Key Points
VWF A3 domain mutations inducing defective collagen binding and impaired protein production.
Abstract
Two unrelated families were recruited in the French Reference Center for von Willebrand Disease with moderate bleeding symptoms associated with low von Willebrand factor (VWF) antigen levels, decreased collagen binding assay, and no or partial response to desmopressin. Genetic analysis showed the presence of heterozygous mutations in the A3 domain away from the collagen-binding surface: 1 never reported previously (p.L1696R) and another (p.P1824H) described in a Spanish family. The mutations were reproduced by site-directed mutagenesis and mutant VWF was expressed in different expression systems, COS-7 cells, baby hamster kidney cells, and in VWF-deficient mice through hydrodynamic injection. p.L1696R and p.P1824H were associated with very low expression levels both in vitro and in vivo, with intracellular retention for p.P1824H. Both homozygous mutants displayed decreased binding to collagen types I and III but also decreased binding to platelet glycoproteins Ib and IIbIIIa. Co-transfections with wild-type VWF partially corrected these defects, except that collagen binding remained abnormal. The in vivo thrombosis response was severely reduced for both heterozygous mutants. In conclusion, we report 2 VWF A3 domain mutations that induce a combined qualitative and quantitative defect.
Introduction
Von Willebrand factor (VWF) is a multimeric plasma glycoprotein composed of identical subunits associated through disulfide bonds. Each mature subunit is organized in 5 types of repeated domains arranged in the order D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK.1 In hemostasis, functions of VWF include recruitment of platelets to sites of vascular injury and protection of coagulation factor VIII (FVIII) from premature clearance.2 The sequence of events leading to the formation of a VWF-dependent platelet plug after initial breach in the vessel wall is: VWF binds to collagens present in the exposed subendothelium, it adopts a platelet-binding conformation, and captures platelets from flowing blood via the glycoprotein (GP) Ib-V-IX complex. This binding step leads to platelet activation and exposition of the platelet receptor GPIIbIIIa. The interaction of GPIIbIIIa with fibrinogen and VWF then promotes firm adhesion of platelets to the vessel wall and thrombus formation.3 In vitro approaches have contributed to identify the functional domains on the VWF molecule involved in the different functions of the protein. The D′-D3 region is critical for FVIII interaction,4 whereas binding of VWF to platelets involves 2 different sites: the A1 domain for binding to GPIb-V-IX and the RGD sequence in the C1 domain for binding to GPIIbIIIa.5,6 As for binding of VWF to collagens, proteolytic fragments and monoclonal antibodies have allowed the identification of several binding sites. The A3 domain (residues 1683-1874) contains the main binding site for fibrillar collagens type I and III,7 whereas the A1 domain binds to nonfibrillar collagen type VI8 and can also interact with collagens I and III in specific conditions.9
Impaired binding of VWF to collagen has been reported to induce type 2 von Willebrand disease (VWD), characterized by qualitative defects in VWF.10 VWD is the most common inherited bleeding disorder, and quantitative defects lead to type 1 or 3 VWD according to their degree of severity. In type 2 VWD, different subtypes exist according to the function that is altered. Point mutations in the sequence of VWF represent the underlying cause of VWD type 2, and the localization of these mutations can normally predict the resulting functional defect.11 For example, mutations in the D′-D3 region are known to affect VWF-FVIII binding, whereas mutations in the A1 domain induce platelet-binding defects.11 Reports of patients harboring mutations in the A3 domain have been, with only 4 mutations characterized: p.S1731T, p.W1745C, p.S1783A, and p.H1786D.12-14 These mutations induce defective collagen binding while not affecting the multimeric pattern of the mutant molecule, classifying them as “true” collagen-binding mutants. Another mutation, p.P1824H, was also reported but not extensively analyzed.15 Here we report the identification of a class of mutations in the A3 domain that induce combined qualitative and quantitative defects. In the homozygous state, the mutations affect the interaction of VWF with multiple ligands, most notably collagen. The patients belong to two unrelated families enrolled in the French National Reference Center for von Willebrand Disease and displayed low VWF antigen levels (VWF:Ag), abnormal collagen binding (VWF:CB) and had partial or no response to desmopressin. Based on the current International Society on Thrombosis and Haemostasis Scientific and Standardization Committee VWD classification, the patients may be classified as VWD type 2M.
Materials and methods
An extensive description of materials and methods can be found provided in the supplemental material on the Blood website, a brief summary of which is given below.
Patients’ family history
Informed consent for phenotypic and genotypic analysis of VWF was obtained from patients enrolled in the study according the Declaration of Helsinki. The study protocol was approved by the local institutional review board. We characterized 6 patients from 2 unrelated families (F1 and F2) with diagnosed VWD who exhibited a heterozygous state for a mutation located within the A3 domain of VWF: p.L1696R (c.5087T>G in exon 29) and p.P1824H (c.5471C>A in exon 32).15 Their clinical bleeding expression was estimated with the bleeding score (BS) for VWD.16
In F1 (mutation p.L1696R), the propositus (I-1) is a 42-year-old man with a moderate bleeding history consisting in 1 episode of posttraumatic joint bleeding and several episodes of bleeding after tooth extraction. None of these episodes required replacement treatment and thus, his BS is not higher than 2. His daughter, age 14 (II-1), has a similar BS at 2 because of menorrhagia requiring either antifibrinolytic agents or a contraceptive pill, whereas his 12-year-old son (II-2) has no bleeding symptoms (BS at 0).
