and host ETPs in the thymi of conditioned and nonconditioned recipient mice 28 weeks after intravenous and intrathymic transplantation of semiallogeneic progenitors. Mean ± SD are presented (*P < .05).
Long-term persistence of semiallogeneic donor-derived ETPs is detected only after intrathymic transplantation of BM progenitors. (A) Bar graph quantification of the percentages of donor-derived DP cells (CD4+CD8+) detected in the thymi of mice 28 weeks after transplantation of intravenous- or intrathymic-administered semiallogeneic progenitors, in the absence or presence of a preconditioning regimen. Data are presented as mean ± SD (***P < .0001). (B) Representative dot plots showing the percentages of ETPs in the thymi of C57Bl/6 (WT) and ZAP-70−/− mice. ETPs were assessed by CD44/c-Kit staining on gated donor-derived CD25-TN thymocytes. The percentages (n = 6) as well as absolute numbers (n = 8) of ETPs were determined, and mean ± SD are presented (P = .003 for percentages and P = .006 for absolute numbers). (C) Representative dot plots showing the percentages of ETPs detected in the thymi of mice transplanted by intravenous or intrathymic administration, in the absence or presence of prior conditioning. Bar graph quantification of the percentages of donor and host ETPs in the thymi of conditioned and nonconditioned recipient mice 28 weeks after intravenous and intrathymic transplantation of semiallogeneic progenitors. Mean ± SD are presented (*P < .05).
We therefore assessed the status of ETPs in transplanted ZAP-70−/− mice. As shown previously,6 the niche occupied by ETPs in ZAP-70−/− mice is significantly reduced compared with WT mice (Figure 4B). The mean percentages of c-Kithigh thymocytes within the CD25- triple-negative (TN) population was 0.5% in ZAP-70−/− mice compared with 1.8% in a WT mouse (n = 8; P = .003), representing a mean absolute number of ETPs of 0.7 × 104 compared with 5.5 × 104, respectively (Figure 4B; P = .006). It is interesting to note that the low level of host ETPs detected in mice undergoing haploidentical transplantation was not significantly modulated at long-term time points after myeloablative conditioning (Figure 4C).
Because of the lack of thymopoiesis in nonconditioned ZAP-70−/− mice receiving intravenous-administered semiallogeneic progenitors, the inability to detect CD44+c-Kithigh ETPs was not surprising (Figure 4B). However, after a preconditioning regimen, low levels of c-Kithigh donor progenitors were detected in mice transplanted intravenously. Significantly, though, the percentages were fourfold higher after intrathymic transplantation, reaching 6.1% of TN cells in the representative mouse shown (Figure 4B). Furthermore, the percentages of ETPs within the donor population was up to 10-fold higher than the relative content of host ETPs present in the thymi of ZAP-70−/− mice before transplantation.
It is very important to note that unlike the situation in intravenous-transplanted mice, myeloablative conditioning did not enhance the long-term persistence of ETPs after intrathymic transplantation. Although ETPs were significantly increased over host levels in conditioned mice treated by intrathymic transplantation, there was actually a lower number of donor ETPs in the presence vs the absence of conditioning (Figure 4B). It is likely that the ETPs detected in the thymus after myeloablative conditioning are the result of BM progenitor engraftment. As shown in Figure 5, donor cells represented a high percentage of lineage-negative progenitors in preconditioned mice, irrespective of whether the semiallogeneic progenitors were injected by the intravenous or intrathymic route. Moreover, a large proportion of these progenitors were c-Kit+ (Figure 5B). These data suggest that in conditioning, there is an increased vascular permeability of the thymus resulting in the settling of transplanted BM precursors in the BM. Therefore, it is likely that the continued thymopoiesis detected in these mice is attributed to the settling of semiallogeneic progenitors in the BM niche.