In F2 (mutation p.P1824H), the propositus (I-1) is a 38-year-old woman who experienced bleeding episodes during surgery and in the postpartum period leading to a BS at 6. Her first sister (I-2), age 35 years, has a significant bleeding history including menorrhagia requiring a specialized hematology consultation and a postpartum bleeding episode requiring replacement therapy by VWF concentrates, leading to a BS at 5. Her second sister (I-3), age 28 years, experienced many bleeding episodes: epistaxis, oral cavity bleeding, bleeding after tooth extraction and from minor wounds, easy bruising, and menorrhagia leading to a BS at 9.
Phenotypic analysis
Citrated whole blood was obtained by venipuncture. Platelet poor plasma was obtained using 2 centrifugations for 15 min at 2500g. Plasma was analyzed for FVIII:C activity, VWF:Ag, VWF ristocetin cofactor activity (VWF:RCo), VWF:CB, and VWF multimers. Detailed description of these assays is provided in the supplemental material. Study of global primary hemostasis was performed using the PFA-100 analyzer (Siemens, Saint-Denis, France) with both ADP- and Epinephrine-collagen cartridges.
The biologic response to desmopressin was evaluated using the defined criteria from the MCMDM-1VWD study.17 Patients were defined as follows: complete responders, both VWF:RCo and FVIII were 50 IU/dL or higher after desmopressin; partial responders, VWF:RCo and FVIII were lower than 50 IU/dL but increased at least threefold; and nonresponders, neither criterion.
Construction of plasmids encoding mutated VWF
Mutations p.L1696R and p.P1824H were introduced in VWF via standard molecular biological procedures using plasmid pNUT-VWF encoding human wild-type (wt)-VWF as a template.6 Both mutations were also introduced in a synthetic VWF complementary DNA (cDNA) encoding a chimeric human VWF protein in which the A1 domain was of murine origin (huVWFmuA1).18 This chimeric construct is cloned into the pLIVE vector (Mirus Bio LLC, Madison, WI). All mutations were verified by DNA sequencing. Other mutants used throughout the study have been described previously: RGD-mutant p.D2509G-rVWF,6 deletion mutant VWF-δ A3 (p.E1673-p.V1876del-rVWF),7 and soluble gain-of-function mutant p.D235Y/M239V-rGpIb.19 Plasma from a compound heterozygous VWD-type 2N patient (p.R768Q/p.R854Q-VWF) was used as a source of VWF with an FVIII binding defect. Detailed information on changes in cDNA for these mutants is provided in the supplemental material.
Cell transfections
Baby hamster kidney (BHK) cells were used to establish stable cell lines, whereas COS-7 cells were used for transient transfection. Detailed description of the procedures is provided in the online supplemental material.
Functional assays
Conditioned medium from cells expressing recombinant wt VWF (wt-rVWF) or mutant VWF (p.L1696R-rVWF or p.P1824H-rVWF) were concentrated 10- to 40-fold and used for various functional assays. These included binding to monoclonal antibodies (Mabs), collagen I and III, FVIII, GpIIbIIIa, and GpIb. Detailed description of these assays is provided in the online supplemental material.
Mice
VWF-deficient mice20 were used on a C57Bl/6 background throughout the study. Housing and experiments were done as recommended by French regulations and the experimental guidelines of the European Community.
Hydrodynamic injections and ferric chloride–induced thrombosis model
VWF-deficient mice were injected with cDNA encoding for the chimeric VWF protein huVWFmuA1 subcloned in pLIVE. To reproduce the heterozygous state of the patients, mice were injected with 150 µg of huVWFmuA1 cDNA together with 150 µg of huVWFmuA1 carrying the p.L1696R or p.P1824H mutation. Injections were done as previously described.21 Ferric chloride injury was induced as described.22 Time to initial thrombus formation (minimal size of 30 μm) and time to occlusion were determined. The choice for the thrombosis model rather than a tail-bleed model was based on the notion that the latter is less sensitive and provides less detailed insight into how thrombus formation is affected by VWF mutations. Furthermore, we have previously shown that impaired collagen binding leaves the tail-bleeding time unaffected.21
Statistical analysis
Data are expressed as means values plus or minus standard deviation (SD). Statistical analyses were performed by 1-way analysis of variance or 2-tailed χ-square test for the in vivo data. A P value less than 0.05 was considered statistically significant. For analysis of variance, in case of P less than .05, pairwise comparisons against the control group were made. Corrections for multiple comparisons were made according to Dunnet.23
Results
Phenotypic and genetic characterization of families with VWD
For all family members, the phenotypic features and the response to desmopressin are summarized in Table 1.
Genotypic and phenotypic features of 6 VWD subjects from 2 unrelated families
| Family . | Members . | VWF mutation . | PFA Epi/ADP (s) . | FVIII:C (IU/dL) . | VWF:Ag (IU/dL) . | VWF:RCo (IU/dL) . | VWF:CB (IU/dL) . | Response to DDAVP . | BS . |
|---|---|---|---|---|---|---|---|---|---|
| F1 | Propositus I-1 | p.L1696R | 300/>300 | 19 | 16 | 16 | 7 | Partial | 2 |
| F1 | Daughter II-1 | p.L1696R | 300/>300 | 32 | 25 | 25 | 12 | Partial | 2 |
| F1 | Son II-2 | p.L1696R | 300/>300 | 43 | 23 | 18 | 12 | Partial | 0 |
| F2 | Propositus I-1 | p.P1824H | 300/>300 | 48 | 12 | 10 | 6 | Not tested | 6 |
| F2 | Sister I-2 | p.P1824H | 300/>300 | 29 | 16 | 10 | 8 | None | 5 |
| F2 | Sister I-3 | p.P1824H | >270/>280 | 26 | 13 | 12 | 3 | None | 9 |
| Family . | Members . | VWF mutation . | PFA Epi/ADP (s) . | FVIII:C (IU/dL) . | VWF:Ag (IU/dL) . | VWF:RCo (IU/dL) . | VWF:CB (IU/dL) . | Response to DDAVP . | BS . |
|---|---|---|---|---|---|---|---|---|---|
| F1 | Propositus I-1 | p.L1696R | 300/>300 | 19 | 16 | 16 | 7 | Partial | 2 |
| F1 | Daughter II-1 | p.L1696R | 300/>300 | 32 | 25 | 25 | 12 | Partial | 2 |
| F1 | Son II-2 | p.L1696R | 300/>300 | 43 | 23 | 18 | 12 | Partial | 0 |
| F2 | Propositus I-1 | p.P1824H | 300/>300 | 48 | 12 | 10 | 6 | Not tested | 6 |
| F2 | Sister I-2 | p.P1824H | 300/>300 | 29 | 16 | 10 | 8 | None | 5 |
| F2 | Sister I-3 | p.P1824H | >270/>280 | 26 | 13 | 12 | 3 | None | 9 |
FVIII:C, FVIII coagulant activity.
In F1, the propositus’ biological phenotype includes infinite closure times (>300 seconds both with epinephrine and ADP cartridges using PFA-100) and significantly decreased plasma VWF levels (VWF:Ag: 16 IU/dL) with a normal VWF:RCo/VWF:Ag ratio (1.0), but a decreased VWF:CB/VWF:Ag ratio (0.44; Table 1). His response to desmopressin is partial. His daughter (II-1) and his son (II-2) have a similar biological phenotype with slightly higher plasma VWF levels (VWF:Ag: 25 and 23 IU/dL, respectively) but similarly decreased VWF:CB/VWF:Ag ratios (0.48 and 0.52, respectively). In both children, the response to desmopressin is also partial.
In F2, the propositus’ biological phenotype includes infinite closure times (>300 seconds both with epinephrine and ADP cartridges using PFA-100) and significantly decreased plasma VWF levels (VWF:Ag: 12 IU/dL) with a VWF:RCo/VWF:Ag ratio at 0.8 but a decreased VWF:CB/VWF:Ag ratio at 0.5. Her sisters (I-2 and I-3) exhibit similar biological phenotypes; neither respond to desmopressin.
Attempts were made to analyze the multimer pattern, which was compromised by the low VWF:Ag levels. Samples that were diluted twofold resulted in a smeary pattern, whereas samples diluted threefold did allow the appearance of distinct bands but resulted in an apparent loss of higher multimers (Figure 1). To test whether these abnormal multimer patterns were due to a limitation of the multimer analysis technique, we analyzed normal plasma mixed with VWF-deficient plasma to a concentration of 15% VWF:Ag. A similar pattern was observed in comparison with the patient samples: the multimers appeared as a smeary pattern when diluted twofold in sample buffer, whereas distinct bands were observed upon a threefold dilution (Figure 1). However, we could not observe an apparent loss in higher multimers in the control sample. Although the analysis of the patient samples does not allow a precise interpretation of their multimer composition, it appears that they are characterized by a modest reduction in the presence of high-molecular-weight multimers. However, this reduction seems less dominant than observed in classical VWD-type 2A patients.
Multimer analysis of patients heterozygous for the p.L1696R or p.P1824H mutation. VWF multimeric pattern from plasma samples of patients and affected family members. A sample from F2 I-1 was not available for analysis. For 2 individuals (F1 I-1 and F2 I-2), samples were analyzed twice using a 1:1 (indicated as 15% Ag a) or 1:2 dilution (indicated as 15% Ag b). Samples of patients F1 II-1 and F1 II-2 were diluted 1:2, whereas plasma of patient F2 I-3 was diluted 1:1. To demonstrate the role of sample dilution on multimer quality, VWF-deficient plasma was mixed with normal pooled plasma to a final concentration of 15% VWF:Ag. This preparation was subject to multimer analysis using a 1:1 (15% Ag a) or a 1:2 (15% Ag b) dilution. NP, normal pooled plasma.
Multimer analysis of patients heterozygous for the p.L1696R or p.P1824H mutation. VWF multimeric pattern from plasma samples of patients and affected family members. A sample from F2 I-1 was not available for analysis. For 2 individuals (F1 I-1 and F2 I-2), samples were analyzed twice using a 1:1 (indicated as 15% Ag a) or 1:2 dilution (indicated as 15% Ag b). Samples of patients F1 II-1 and F1 II-2 were diluted 1:2, whereas plasma of patient F2 I-3 was diluted 1:1. To demonstrate the role of sample dilution on multimer quality, VWF-deficient plasma was mixed with normal pooled plasma to a final concentration of 15% VWF:Ag. This preparation was subject to multimer analysis using a 1:1 (15% Ag a) or a 1:2 (15% Ag b) dilution. NP, normal pooled plasma.
When looking at the position of the 2 mutations within the crystal structure of the VWF A3 domain,24 they appeared to be located inside the A3 domain, not exposed to solvents (supplemental Figure 1). This is in contrast to previously identified collagen-binding mutations (orange) or to residues identified in vitro to be critical for collagen binding (purple),25,26 all located on the same face of the A3 domain and most exposed to the surface.