Myeloablative conditioning results in long-term engraftment of semiallogeneic c-Kit+ BM progenitors administered by intravenous as well as intrathymic routes. (A) Quantification of the percentages of semiallogeneic donor cells detected within the lineage-negative BM population. Mice were either nonconditioned or preconditioned with busulphan/cyclophosphamide (cond). At 28 weeks posttransplantation, BM was isolated and the presence of donor cells within the lineage-negative progenitor gate was determined by flow cytometry. Each point represents data from a single mouse, and mean levels are indicated by a horizontal line. (B) Expression of the stem cell receptor c-Kit was assessed on lineage-negative donor progenitors in each group, and a representative histogram from 1 mouse in each group is presented.
Myeloablative conditioning results in long-term engraftment of semiallogeneic c-Kit+ BM progenitors administered by intravenous as well as intrathymic routes. (A) Quantification of the percentages of semiallogeneic donor cells detected within the lineage-negative BM population. Mice were either nonconditioned or preconditioned with busulphan/cyclophosphamide (cond). At 28 weeks posttransplantation, BM was isolated and the presence of donor cells within the lineage-negative progenitor gate was determined by flow cytometry. Each point represents data from a single mouse, and mean levels are indicated by a horizontal line. (B) Expression of the stem cell receptor c-Kit was assessed on lineage-negative donor progenitors in each group, and a representative histogram from 1 mouse in each group is presented.
In marked contrast, BM engraftment by lineage-negative progenitors was very low in the absence of conditioning and this was the case for both intrathymic and intravenous transplantations (Figure 5A). Furthermore, the few detected lineage-negative cells in these mice did not express the c-Kit progenitor marker. This finding was in marked contrast with preconditioned mice in which approximately 50% of progenitors were c-Kithigh. Thus, these data strongly suggest that the expansion of a large c-Kithigh donor thymocyte pool in nonconditioned mice receiving an intrathymic injection of progenitors is not the result of BM engraftment. Rather, thymocyte progenitor expansion is a specific characteristic of intrathymic-transplanted mice resulting in long-term thymopoiesis across histocompatibility barriers.
Extended myeloid potential of ETPs differentiating from intrathymically administered hematopoietic progenitors
Given the unique generation of a large population of early c-Kithigh donor precursors after intrathymic transplantation, it was of interest to assess whether these ETPs could be distinguished from those detected in the thymi of WT control mice. Therefore, we assessed the myeloid potential of sorted donor progenitor cells from the thymi of WT and intrathymic-reconstituted mice (isolated 16 weeks after intrathymic transplantation), with the latter herein referred to as intrathymic ETPs. Colony-forming units in vitro differentiation assays on MethoCult medium revealed a significantly higher number of colony-forming units originating from intrathymic ETPs compared with WT ETPs (Figure 6A). Furthermore, the former were significantly more diverse as shown by their ability to form granulocyte-macrophage and granulocyte colony-forming units, as well as erythroid burst-forming units (Figure 6B).
Only ETPs differentiating from intrathymically administered hematopoietic progenitors retain significant ex vivo myeloid potential. Mice reconstituted with intrathymically injected WT BM progenitors were sacrificed 16 weeks posttransplantation, and ETPs were sorted from the thymi of mice undergoing thymopoiesis. (A) ETPs (5 × 102) from WT and intrathymic-reconstituted (intrathymic-ETPs) mice were cultured in MethoCult media to assess myeloid potential. The total numbers of colonies derived from WT and intrathymic ETPs in a representative experiment are shown. (B) Colony morphologic features were assessed 7 days after culture, and representative photographs of granulocyte/macrophage and granulocyte colony-forming units as well as erythroid burst-forming units are shown. (C) The expression of myeloid, erythroid, and progenitor cell surface markers was assessed on WT- and intrathymic-ETP–derived colony-forming units. Representative plots showing expression of CD11b, Gr-1, c-Kit, CD25, and Ter119 are presented for cells derived from single colonies.