Expression of recombinant mutated VWF
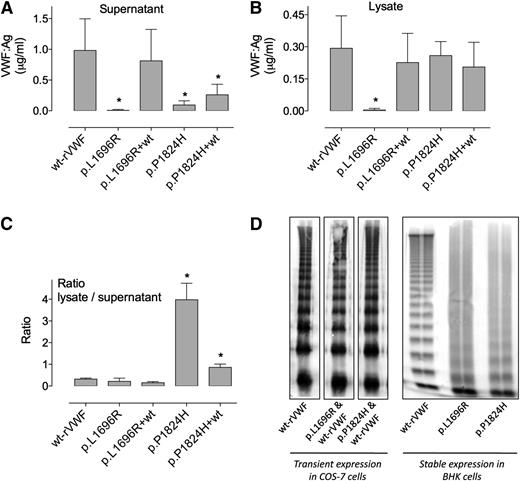
Transient transfections using cDNAs encoding for p.L1696R-rVWF and p.P1824H-rVWF were performed using COS-7 cells. To reproduce the heterozygous state of the patient, contransfection with a 1:1 ratio of mutant VWF cDNA and wt-VWF cDNA were undertaken. Results of expression levels measured both in cell lysate and cell supernatant are shown in Figure 2. When transfected homozygously, small amounts of p.L1696R-rVWF and p.P1824H-rVWF were found in the supernatant (7.1 ± 15 ng/mL and 93 ± 69 ng/mL, respectively, compared with 982 ± 510 ng/mL for wt-rVWF [mean ± SD]) (P < .01) (Figure 2). In relative terms compared with wt-rVWF, this corresponds to 0.7 ± 1.5% and 9.5 ± 7% for p.L1696R-rVWF and p.P1824H-rVWF, respectively. In cell lysates, homozygous p.L1696R-rVWF was also very low (4.3 ± 7 ng/mL or 1.5 ± 2.4% compared with wt-rVWF), whereas p.P1824H-rVWF was expressed at the same level as wt-rVWF (259 ± 65 ng/mL and 290 ± 150 ng/mL, respectively; Figure 2B). The calculated lysate/supernatant ratio was 0.3 for wt-rVWF, and this ratio was particularly increased for mutant p.P1824H-rVWF (ratio > 3.0, P < .0001 compared with wt-rVWF), suggesting intracellular retention for this mutant (Figure 2C). Of note, the total VWF production (supernatant + lysate) was reduced about threefold for p.P1824H-rVWF compared with wt-rVWF (383 ng/mL vs 1241 ng/mL, respectively).
Multimeric analysis and expression levels of recombinant mutant VWF.VWF cDNAs encoding wt, p.L1696R, or p.P1824H mutants were transfected in COS-7 cells. Cotransfections (1:1) of the mutant with wt cDNA were also performed in COS-7 cells. VWF:Ag levels resulting from these different transfections were measured by enzyme-linked immunosorbent assay in both cell supernatants (A) and cell lysates (B) and are expressed as micrograms per milliliter. The ratio of VWF:Ag in lysates over supernatants (SN) is also represented (C). Data represent means ± SD of 3 independent experiments. *P values < .05 compared with wt values. The multimeric profile of the various recombinant proteins was analyzed through 2% sodium dodecyl sulfate-agarose gel electrophoresis (D).
Multimeric analysis and expression levels of recombinant mutant VWF.VWF cDNAs encoding wt, p.L1696R, or p.P1824H mutants were transfected in COS-7 cells. Cotransfections (1:1) of the mutant with wt cDNA were also performed in COS-7 cells. VWF:Ag levels resulting from these different transfections were measured by enzyme-linked immunosorbent assay in both cell supernatants (A) and cell lysates (B) and are expressed as micrograms per milliliter. The ratio of VWF:Ag in lysates over supernatants (SN) is also represented (C). Data represent means ± SD of 3 independent experiments. *P values < .05 compared with wt values. The multimeric profile of the various recombinant proteins was analyzed through 2% sodium dodecyl sulfate-agarose gel electrophoresis (D).
Cotransfection with wt-VWF cDNA normalized the expression levels both in lysate and in supernatant for mutant p.L1696R-rVWF (supernatant: 810 ± 180 ng/mL or 83 ± 18%; lysate: 230 ± 50 ng/mL or 79 ± 17%). For p.P1824H-rVWF, a modest 2.8-fold increase in the amount of secreted protein was observed (260 ± 60 ng/mL or 27 ± 6%), whereas the amount of intracellular VWF remained unchanged (210 ± 40 µg/mL) upon co-expression with wt-rVWF. To ensure that the proteins produced upon co-expression with wt-rVWF indeed contain mutated subunits, an assay was used based on the fact that both mutants do not interact with anti-VWF Mab200, whereas binding to Mab418 is unaffected (Figure 3). As such, the ratio Mab200/Mab418 is related to the heterozygosity of the protein: it is 1 for wt-rVWF, 0 for mutant VWF, and somewhere between 0 and 1 for the heterozygous protein. Using this assay, we found a Mab200/Mab418 ratio of 0.57 and 0.38 for p.L1696R-rVWF and p.P1824H-rVWF upon co-expression with wt-rVWF, respectively (supplemental Figure 2). This shows that approximately half of the subunits of the secreted VWF proteins comprise the mutation. Of note, a similar heterozygosity was also observed when analyzing plasma samples of the patients (ratio ranging between 0.43 and 0.55).
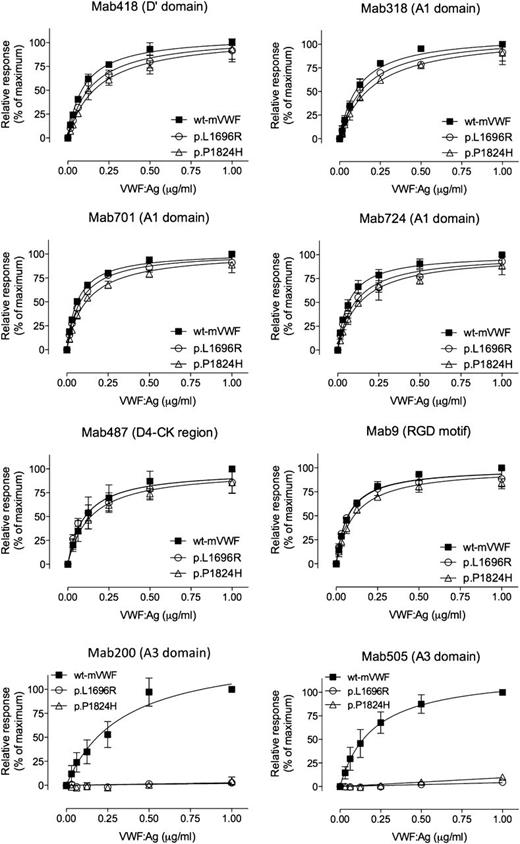
Binding of VWF mutants to different Mabs. Various concentrations (0-1 μg/mL) of wt-rVWF (▪), p.L1696R-rVWF (○), or p.P1824H-VWF (△) were incubated with microtiter wells coated with 1 of the indicated Mabs (418, 318, 701, 724, 487, 9, 200, or 505). Bound VWF was probed using horseradish peroxidase–labeled polyclonal anti-VWF antibodies and detected via peroxidase hydrolysis of Tetramethylbenzidine. Presented is the relative response (% of binding by wt-rVWF at 1 μg/mL) vs VWF:Ag concentration (μg/mL). The drawn lines represent the best fit using an equation for one-site–specific binding using GraphPad Prism software. Data represent the mean ± SD of 3 independent measurements.
Binding of VWF mutants to different Mabs. Various concentrations (0-1 μg/mL) of wt-rVWF (▪), p.L1696R-rVWF (○), or p.P1824H-VWF (△) were incubated with microtiter wells coated with 1 of the indicated Mabs (418, 318, 701, 724, 487, 9, 200, or 505). Bound VWF was probed using horseradish peroxidase–labeled polyclonal anti-VWF antibodies and detected via peroxidase hydrolysis of Tetramethylbenzidine. Presented is the relative response (% of binding by wt-rVWF at 1 μg/mL) vs VWF:Ag concentration (μg/mL). The drawn lines represent the best fit using an equation for one-site–specific binding using GraphPad Prism software. Data represent the mean ± SD of 3 independent measurements.
Both mutants had a smeary multimeric pattern when analyzed as homozygous proteins, whereas the multimeric pattern appeared normal upon cotransfection with wt-VWF cDNA (Figure 2D).
Interaction of VWF and its mutants with anti-VWF antibodies
To allow functional analysis of the mutants, larger amounts of the proteins were produced using stably transfected BHK-furin cell lines. Large amounts of supernatant were collected, containing 0.14 ± 0.03 µg/mL and 0.2 ± 0.01 µg/mL for p.L1696R-rVWF and p.P1824H-rVWF, respectively, vs 2.0 ± 0.85 µg/mL for wt-rVWF. VWF levels of the mutants were increased to 2.1 to 3 µg/mL after concentration. We then compared binding of wt-rVWF and VWF mutants to a series of Mabs (Figure 3). Mutants p.L1696R-rVWF and p.P1824H-rVWF were similar to wt-rVWF in their interaction with Mabs against the D′ domain (Mab418), the A1 domain (Mab318, Mab701, and Mab724), the D4-CK region (Mab487), and the RGD motif (Mab9). No statistical significant difference in 50% effective concentration (EC50) or Bmax was found for the interaction with these antibodies. In contrast, binding of the mutants to 2 antibodies recognizing the A3 domain (Mab200 and Mab505) was severely disturbed, with virtually no response being detected (Figure 3). Thus, the overall structure of the mutant proteins seems to be maintained, whereas local disruption of the A3 domain structure prevents interaction with antibodies Mab200 and Mab505.
Binding of recombinant mutant VWF to collagens type I and III
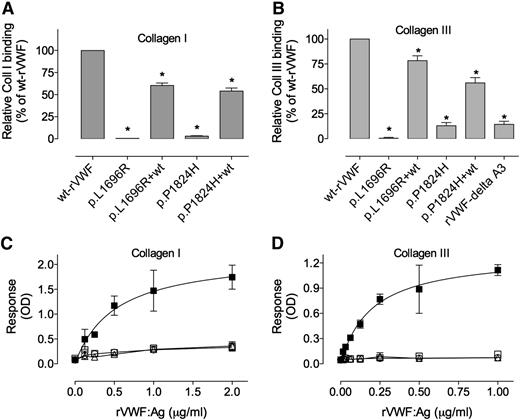
Given the inability of the mutants to interact with Mabs against the A3 domain, we then tested the mutants for collagen binding. Both homozygous mutants p.L1696R-rVWF and p.P1824H-rVWF were unable to bind either collagen I or collagen III (Figure 4). Their binding was as low as that of a VWF variant deleted from the A3 domain (5.6 ± 1.4% of wt values for collagen type I and 14 ± 3% of wt values for collagen type III). The EC50 of wt-rVWF binding to collagen I and III was 0.5 ± 0.1 μg/mL and 1.6 μg/mL, respectively (Figure 4C-D). Because of the nonsaturating response, no reliable EC50 could be calculated for any of the mutants, and was estimated to be >10-fold the EC50 calculated for wt-rVWF. To investigate whether co-expression with wt-rVWF could rescue collagen binding, we also analyzed concentrated supernatants of transiently expressing cells. Cotransfections with wt-VWF resulted in a significant improvement of binding to collagen I or III for both p.L1696R-rVWF (60.4 ± 7.7% and 78.4 ± 4.9%) and p.P1824H-rVWF (54.4 ± 9.6% and 56.0 ± 5.3%), although binding was never as high as obtained for wt-VWF (Figure 4A). These data indicate that both mutations are associated with a severe defect in collagen binding.