Only ETPs differentiating from intrathymically administered hematopoietic progenitors retain significant ex vivo myeloid potential. Mice reconstituted with intrathymically injected WT BM progenitors were sacrificed 16 weeks posttransplantation, and ETPs were sorted from the thymi of mice undergoing thymopoiesis. (A) ETPs (5 × 102) from WT and intrathymic-reconstituted (intrathymic-ETPs) mice were cultured in MethoCult media to assess myeloid potential. The total numbers of colonies derived from WT and intrathymic ETPs in a representative experiment are shown. (B) Colony morphologic features were assessed 7 days after culture, and representative photographs of granulocyte/macrophage and granulocyte colony-forming units as well as erythroid burst-forming units are shown. (C) The expression of myeloid, erythroid, and progenitor cell surface markers was assessed on WT- and intrathymic-ETP–derived colony-forming units. Representative plots showing expression of CD11b, Gr-1, c-Kit, CD25, and Ter119 are presented for cells derived from single colonies.
For further characterization of individual colonies, they were isolated, and cell surface markers were assessed by flow cytometry. ETPs sorted from WT mice demonstrated a limited differentiation potential; only the CD11b myeloid marker was detected in all assessed colonies (Figure 6C and data not shown). In contrast, the c-Kithigh donor ETPs isolated from intrathymic-reconstituted mice (intrathymic ETPs), were capable of differentiating into a much wider range of lineages. This is shown by expression of distinct levels of CD11b, expression of the Ter119 erythroid marker, and even expression of CD25, associated with early B-cell differentiation, on cells isolated from different colonies (Figure 6C). These experiments demonstrate that the c-Kithigh intrathymic ETPs, persisting in the thymi of intrathymic-injected mice, are distinct from their WT equivalents, with a much more extensive lineage potential than their WT equivalents.
Discussion
The ideal treatment of patients with SCID would result in engraftment of HSCs/progenitors, a rapid lymphocyte/immune reconstitution, and a long-lasting thymocyte differentiation, all in the absence of graft-versus-host disease. The current belief is that the BM, but not the thymus, can provide an environment in which long-term progenitor cell engraftment and renewal can occur. As such, many translational research efforts, designed to improve the outcome of patients with SCID, have focused on enhancing the homing of progenitors to the BM and creating conditions in which the BM stem cell niche promotes an optimal balance between self-renewal and differentiation. However, the experiments reported here indicate that hematopoietic progenitors capable of thymocyte renewal and T-cell differentiation may persist in the thymus after intrathymic administration, even across major histocompatibility barriers. The long-term expansion of c-Kithigh ETPs, in the absence of myeloablative conditioning, highlights the capacity of a thymus with available stem cell niches to support long-term renewal potential.
In the absence of conditioning, persistent donor-derived thymopoiesis by semiallogeneic progenitors was detected only in intrathymically transplanted ZAP-70−/− mice, and this differentiation was associated with a massive expansion of c-Kithigh ETPs (Figure 4). Furthermore, in these mice, BM engraftment by lineage-negative c-Kithigh progenitors was not detected (Figure 5). These data challenge the long-held belief that thymocyte differentiation requires a continuous replenishment by BM-derived progenitors. Indeed, recent data, including experiments from our own laboratory, strongly suggest that under specific conditions, intrathymic populations can harbor the capacity to support a long-term thymus-autonomous T-cell differentiation.6-8 This occurs under conditions in which a paucity of BM progenitors exists, thereby limiting the possibility of replenishment, and a thymic progenitor niche(s) is available. In ZAP-70–deficient mice, we detected this thymocyte renewal after injection of a heterogeneous WT lineage-negative progenitor pool, harboring true stem cells, as well as after intrathymic injection of a more downstream multipotent progenitor population (characterized as Lin-Sca-1+c-Kit+Flt3+; unpublished data). It is notable that this population has been elegantly shown to promote the most efficient thymopoiesis after intravenous transfer into nonirradiated WT mice.19
Long-term in situ thymocyte differentiation seems to require an available thymic progenitor niche as well as an immunodeficiency. Thymus-autonomous T-cell differentiation is suppressed in mice with mutations in c-Kit alone but is observed at high frequencies in mice with combined mutations in c-Kit and Rag.17 Furthermore, hematopoietic precursors do not seem to self-renew in nonimmunodeficient CC chemokine receptor CCR7/CCR9 double knockout mice,20 although these mice show a 1000-fold defect in ETPs.20,21 Given that the steady-state level of ETPs is significantly reduced in ZAP-70–deficient vs WT mice, with a fivefold decrease in their absolute number (Figure 4), it will be important to determine whether ETPs are also decreased in other immunodeficiencies and, furthermore, to assess whether intrathymic transplantation of HSCs will result in thymocyte self-renewal.