Binding of VWF mutants to collagens type I and III. A fixed concentration of (A) 0.25 μg/mL and (B) 0.125 μg/mL of rVWF obtained from transiently transfected COS-7 cells, or various concentrations of (C) 0 to 2 μg/mL and (D) 0 to 1 μg/mL of rVWF obtained from stable transfected BHK cells was added to wells coated with collagen I (A,C) or collagen III (B,D). Bound VWF was probed with horseradish peroxidase–labeled polyclonal anti-VWF antibodies and detected via peroxidase hydrolysis of Tetramethylbenzidine (A,C) and o-Phenylenediamine dihydrochloride for (B,D). Symbols represent wt-rVWF (▪), rVWF-δ A3 (□), p.L1696R-VWF (○), and p.P1824H-rVWF (△). (A,B) Binding of homozygous and cotransfected (1:1) mutants are represented as % of wt-VWF binding. Data represent means ± SD of 3 independent experiments. *P values < .05 as compared with wt values. OD, optical density; NP, normal pooled plasma.
Binding of VWF mutants to collagens type I and III. A fixed concentration of (A) 0.25 μg/mL and (B) 0.125 μg/mL of rVWF obtained from transiently transfected COS-7 cells, or various concentrations of (C) 0 to 2 μg/mL and (D) 0 to 1 μg/mL of rVWF obtained from stable transfected BHK cells was added to wells coated with collagen I (A,C) or collagen III (B,D). Bound VWF was probed with horseradish peroxidase–labeled polyclonal anti-VWF antibodies and detected via peroxidase hydrolysis of Tetramethylbenzidine (A,C) and o-Phenylenediamine dihydrochloride for (B,D). Symbols represent wt-rVWF (▪), rVWF-δ A3 (□), p.L1696R-VWF (○), and p.P1824H-rVWF (△). (A,B) Binding of homozygous and cotransfected (1:1) mutants are represented as % of wt-VWF binding. Data represent means ± SD of 3 independent experiments. *P values < .05 as compared with wt values. OD, optical density; NP, normal pooled plasma.
Binding of recombinant mutant VWF to other VWF ligands
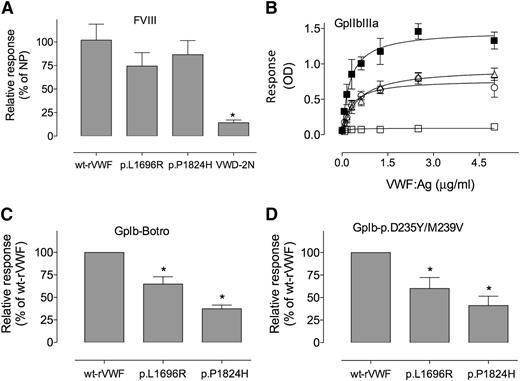
We next undertook a more detailed characterization of the homozygous recombinant mutants in relation to other ligands for VWF (Figure 5). First, binding to FVIII was assessed, and binding of mutants that was similar to wt-rVWF was observed. In contrast, plasma obtained from a VWD-type 2N patient (p.R768Q/p.R854Q-VWF) displayed strongly reduced binding to FVIII (Figure 5A).
Binding of VWF mutants to FVIII, GPIb and GPIIbIIIa. Recombinant VWF, wt (▪), p.D2509G (□), or p.L1696R (○), and p.P1824H (△) produced in BHK cells were tested for binding to FVIII (A), GPIIbIIIa (B), wt-GPIb in the presence of botrocetin (C), or to a GPIb carrying p.D235Y/M239V mutations (D) as described in “Materials and methods.” Data represent means ± SD of 3 independent experiments, except for binding to FVIII, where means ± SEM are represented. *P values < .05 as compared with wt values. OD, optical density; NP, normal pooled plasma.
Binding of VWF mutants to FVIII, GPIb and GPIIbIIIa. Recombinant VWF, wt (▪), p.D2509G (□), or p.L1696R (○), and p.P1824H (△) produced in BHK cells were tested for binding to FVIII (A), GPIIbIIIa (B), wt-GPIb in the presence of botrocetin (C), or to a GPIb carrying p.D235Y/M239V mutations (D) as described in “Materials and methods.” Data represent means ± SD of 3 independent experiments, except for binding to FVIII, where means ± SEM are represented. *P values < .05 as compared with wt values. OD, optical density; NP, normal pooled plasma.
We also measured binding to the GPIIbIIIa platelet receptor. Both mutants showed impaired binding to this ligand compared with wt-rVWF (Figure 5B). However, binding was not as low as with a VWF variant mutated in the RGD site (p.D2509G-rVWF).
Binding to platelet GPIb was assessed using 2 different systems in the presence of botrocetin and using a mutant GPIb able to bind spontaneously to VWF (p.D235Y/p.M239V-rGPIb). Similar results were obtained in both assays with reduced binding of both VWF mutants relative to wt-rVWF (Figure 5C-D). In botrocetin-induced GPIb binding, the p.L1696R-rVWF mutant showed intermediate binding (64.8 ± 8.1%) between wt-rVWF (100%) and the other mutant p.P1824H-rVWF (37.3 ± 4.1%). These results were confirmed using p.D235Y/p.M239V-rGPIb.
The observed defects in the interaction with these homozygous VWF ligands are mild to modest. Indeed, the patient samples containing the heterozygous mutants were characterized by a normal VWF:RCo/VWF:Ag ratio. FVIII:C levels were reduced in all patients but to a lesser extent than the VWF:Ag levels, which points to normal FVIII binding by the patients` VWF.
Effect of mutations on VWF function in vivo
In the last series of experiments, we explored the consequences of the 2 mutations in vivo. After hydrodynamic injection in VWF-deficient mice of huVWFmuA1 cDNA carrying the different mutations, expression levels obtained in the mice were too low (<5% VWF:Ag) to perform functional studies. Therefore we injected mice with a 50:50 mix of wt-huVWFmuA1 cDNA and mutant-huVWFmuA1 cDNA, thus reproducing the heterozygous state of the patients. As in the in vitro expression studies, co-expression with wt-VWF resulted in a marked increased antigen levels (Table 2). Antigen levels of 259 ± 26% were detected for wt-huVWFmuA1 (as compared with normal pooled human plasma), whereas antigen levels were 2.5- to 5-fold lower for the p.L1696R-huVWFmuA1/wt-huVWFmuA1 and p.P1824H-huVWFmuA1/wt-huVWFmuA1 (94 ± 66% and 43 ± 22%, respectively) combinations. The Mab200/Mab418 ratio was 1 for wt-huVWFmuA1 and 0.5 ± 0.1 for both mutants (supplemental Figure 2). Thus, the mouse model represents faithfully the patient situation, with proportionally reduced antigen levels compared with normal VWF and half of the circulating subunits being mutated.
In vivo analysis of wt-VWF and mutants p.L1696R and p.P1824H
| cDNA injected . | VWF:Ag (homozygous expression) . | VWF:Ag (heterozygous expression) . | Fold increase . | Formation of thrombi >30 µm . | Occlusion . | ||
|---|---|---|---|---|---|---|---|
| Veins . | Arterioles . | Veins . | Arterioles . | ||||
| wt-huVWFmuA1 | 259 ± 26% | NA | NA | 7/7 | 7/7 | 6/7 | 7/7 |
| p.L1696R-huVWFmuA1 ± wt-huVWFmuA1 | <5% | 94 ± 66% | 26 | 7/7 | 7/7 | 5/7 | 2/7* |
| p.P1824H-huVWFmuA1 ± wt-huVWFmuA1 | <5% | 43 ± 22% | 8 | 6/6 | 5/6 | 4/6 | 0/6** |
| cDNA injected . | VWF:Ag (homozygous expression) . | VWF:Ag (heterozygous expression) . | Fold increase . | Formation of thrombi >30 µm . | Occlusion . | ||
|---|---|---|---|---|---|---|---|
| Veins . | Arterioles . | Veins . | Arterioles . | ||||
| wt-huVWFmuA1 | 259 ± 26% | NA | NA | 7/7 | 7/7 | 6/7 | 7/7 |
| p.L1696R-huVWFmuA1 ± wt-huVWFmuA1 | <5% | 94 ± 66% | 26 | 7/7 | 7/7 | 5/7 | 2/7* |
| p.P1824H-huVWFmuA1 ± wt-huVWFmuA1 | <5% | 43 ± 22% | 8 | 6/6 | 5/6 | 4/6 | 0/6** |
VWF-deficient mice were injected hydrodynamically with wt- or mutant-huVWFmuA1 cDNA or with a 50:50 mix of both cDNAs. Four days after injection, mice expressing heterozygous VWF were tested in a FeCl3-induced thrombosis model. Thrombus formation and vessel occlusion was recorded for each vessel, vein, or arteriole. Statistical analysis was performed using 2-tailed χ-square test. Antigen levels are in comparison with normal pooled human plasma. Fold increase refers to the increase in Ag levels in the heterozygous expression compared with homozygous expression.
NA, not applicable.
*0.0053, **0.0003 compared with wt-huVWFmuA1.
The ability of the different proteins to support thrombus formation in a ferric chloride–induced thrombosis model was then assessed. Expression of the chimeric molecule wt-huVWFmuA1 allowed occlusion of both venous and arterial mesenteric vessels (Table 2). Expression of the 2 VWF mutants together with wt-huVWFmuA1 led to an almost normal thrombotic response in veins, whereas thrombus formation in arterioles was compromised. The most severe phenotype was observed for the p.P1824H mutation where no arteriolar occlusion was obtained in all 6 tested mice. The vessel occlusion occurring in 2 of the 7 tested mice for p.L1696R-rVWF was strongly delayed compared with wt-huVWFmuA1–expressing mice.
Discussion
We report the identification of 2 mutations located in the VWF A3 domain leading to a combined qualitative and quantitative defect in the protein. The mutations were identified in a heterozygous state in families recruited via the French National Reference Center for von Willebrand Disease because of moderate bleeding symptoms and low VWF:Ag levels. Genetic analysis identified the missense mutations p.L1696R and p.P1824H. Localization of these mutations into the crystal structure of the VWF A3 domain revealed that they were not in close proximity to the collagen binding site, in contrast to previously reported VWF mutations in this A3 domain.12-14 This was a first indication that these mutations may induce defects different than those described for regular collagen binding mutants. We therefore decided to perform a detailed analysis of these mutants.