The c-Kithigh progenitors persisting after intrathymic administration seemed to be distinct from those progenitors detected in WT mice. To assess their progenitor potential, we took advantage of previous work showing that ETPs harbor in vitro myeloid potential.22-24 Although some controversy exists whether this myeloid differentiation occurs in the thymus itself,25 a very recent study has found that the data emerging from ex vivo methylcellulose assays are recapitulated in vivo.26 Therefore, we used methylcellulose colony-forming assays as a measure of myeloid differentiation potential.
In our experiments, an in vitro myeloid differentiation of WT ETPs was detected, but this differentiation occurred at significantly lower levels than that detected for intrathymic ETPs (Figure 6). Colony-forming units generated from intrathymic ETPs were also larger and harbored cells with significantly more diverse phenotypes than those generated from physiologic ETPs isolated from WT thymi. Furthermore, only intrathymic ETPs gave rise to cells expressing erythroid lineage markers. This finding is of significant interest because it places these intrathymic progenitors at an earlier stage of differentiation than physiologic ETPs. ETPs in the neonatal thymus have elegantly been shown to harbor combined, T, B, and GM lineage potential but are completely devoid of megakaryocyte-erythroid lineage potential.24 These data indicate that the BM precursors that are forced into the thymus retain a more expanded lineage potential than the BM thymic-settling cells that normally seed the thymus.
It is notable, though, that the intrathymically administered BM progenitors, intrathymic ETPs, did not seem to demonstrate nonlymphoid potential in vivo, as only donor lymphocytes with a T-cell phenotype were detected after intrathymic administration of HSCs (Figure 2). The intrathymic-injected progenitors differentiated in a physiologic manner, giving rise to a normal partition of CD4/CD8 thymocytes as well as T cells. It is important to also note that, unlike the situation observed in the absence of any BM progenitor migration, these intrathymic-persisting progenitors did not give rise to any thymomas, leukemias, or lymphomas.7,8 We hypothesize that this important difference arises because in our system, all progenitor cells were capable of responding to trophic signals such as Kit ligand and interleukin 7 (IL-7). As such, the selection for progenitors capable of surviving in the absence of these growth factors would be significantly lower than that existing in mice with defects in the receptors for these cytokines. Additional studies will be needed to test this experimentally.
It was quite surprising to note that the persistence of c-Kithigh ETPs was reduced after myeloablative conditioning (Figure 4). This is likely because of changes in thymus stromal cell composition as well as in gene expression in resistant stromal cell populations after conditioning.27-29 It was also initially surprising to detect intrathymic-injected donor progenitors in the BM of conditioned mice, as the “inverse” migration of progenitors from the thymus to the BM has not been described, to the best of our knowledge. However, the high level of progenitor engraftment that we detected in the BM of intrathymic-transplanted mice is probably the result of an increased vascular permeability of the thymus. Indeed, it has recently been shown that thymus permeability is increased by more than fivefold after myeloablative conditioning.30 In our experiments, BM engraftment of conditioned mice treated by intravenous transplantation reached levels of more than 90%, but BM donor cells in intrathymic-transplanted mice were also elevated, accounting for at least 30% of lineage-negative BM progenitors (Figure 5). The high BM engraftment of progenitors was associated with the maturation of donor B lymphocytes, although ZAP-70–deficient host precursors are completely capable of B-cell differentiation (Figure 2). Thus, in marked contrast to the situation in nonconditioned mice, engraftment of semiallogeneic stem cells in the BM of conditioned mice plays a critical role in contributing to ongoing thymopoiesis.
After conditioning, the so-called quality of the available thymocyte precursor niche is likely to be altered. Under physiologic conditions, thymic settling of precursors is dependent on CCR7 or CCR9 as well as on the P-selectin glycoprotein ligand-1.20,21,31,32 Conditions that increase expression of the CCR9 ligand, CCL25, on thymic epithelial cells will also enhance ETP immigration to the thymus.33 In this context, it is interesting to note that the levels of CCL25 and P-selectin are decreased by 40% in ZAP-70–deficient vs WT thymi (as detected by quantitative reverse-transcription polymerase chain reaction; R.V. and V.Z., unpublished data), likely accounting for the decreased steady-state level of ETP in these mice. However, it is striking that immediately after conditioning by irradiation, the requirement for CCR7/CCR9 is abolished.30 Thus, it is possible that in our conditioned ZAP-70–deficient mice, the absence of donor progenitor cell expansion and/or long-term persistence after intrathymic transplantation is because of an early competition from incoming endogenous progenitors as well as the presence of a less favorable thymic niche.
A recent elegant study showed the importance of IL-23–induced IL-22 production in thymic reconstitution, with IL-22 promoting the entry of ETPs.34 Of interest, IL-22 levels were found to be inversely proportional to the number of DP thymocytes in various murine models of genetic T-cell immunodeficiencies.34 Approximately normal levels of DP thymocytes are present in both humans and mice with ZAP-70 immunodeficiency,35-40 and as such, intrathymic IL-22 levels would be expected to be low, diminishing the potential replenishment of ETPs with BM progenitors. As administration of recombinant IL-22 increased thymic cellularity by endogenous progenitors after irradiation,34 it will be important to determine whether this cytokine enhances or alternatively hampers thymopoiesis by WT precursors administered by intrathymic or intravenous injection into ZAP-70–deficient mice.
Therapies enhancing immune reconstitution in patients with SCID undergoing fully histocompatible or partially histocompatible HSC transplantations would significantly improve outcomes for these patients. It is still not clear whether the use of a myeloablative-conditioning regimen is preferable, as elegant recent studies showed that the survival rate of adenosine deaminase–deficient patients is superior in the absence of conditioning (81% vs 54%),18 whereas survival duration of a heterogeneous group of patients with SCID undergoing transplantation is improved by myeloablative conditioning.17 Our data indicate that in recipients without pretransplant conditioning, donor-derived thymocyte renewal and associated long-term T-cell differentiation across histocompatibility barriers are only detected if progenitors are administered intrathymically. Furthermore, the kinetics of T-cell reconstitution is significantly more rapid after intrathymic vs intravenous progenitor cell administration; donor cells accounted for 12% of cells in the peripheral blood 6 weeks after intrathymic transplantation vs only 1% after intravenous transplantation. Thus, under conditions in which histocompatible donors are not available and pretransplant conditioning is not advisable, intrathymic administration of partially histocompatible hematopoietic progenitors may have a high therapeutic value, providing for long-term T-cell differentiation for certain groups of patients with SCID.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all members of our laboratory for their scientific critique and support and are grateful to A. Singer for his scientific input and critical reading of the manuscript.
This work was supported by grant R01AI059349 from the National Institute of Allergy and Infectious Diseases, the Association Française contre le Myopathies (AFM) (S.C.d.B., R.V., fellowship), the European Community (contract LSHC-CT-2005-018914 “ATTACK”), ANR, ARC, INCA, the Portuguese Foundation for Science and Technology (SFRH/BD/23553/2005) (R.V.), INSERM (C.J., N.T.), and CNRS (K.C., V.Z.).
National Institutes of Health
Authorship
Contribution: S.C.d.B. performed most of the experimental work; R.V. performed extensive initial experiments; K.C. and C.J. performed mice experiments; S.C.d.B., R.V., V.Z., and N.T. planned the project and analyzed data; and S.C.d.B., V.Z., and N.T. prepared the manuscript.
Conflict-of-Interest disclosure: The authors declare no competing financial interests.
Correspondence: Valérie S. Zimmermann and Naomi Taylor, IGMM, 1919 Route de Mende, 34293 Montpellier, Cedex 5, France; e-mail: zimmermann@igmm.cnrs.fr, taylor@igmm.cnrs.fr.