The first mutant p.L1696R led to unusually low VWF:Ag levels in both the supernatant and cell lysate (Figure 2), suggesting a defect that prevents synthesis of the full protein. We considered the option that splicing defects could contribute to the lack of protein production. This possibility was ruled out by the use of cDNA rather than genomic DNA. Furthermore, in silico analysis (NetGene2 server, www.cbs.dtu.dk/services/NetGene2) revealed that the mutations did not induce new or modulate existing splicing sites. However, we cannot exclude that the transcribed mRNA is unstable and not translated. Alternatively, the protein might be degraded during its synthesis within the endoplasmic reticulum. Surprisingly, production of the mutant was rescued upon co-expression with wt-VWF. Theoretically, the produced protein could consist uniquely of wt subunits. However, we could demonstrate that approximately half of the subunits contained the mutation by using specific antibodies that recognize the mutant VWF either normally (Mab418) or not at all (Mab200; Figure 3, supplemental Figure 2). Of note, our assay does not make the distinction between multimers containing both wt and mutant subunits into 1 multimer vs a mixture of multimers containing either mutant or wt subunits. The normalization of the expression levels seems in partial disagreement with the in vivo situation, where levels of the heterozygous protein are markedly lower compared with normal VWF, both in the patient and in our mouse model. These results suggest that in patients as well as in mice, the p.L1696R mutation has a dominant negative effect that cannot be clearly reproduced in cellular expression systems, showing the limits of such systems. It is possible that the mutant protein is cleared more rapidly than normal VWF in vivo, a possibility that cannot be addressed in vitro. In functional studies, the homozygous protein displayed defects in the interaction with the VWF ligands GpIIbIIIa and GpIbα (Figure 5). However, the defects were mild to modest at best, and the GpIb defect is not observed when analyzing the heterozygous patient proteins. Indeed, normal binding to several antibodies that recognize various parts of the VWF molecule was observed (Figure 3). In contrast, a profound defect in collagen binding was observed, paralleling the absence of binding to 2 antibodies that recognize the A3 domain. The reduced collagen binding was only partially recovered in the heterozygous protein, corresponding to the collagen-binding defect that was observed for the patient samples (Figure 4 and Table 1). The collagen-binding defect in combination with the reduced VWF:Ag levels could explain the bleeding phenotype of the patients. The data obtained using our mouse model are in agreement with this possibility, because vessel occlusion was impaired in arterioles of mice expressing the heterozygous mutant (supplemental Table 1). Indeed, we have previously shown that a collagen-binding defect prevents vessel occlusion but not initial thrombus formation in the ferric chloride–induced thrombosis model.18,21
The second mutation, p.P1824H,15 leads to a slightly different picture. Although low antigen levels are also observed with this mutant, it appears from our expression studies that this is due to intracellular retention and that cotransfection does not correct the defect, again suggesting a dominant negative effect of the mutant protein. Furthermore, a more pronounced defect compared with mutation p.L1696R in vessel occlusion of arterioles was observed. In other aspects, the mutant seems similar to mutant p.L1696R, displaying reduced binding to VWF ligands GPIIbIIIa and GpIbα, having normal interaction with a series of Mabs, and having a profound defect in collagen binding (Figures 3,Figure 4-5).
The mutants described in the present study are distinct from other mutants described previously, including those concerning the A3 domain.12-14 The mutations (1) impair collagen binding, despite being located away from the collagen binding surface; (2) induce a combined qualitative and quantitative defect; (3) affect multiple VWF-ligand interactions, at least when in the homozygous context; (4) are associated with a partial or complete lack of response to desmopressin treatment; and (5) induce a modest reduction in the quantity of high-molecular-weight multimers in the patients, with the deficiency being less pronounced than observed in classical VWD-type 2A patients.
Both mutations are exemplary for the complexity that underlies the classification of VWF mutants. Based on a minimalist laboratory analysis (VWF:Ag and VWF:RCo), both mutants would be misclassified as VWD-type 1. The multitude of functional defects of the homozygous mutants in combination with the defective biosynthesis does not fit with the current International Society on Thrombosis and Haemostasis Scientific and Standardization Committee VWD classification. However, part of the defect is compensated for in the heterozygous state that characterizes the patients. Despite patients displaying normal VWF:RCo/VWF:Ag ratios, we feel that the defective binding to collagen in the subendothelium and their poor desmopressin response could justify description under the VWD-type 2M subtype.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Elizabeth Benz-Lemoine (Centre Hospitalier Universitaire [CHU] Poitiers), Katia Pouymayou (CHU La Timone, Marseille), and Marc Trossaert (Laboratoire d’Hématologie, CHU Hôtel-Dieu, Nantes, French Reference Center for von Willebrand disease) for their help with characterization of the patients.
This work was funded by the Institut National de la Santé et de la Recherche Médicale, Laboratoires Français de Fractionnement et des Biotechnologies, and grants from Agence Nationale de la Recherche (ANR-08-EBIO-026-01 [C.V.D.] and ANR-08-CEXC-018-01 [P.J.L.]) and Fondation pour la Recherche Médicale (FRM-SPF2010220866) (P.J.L., C. Casari).
Authorship
Contribution: P.L., A.-M.N., J.R., P.B., C.T., C. Casari, C. Caron, C.V.D., and O.D.C. performed experiments and analyzed data; P.B., C.T., C. Caron, and J.G. provided patient material; E.F., A.V., P.J.L., C.V.D., and O.D.C. designed research and wrote manuscript; all authors contributed to the editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter J. Lenting, INSERM U770, 80 rue du General Leclerc, 94276 Le Kremlin-Bicêtre, France; e-mail: peter.lenting@inserm.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal