Key Points
PGE2 signaling positively regulates hematopoietic stem cells both directly and via activation of a nonhematopoietic cell population.
EP4 is a major receptor for the PGE2-mediated regulation of hematopoietic stem and progenitor cells.
Abstract
Prostaglandin E2 (PGE2) regulates hematopoietic stem/progenitor cell (HSPC) activity. However, the receptor(s) responsible for PGE2 signaling remains unclear. Here, we identified EP4 as a receptor activated by PGE2 to regulate HSPCs. Knockdown of Ep4 in HSPCs reduced long-term reconstitution capacity, whereas an EP4-selective agonist induced phosphorylation of GSK3β and β-catenin and enhanced long-term reconstitution capacity. Next, we analyzed the niche-mediated effect of PGE2 in HSPC regulation. Bone marrow mesenchymal progenitor cells (MPCs) expressed EP receptors, and stimulation of MPCs with PGE2 significantly increased their ability to support HSPC colony formation. Among the EP receptor agonists, only an EP4 agonist facilitated the formation of HSPC colonies after the coculture with MPCs. PGE2 up-regulated the expression of cytokine-, cell adhesion-, extracellular matrix-, and protease-related genes in MPCs. We also examined the function of PGE2/EP4 signaling in the recovery of the HSPCs after myelosuppression. The administration of PGE2 or an EP4 agonist facilitated the recovery of HSPCs from 5-fluorouracil (5-FU)–induced myelosuppression, indicating a role for PGE2/EP4 signaling in this process. Altogether, these data suggest that EP4 is a key receptor for PGE2-mediated direct and indirect regulation of HSPCs.
Introduction
Hematopoietic stem cells (HSCs) maintain a balance between self-renewal and differentiation to replenish all blood cell types. HSC regulation requires both intrinsic and extrinsic signals. Cytokines, chemokines, adhesion molecules, proteolytic enzymes, and neurotransmitters are produced by environmental niches functioning together to regulate HSC function and fate.1-3
Prostaglandin E2 (PGE2) is synthesized from arachidonic acid by cyclooxygenases (COX) and prostaglandin E synthases.4 PGE2 modulates various pathologic and physiologic activities, such as cancer, fever, inflammation, atherosclerosis, blood pressure, stroke, and reproduction, as recently reviewed by Legler et al.5 PGE2 also modulates hematopoiesis.6-14 PGE2 is involved in the production of definitive HSCs in the aorta-gonad-mesonephros region and in HSC engraftment.15 PGE2 enhances HSC homing, survival, and proliferation.16 More recently, a stable derivative of PGE2, 16,16-dimethyl PGE2 (dmPGE2), was shown to significantly enhance the engraftment of human cord blood cells in a xenotransplantation model.17
Although PGE2 could be used in a clinical setting to improve hematopoietic transplantation therapy, the expression profiles of its 4 receptors (EP1, EP2, EP3, and EP4) vary within different organs and tissues.18 EP signaling pathways differ according to EP subtypes. Binding of PGE2 to EP2 and EP4 induces production of 3′-5′-cyclic adenosine monophosphate (cAMP) leading to protein kinase A (PKA)–dependent glycogen synthase kinase-3β (GSK-3β) phosphorylation and β-catenin activation. EP2 couples strongly with Gs protein and increases cAMP levels more effectively than EP4.19,20 EP4 activates β-catenin signaling through phosphoinositide 3-kinase (PI3K) as well as cAMP/PKA.19,20 In contrast to EP2 and EP4, an activation of EP1 regulates Ca2+ channel gating via an unidentified G protein.21 The activation of EP3 inhibits adenylate cyclase via Gi protein and decreases cAMP levels.18 A recent study demonstrated that crosstalk between the PGE2 and Wnt signaling at the level of β-catenin stabilization in hematopoietic stem/progenitor cells (HSPCs) contributes to the regulation of HSPC development and regeneration.22 This finding suggests that the increase in cAMP concentrations and GSK-3β phosphorylation via EP2 or EP4 signaling are key events during PGE2-mediated HSC regulation. However, it is not completely clear whether EP2 or EP4 is primarily responsible for the regulation of adult HSCs.
PGE2 also affects the bone marrow (BM) microenvironment.23,24 When mice were continuously administered PGE2, they exhibited less bone trabeculae and more short-term HSCs and multipotent progenitors.24 Although it was suggested that these effects are the result of enlargement of the physical space in the BM, PGE2 may also stimulate a nonhematopoietic cell population to indirectly regulate HSPCs.
Here, we analyzed the ability of PGE2 signaling to directly regulate HSPCs, and we also studied the indirect regulation of HSPC activity by PGE2 via stimulation of a nonhematopoietic cell population. We found that PGE2 signaling positively regulates HSCs both directly and via activation of a nonhematopoietic cell population, and that EP4 is a major receptor for the PGE2-mediated regulation of HSPCs.
Methods
Mice
C57BL/6 mice were purchased from Japan SLC. C57BL/6 mice congenic for the Ly5 locus (B6-Ly5.1) were purchased from Sankyo-Laboratory Service. Because EP4 knockout (KO) mice do not survive on a C57BL/6 background,25,26 they were maintained on a mixed background of 129/Ola × C57BL/6. Animals were cared for in accordance with the guidelines of the Keio University School of Medicine for animal and recombinant DNA experiments. All animal experiments were approved by the ethical review board of the Keio University School of Medicine.
Antibodies
Antibodies used for fluorescence-activated cell sorter (FACS) analyses and immunoblotting are listed in the supplemental data.
Reagents
PGE2 was purchased from Sigma-Aldrich (St. Louis, MO), and dmPGE2 was purchased from Enzo Life Sciences (Farmingdale, NY). The EP1-selective agonist (ONO-DI-004: 17S,17,20-dimethyl-2,5-ethano-6-oxo-PGE1), the EP2-selective agonist (16s-9-deoxy-9β-chloro-15-deoxy-16-hydroxy-17,17-propano-19,20-didehydro-PGE2), the EP3-selective agonist (ONO-AE-248: 11,15-O-dimethyl-PGE2), and the EP4-selective agonist (16-(3-methoxymethyl)phenyl-ω-tetranor-3,7-dithia prostaglandin E1) were provided by Ono Pharmaceutical (Osaka, Japan).
Cell preparation and sorting
The preparation of BM cells and immunostaining for flow cytometry have been described.27 The isolation of endosteal cell populations on the bone surface has been described.28 Stained cells were analyzed and sorted using a FACSvantage DiVa flow cytometer (BD Biosciences, San Jose, CA) and FACSAria (BD Biosciences).
Analyses with quantitative reverse-transcription polymerase chain reaction
Methods for conventional quantitative reverse-transcription polymerase chain reaction (qRT-PCR, Digital PCR, and Digital Array; Fluidigm), and qPCR array (Dynamic Array, Fluidigm) analyses are described in the supplemental data.
Colony assay
To evaluate the colony-forming abilities of Lin−Sca1+cKit+ (LSK) cells from EP2 KO or EP4 KO mice, LSK cells were sorted from EP2 KO, EP4 KO, or wild-type (WT) mice and cultured in MethoCult GF M3434 medium (StemCell Technologies, Vancouver, BC). The number of colony-forming units in culture (CFU-Cs) containing more than 50 cells was scored after 7 days of culture. To evaluate the number of high proliferative potential colony-forming cells (HPP-CFCs), the number of colonies more than 2 mm in diameter was counted after 2 weeks of culture. To analyze the effects of EP agonists on colony-forming ability, LSK cells were treated with control (ethanol), PGE2 (0.1, 1, 10, or 100 μM), an EP2 agonist (0.1, 1, 10, or 100 μM), or EP4 agonist (0.1, 1, 10, or 100 μM) in 2% fetal calf serum phosphate-buffered saline (FCS-PBS) for 2 hours on ice. After incubation, treated cells were cultured in MethoCult GF M3434 medium (StemCell Technologies). To determine whether PEG2 or EP agonists have an effect to stimulate BM niche cell compartment to regulate HSPCs, endosteal fractions (ALCAM+Sca-1–, ALCAM–Sca-1–, and ALCAM–Sca-1+ cells) were isolated and were cultured on type 1 collagen–coated plates (BD Biocoat Cellware; BD Biosciences) (7000 cells/well) in MSCBM (Lonza, Walkersville, MD). After 4 days of culture, each endosteal cell fraction was treated with dmPGE2 (10 μM), EP agonists (0.1, 1, and 10 µM), or ethanol for 2 hours at 37°C and then washed with PBS. LSK cells (400 cells) were cocultured on each endosteal population for 3 days in MyeloCult (StemCell Technologies) without cytokines. Cells were then collected for the colony assay.
Immunoblot analysis
To evaluate the effects of treatments on the levels of phosphorylated GSK-3β, nonphosphorylated GSK-3β, and phosphorylated β-catenin in HSPCs, BM Lin–c-Kit+ cells were treated with dmPGE2 (10 μM), the EP4 agonist (10 or 100 μM), or the EP2 agonist (10 or 100 μM) in 2% FCS-PBS for 30 minutes at room temperature. Cells were lysed and subjected to sodium dodecyl sulfate and polyacrylamide gel electrophoresis and immunoblotting using antibodies against phospho-GSK-3β (Ser9) (D85E12, Cell Signaling Technology, Danvers, MA) (1:1000), nonphosphorylated GSK-3β (27C10, Cell Signaling) (1:1000), or phosphorylated β-catenin (Ser675) (D2F1, Cell Signaling) (1:100). To assess downstream signaling, BM Lin–c-Kit+ cells were treated with dmPGE2 (10 μM), EP4 agonist (100 μM), or EP2 agonist (100 μM) in the presence of PKA (10-μM H89, Sigma-Aldrich) or PI3K (10-μM LY294002, Sigma-Aldrich) inhibitors in 2% FCS-PBS for 30 minutes at room temperature. Densitometric quantification was performed on scanned immunoblot images using Multi Gauge Version 3.1 (FujiFilm, Tokyo, Japan).
EP4 shRNA sequences and preparation of shEP4 retrovirus
EP4 short hairpin RNA (shRNA) sequences were as follows: shEP4-1, 5′-CGAGTGTTCATTAACCAGTTA-3′ and shEP4-2, 5′-CCACCTCGCTGAGAACTTT-3′. The sequence was separated by a 9-nucleotide noncomplementary spacer (TTCAAGAGA) from the corresponding reverse complement of the same 21-nucleotide sequence. A scrambled sequence (5′-GTCCCTATCGCCATTTACT-3′) served as a control. Oligonucleotides were cloned into BglII and HindII sites of the pReGS retrovirus vector provided by Dr. Hara (Tokyo Metropolitan Institute of Medical Science). Retrovirus-shEP4 was produced using the PlatE cell line.
Retroviral transduction
LSK cells were precultured for 1 day, transfected with retrovirus-shEP4 on RetroNectin (Takara Bio, Shiga, Japan) using Magnetofection (OZ Biosciences, Marseille, France), and were incubated for an additional 2 days at 37°C in 5% CO2. Cells were maintained in serum-free medium (SF-O3) containing 1.0% bovine serum albumin, 100 ng/mL of stem cell factor, 100 ng/mL of thrombopoietin, and 10−5 M of 2-mercaptoethanol.
BM reconstitution assay
Donor cells were transplanted into lethally irradiated recipient mice. Peripheral blood (PB) chimerism was analyzed every month. The percentage of donor-derived cells in BM and the ability of donor-derived cells to differentiate into various cell types were analyzed 3 to 4 months after BM transplant (BMT). Details of the BM reconstitution assay are described in the supplemental data.
Injection of 5-FU and in vivo PGE2 and EP4 agonist treatment
To induce myelosuppression, 8-week-old C57BL/6 mice were injected with 5-FU (150 mg/kg body weight, intraperitoneal [IP] route). To evaluate the effects of PGE2 treatment, 5-FU–treated mice were injected intraperitoneally twice daily for the indicated days with either PGE2 (6 mg/kg body weight, IP) or vehicle (4% ethanol in PBS) from the day after 5-FU injection. In some cases, 5-FU–injected mice were treated with the EP4 selective agonist (50 μg/kg body weight, IP) or vehicle twice per day for 7 days. BM mononuclear cells (MNCs) were harvested 12 hours after the final injection for analysis.
Statistical analysis
Statistical significance was determined by a 2-tailed Student t-test, the Tukey test, or the Dunnett test.
Results
Expression of EP receptors in HSPCs
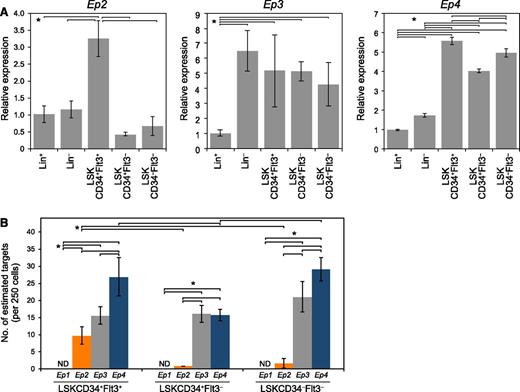
To determine the expression of EP receptors in fractionated mouse BM cells, we undertook qPCR analysis (Figure 1A). We found that Ep4 was highly expressed in LSKCD34-Flt3–, LSKCD34+Flt3–, and LSKCD34+Flt3+ cells compared with Lin– or Lin+ fractions. Ep2 messenger RNA (mRNA) expression was highest in LSKCD34+Flt3+. EP3, a negative receptor that decreased cAMP,18 was expressed in Lin–, LSKCD34-Flt3–, LSKCD34+Flt3–, and LSKCD34+Flt3+ cells. EP1 was not expressed in hematopoietic cells. Next, we investigated the mRNA copy numbers of Ep2, Ep3, and Ep4 by Digital Array and found that the level of Ep2 transcripts in HSPCs was much lower than that of EP3 and EP4 transcripts (Figure 1B). The estimated copy numbers of Ep2, EP3, and Ep4 were 9.75 ± 2.48, 15.57 ± 2.59, and 26.94 ± 5.58 in LSKCD34+Flt3+ cells (per 250 cells), 0.79 ± 0.05, 16.07 ± 2.44, and 15.72 ± 1.63 in LSKCD34+Flt3– cells (per 250 cells), 1.55 ± 1.42, 21.06 ± 4.46, and 29.14 ± 3.39 in LSKCD34–Flt3– cells (per 250 cells), respectively.
Expression of EP receptors in hematopoietic cells. (A) qPCR analysis of Ep2, Ep3, and Ep4 mRNA expression in Lin+, Lin–, LSKCD34+Flt3+, LSKCD34+Flt3–, and LSKCD34–Flt3– fractions. Data represent means ± SD (*P < .05, n = 4). Representative data from 4 independent experiments are shown. Ep1 expression was not detected in hematopoietic cells. (B) Copy number determination of Ep2, Ep3, and Ep4 in LSKCD34+Flt3+, LSKCD34+Flt3–, and LSKCD34–Flt3– cells (250 cells/sample). The numbers of estimated target genes were analyzed by Digital Array. More Ep4-positive signals were observed in LSKCD34+Flt3+ and LSKCD34–Flt3– cells than Ep2- and Ep3-positive signals. Data represent means ± SD (*P < .01, n = 3). Representative data from 2 independent experiments are shown. ND, not detected.
Expression of EP receptors in hematopoietic cells. (A) qPCR analysis of Ep2, Ep3, and Ep4 mRNA expression in Lin+, Lin–, LSKCD34+Flt3+, LSKCD34+Flt3–, and LSKCD34–Flt3– fractions. Data represent means ± SD (*P < .05, n = 4). Representative data from 4 independent experiments are shown. Ep1 expression was not detected in hematopoietic cells. (B) Copy number determination of Ep2, Ep3, and Ep4 in LSKCD34+Flt3+, LSKCD34+Flt3–, and LSKCD34–Flt3– cells (250 cells/sample). The numbers of estimated target genes were analyzed by Digital Array. More Ep4-positive signals were observed in LSKCD34+Flt3+ and LSKCD34–Flt3– cells than Ep2- and Ep3-positive signals. Data represent means ± SD (*P < .01, n = 3). Representative data from 2 independent experiments are shown. ND, not detected.
Expression of genes encoding PGE2 synthesis–related enzymes in the endosteal niche compartment
Osteoblasts in BM reportedly express PGE2.29-31 However, because cells in the endosteum are heterogeneous, it is unclear which populations are responsible for PGE2 production. Therefore, we analyzed the expression of genes encoding enzymes that participate in PGE2 synthesis, namely, Cox1, Cox2, prostaglandin E synthase 1 (Ptges1), Ptges2, and Ptges3, in BM endosteal cell populations. Proinflammatory stimuli markedly induce expression levels of Ptges1 and Cox2, accompanied by a marked increase in PGE2 production. Prostaglandin E synthase 1 is also reportedly functionally coupled with COX2 in marked preference to COX1.32,33 In contrast, Ptges2 is constitutively expressed primarily in the brain, heart, skeletal, kidney, and liver, and Prostaglandin E synthase 2 is functionally coupled with both COX1 and COX2. The endosteal cell fraction (CD45–CD31–Ter119–) was subdivided into 3 fractions based on activated leukocyte cell adhesion molecule (ALCAM) and Sca-1 expression (supplemental Figure 1). We previously reported that mature osteoblasts, immature osteoblasts, and MPCs were enriched in ALCAM+Sca-1–, ALCAM–Sca-1–, and ALCAM–Sca-1+ cells, respectively.28 Cox1 was highly expressed in ALCAM–Sca-1– cells, whereas Ptges2 was more highly expressed in ALCAM+Sca-1– and ALCAM–Sca-1+ cells than in ALCAM–Sca-1– cells. Cox2 was highly expressed in ALCAM–Sca-1– and ALCAM–Sca-1+ cells. It is interesting to note that Ptges1 was highly expressed in ALCAM–Sca-1+ MPCs (supplemental Figure 1). These data suggest that all endosteal cell populations contribute to PGE2 production to varying extents.
PGE2/EP4 signaling functions in HSPC differentiation and LTR capacity
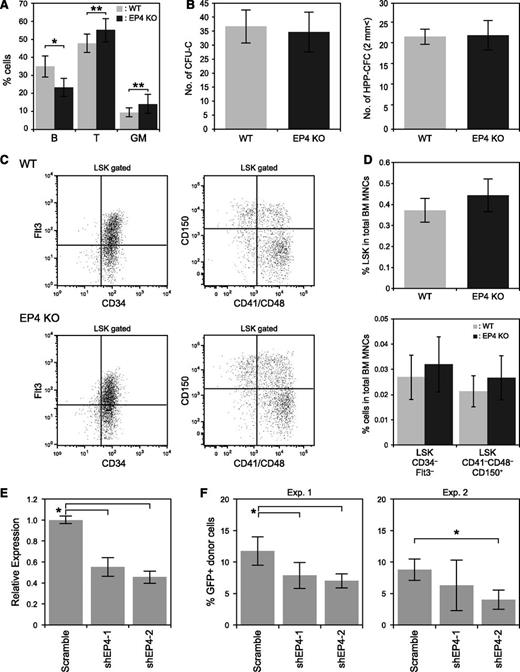
Next, we examined EP4 KO mice to analyze the role of EP4 in PGE signaling in hematopoiesis. Although PB counts were normal, analysis of T, B, and myeloid cells in PB revealed that EP4 KO mice exhibited a slightly higher percentage of T cells (47.7 ± 5.0% in WT and 55.1 ± 6.5% in EP4 KO) and myeloid cells (9.3 ± 2.5% in WT and 14.0 ± 5.2% in EP4 KO) and a slightly lower percentage of B cells compared with WT controls (34.8 ± 5.8% in WT and 23.3 ± 5.0% in EP4 KO) (Figure 2A and supplemental Table 1). The number of CFU-Cs and HPP-CFCs did not significantly differ between WT and EP4 KO LSK cells (Figure 2B). Percentages of LSK, LSKCD34–Flt3–, and LSKCD41–CD48–CD150+ cells in EP4 KO mice were comparable to those seen in WT mice (Figure 2C-D).
Analysis of hematopoiesis in the EP4 KO mouse. (A) PB analysis of B (B220), T (CD4/CD8), and myeloid (Mac-1/Gr-1) cells in 8-week-old WT (n = 13) and EP4 KO (n = 11) mice. Data represent means ± SD (*P < .01; **P < .05). (B) The number of CFU-Cs and HPP-CFCs. Fifty LSK cells were cultured per dish. Data represent means ± SD (n = 3). (C) Representative FACS profiles of LSKCD34–Flt3– cells (left panels) and LSKCD41–CD48–CD150+ cells (right panels) in WT (upper panels) and EP4 KO mice (lower panels). (D) Percentage of LSK cells, LSKCD34–Flt3– cells, and LSKCD41–CD48–CD150+ cells among the total BM MNCs. Data represent means ± SD (n = 7/group). (E) Efficiency of EP4 KD by shEP4-1 and shEP4-2 in LSK cells. Two days after transduction of shEP4-1, shEP4-2, or scrambled shRNA into LSK cells, GFP+LSK cells were sorted and cultured for 1 week. GFP+LSK cells were then resorted, and EP4 expression was analyzed by qPCR. Data represent means ± SD (*P < .01, n = 4). (F) Effects of EP4 shRNA on the LTR capacity of LSK cells. Percentages of GFP+ donor-derived (Ly5.1+) cells in recipient mice 3 months after BMT are shown. Data represent means ± SD (*P < .01, n = 5/group). Data are representative of 2 independent experiments.
Analysis of hematopoiesis in the EP4 KO mouse. (A) PB analysis of B (B220), T (CD4/CD8), and myeloid (Mac-1/Gr-1) cells in 8-week-old WT (n = 13) and EP4 KO (n = 11) mice. Data represent means ± SD (*P < .01; **P < .05). (B) The number of CFU-Cs and HPP-CFCs. Fifty LSK cells were cultured per dish. Data represent means ± SD (n = 3). (C) Representative FACS profiles of LSKCD34–Flt3– cells (left panels) and LSKCD41–CD48–CD150+ cells (right panels) in WT (upper panels) and EP4 KO mice (lower panels). (D) Percentage of LSK cells, LSKCD34–Flt3– cells, and LSKCD41–CD48–CD150+ cells among the total BM MNCs. Data represent means ± SD (n = 7/group). (E) Efficiency of EP4 KD by shEP4-1 and shEP4-2 in LSK cells. Two days after transduction of shEP4-1, shEP4-2, or scrambled shRNA into LSK cells, GFP+LSK cells were sorted and cultured for 1 week. GFP+LSK cells were then resorted, and EP4 expression was analyzed by qPCR. Data represent means ± SD (*P < .01, n = 4). (F) Effects of EP4 shRNA on the LTR capacity of LSK cells. Percentages of GFP+ donor-derived (Ly5.1+) cells in recipient mice 3 months after BMT are shown. Data represent means ± SD (*P < .01, n = 5/group). Data are representative of 2 independent experiments.
Because EP4 KO mice on a C57BL/6 background die of patent ductus arteriosus,25,26 we performed shRNA-mediated knockdown (KD) of EP4 in HSPCs. First, LSK cells were transduced with retrovirus expressing 2 different shRNAs, shEP4-1, or shEP4-2. Both suppressed EP4 expression in that cell type (Figure 2E). We then examined the LTR capacities of LSK cells transduced with scrambled shRNA, shEP4-1, or shEP4-2. The engraftment of donor cells 3 months after BMT was reduced after transduction of shEP4-1 (6.3 ± 4.0%) or shEP4-2 (4.0 ± 1.5%) compared with LSK cells transduced with the scrambled shRNA (9.2 ± 1.6%) at 3 months after BMT (Figure 2F).
We examined the differentiation capacity of donor-derived cells 4 months after BMT. Similar to the BM of EP4 KO mice, mice transplanted with EP4 KD LSK cells had higher percentages of T and myeloid cells and a lower percentage of B cells, compared with mice transplanted with scrambled shRNA-transduced LSK cells (supplemental Figure 2A-B).
EP2 KO mice exhibit normal hematopoietic activity
We next evaluated EP2 receptor function in HSPC regulation by analyzing EP2 KO mice. These mice had normal PB cell counts and a normal differentiation capacity (supplemental Figure 3A and Table 2). The number of CFU-Cs and HPP-CFCs did not differ significantly between WT and EP2 KO LSK cells (supplemental Figure 3B). WT and EP2 KO mice had similar proportions of LSK, LSKCD34–Flt3–, and LSKCD41–CD48–CD150+ cells (supplemental Figure 3C).
When the LTR capacity of EP2 KO LSK and LSKCD34– cells was analyzed, no defect in BM reconstitution was detected (supplemental Figure 3D). Short-term treatment with an EP2 agonist did not change the number of CFU-Cs and HPP-CFCs, suggesting that the colony-forming activity of LSK cells was unaffected (supplemental Figure 4). These data suggest that PGE2/EP2 signaling does not affect the self-renewal activity or proliferation activity of HSPCs.
EP4 stimulation increases HSPC colony formation and LTR capacity
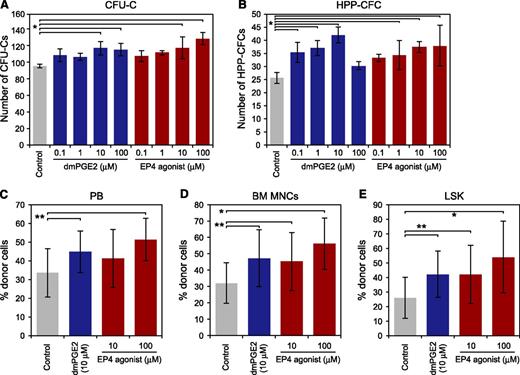
To evaluate the role of PGE2 signaling through EP4 in HSPCs, we assessed the effect of the EP4 agonist on HSPC colony-forming activity. When LSK cells were briefly treated with dmPGE2 or the EP4 agonist and cultured in methylcellulose medium, the number of CFU-Cs increased relative to the control (Figure 3A). In addition, dmPGE2 and EP4 agonist treatments increased the number of HPP-CFCs (Figure 3B). A high dose (100 μM) of dmPGE2 was less effective for HPP-CFC formation compared with the 0.1, 1, or 10 μM of dmPGE2. This was not the case for the EP4 agonist.
Effects of the EP4 agonist on HSPC regulation. (A and B) Effects of dmPGE2 and the EP4 agonist on colony formation of LSK cells (300 cells/dish). Numbers of CFU-Cs (A) and HPP-CFCs (B) are shown. Data represent means ± SD (*P < .01; **P < .05, n = 3). Representative data from 3 independent experiments are shown. (C-E) Effect of dmPGE2 and EP4 agonist treatment on HSPC engraftment. LSK cells (1 × 103 cells/mouse) were treated with 10 μM of dmPGE2, 10 μM, or 100 μM of the EP4 agonist or control were transplanted into lethally irradiated recipient mice. After 4 months of BMT, the frequencies of donor-derived cells in recipient mice were analyzed. Percentages of donor-derived cells engrafted in the PB (C), BM (E), and among BM LSK cells (F). Data represent means ± SD (*P < .01; **P < .05, control: n = 16, dmPGE2: n = 11, 10 μM of EP4 agonist: n = 9, 100 μM of EP4 agonist: n = 9).
Effects of the EP4 agonist on HSPC regulation. (A and B) Effects of dmPGE2 and the EP4 agonist on colony formation of LSK cells (300 cells/dish). Numbers of CFU-Cs (A) and HPP-CFCs (B) are shown. Data represent means ± SD (*P < .01; **P < .05, n = 3). Representative data from 3 independent experiments are shown. (C-E) Effect of dmPGE2 and EP4 agonist treatment on HSPC engraftment. LSK cells (1 × 103 cells/mouse) were treated with 10 μM of dmPGE2, 10 μM, or 100 μM of the EP4 agonist or control were transplanted into lethally irradiated recipient mice. After 4 months of BMT, the frequencies of donor-derived cells in recipient mice were analyzed. Percentages of donor-derived cells engrafted in the PB (C), BM (E), and among BM LSK cells (F). Data represent means ± SD (*P < .01; **P < .05, control: n = 16, dmPGE2: n = 11, 10 μM of EP4 agonist: n = 9, 100 μM of EP4 agonist: n = 9).
Next, we examined the effect of the EP4 agonist on the LTR capacity of LSK cells. LSK cells were treated with dmPGE2 (10 μM) and the EP4 agonist (10 or 100 μM) for 2 hours on ice and were transplanted into recipient mice. In the PB, 10 μM of dmPGE2- and 100 μM of EP4 agonist–treated cells showed higher chimerism of donor-derived cells than the control cells 4 months after BMT, whereas no statistically significant difference was observed in the frequency of donor-derived cells between controls and 10 μM of EP4 agonist-treated cells (Figure 3C). On the other hand, in comparison with control cells, 10 μM of dmPGE2 and EP4 agonist (10 or 100 μM)–treated cells showed greater engraftment in BM MNCs (Figure 3D) and in the LSK fraction (Figure 3E) at 4 months after BMT. These results indicate that PGE2/EP4 signaling positively affects the engraftment of hematopoietic cells.
PGE2 signaling through EP4 induces phosphorylation of GSK-3β and β-catenin in HSPCs
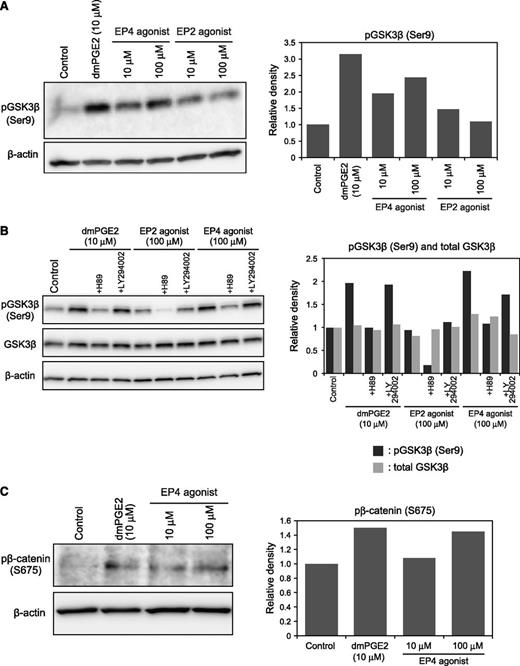
Next, we evaluated the function of PGE2/EP4 signaling in the stabilization of β-catenin in HSPC. PGE2 signaling reportedly interacts with the Wnt/β-catenin pathway.22 GSK-3β is a component of the β-catenin destruction complex, and its phosphorylation at Ser9 decreases its ability to associate with other complex members. GSK-3β phosphorylation was monitored in Lin–c-Kit+ cells after treatment with EP4 or EP2 agonists, or with dmPGE2. Immunoblot analysis showed that treatment with the EP4 agonist, but not the EP2 agonist, increased GSK-3β phosphorylation in Lin–c-Kit+ cells in a dose-dependent manner (Figure 4A). To determine which pathway is activated by PGE2/EP4 signaling, Lin–c-Kit+ cells were briefly treated with dmPGE2 or the EP4 agonist in the presence of a PKA inhibitor (H89) or a PI3K inhibitor (LY294002). H89 treatment significantly decreased GSK-3β phosphorylation in Lin–c-Kit+ cells (Figure 4B). These data indicate that the cAMP/PKA pathway was activated by PGE2/EP4 signaling in HSPCs. In addition, treatment with dmPGE2 or the EP4 agonist increased phosphorylation of β-catenin at Ser675, indicating that β-catenin was stabilized (Figure 4C).34
Effects of dmPGE2 or an EP4 agonist on the regulation of β-catenin. (A) Immunoblot analysis of GSK-3β phosphorylation (Ser9) in Lin–c-Kit+ cells (left panel). Densitometry analysis of the immunoblot is shown in the right panel. Representative data from 2 independent experiments are shown. (B) Immunoblot analysis of GSK-3β phosphorylation (Ser9) in Lin–c-Kit+ cells (left panel). Densitometry analysis of the immunoblot is shown in the right panel. (C) Immunoblot analysis of β-catenin phosphorylation (Ser675) in Lin–c-Kit+ cells (left panel). Densitometry analysis of the immunoblot is shown in the right panel. Representative data from 3 independent experiments are shown.
Effects of dmPGE2 or an EP4 agonist on the regulation of β-catenin. (A) Immunoblot analysis of GSK-3β phosphorylation (Ser9) in Lin–c-Kit+ cells (left panel). Densitometry analysis of the immunoblot is shown in the right panel. Representative data from 2 independent experiments are shown. (B) Immunoblot analysis of GSK-3β phosphorylation (Ser9) in Lin–c-Kit+ cells (left panel). Densitometry analysis of the immunoblot is shown in the right panel. (C) Immunoblot analysis of β-catenin phosphorylation (Ser675) in Lin–c-Kit+ cells (left panel). Densitometry analysis of the immunoblot is shown in the right panel. Representative data from 3 independent experiments are shown.
Treatment with PGE2 and the EP4 agonist enhanced the ability of ALCAM-Sca-1+ MPCs to support HSPCs
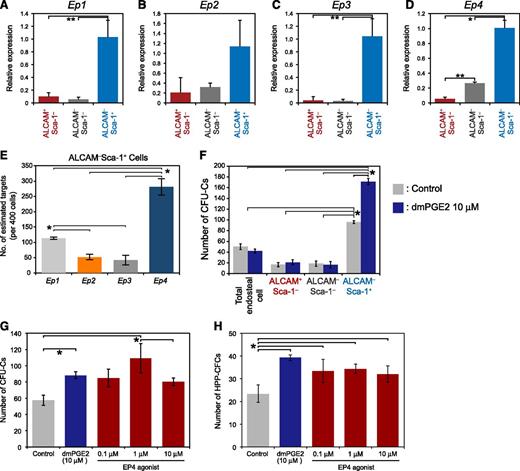
Next, we examined the ability of PGE2 signaling to regulate HSPCs by modulating the activity of MPCs. qPCR analysis demonstrated that EP1-4 was expressed more highly in ALCAM–Sca-1+ MPCs than in the other fractions (Figure 5A-D). These findings indicate that MPCs in adult BM may be particularly sensitive to PGE2. Digital Array analysis was performed to determine the mRNA level of each EP receptor in ALCAM–Sca-1+ MPCs (Figure 5E). A high copy number of Ep4 was detected (280.9 ± 26.9 copies/400 cells). The copy number of the Ep1 receptor (113.92 ± 4.2 copies/400 cells) was higher than that of Ep2 (52.03 ± 8.6 copies/400 cells) and Ep3 (41.87 ± 16.18 copies/400 cells), but was lower than that of Ep4. We investigated whether the hematopoietic-supporting activity of each endosteal cell population was enhanced by PGE2. When unstimulated, cells formed a greater number of CFU-Cs when cocultured with ALCAM–Sca-1+ than when they were cocultured with other fractions or with total endosteal cells (Figure 5F). It is noteworthy that the pretreatment of ALCAM–Sca-1+ MPCs with dmPGE2 significantly enhanced CFU-C and HPP-CFC formation of LSK cells (Figure 5F), whereas treatment of ALCAM+Sca-1–, ALCAM–Sca-1–, or total endosteal cells with PGE2 had no effect on the colony-forming capacity of cocultured LSK cells. These data suggest that the MPC fraction promotes HSPC proliferation after dmPGE2 stimulation. Because ALCAM–Sca-1+ MPCs expressed a higher level of EP4 compared with the other EP receptors, we tested whether the EP4 agonist increased the activity of ALCAM–Sca-1+ MPCs to drive CFU-C and HPP-CFC formation of LSK cells. Treatment of ALCAM–Sca-1+ MPCs with dmPGE2 or the EP4 agonist increased the number of CFU-Cs and HPP-CFC formed (Figure 5G-H). Colony formation was unaffected when LSK cells were cocultured with MPCs that had been pretreated with the other EP agonists (supplemental Figure 5). These data suggest that PGE2 signaling via EP4 activates a niche cell population to support HSPC proliferation, and in this way, PGE2 indirectly regulates HSPC activity.
ALCAM-Sca-1+ MPCs enhance the colony-forming capacity of HSPCs after stimulation with dmPGE2 or the EP4 agonist. (A-D) Expression of EP receptors in endosteal cell fractions. The expression of the PGE2 receptors (A) Ep1, (B) Ep2, (C) Ep3, and (D) Ep4 in each fraction was analyzed. All EP receptors were highly expressed in ALCAM–Sca-1+ MPCs compared with the other fractions. The ALCAM–Sca-1– fraction served as the reference sample. Actb was used to normalize target gene expression. Data represent means ± SD (*P < .01; **P < .05, n = 3). Representative data from 2 independent experiments are shown. (E) Digital Array analysis of Ep1, Ep2, Ep3, and Ep4 expression in ALCAM–Sca-1+ MPCs (400 cells/sample). The numbers of estimated target genes are shown. Data represent means ± SD (*P < .01, n = 3). Representative data from 2 independent experiments are shown. (F) Effect of dmPGE2 on HSPCs via endosteal cells. LSK cells were cocultured with drug-stimulated endosteal cell populations, and the colony-forming capacity was evaluated. Numbers of CFU-Cs and HPP-CFCs are shown. Data represent means ± SD (*P < .05, n = 3). Representative data from 2 independent experiments are shown. (G and H) The effects of dmPGE2 and other EP4 agonists on the formation of CFU-Cs and HPP-CFCs from LSK cells after coculture with ALCAM–Sca-1+ MPCs. Numbers of CFU-Cs (G) and HPP-CFCs (H) are shown. Data represent means ± SD (*P < .05, n = 3). Representative data from 3 independent experiments are shown.
ALCAM-Sca-1+ MPCs enhance the colony-forming capacity of HSPCs after stimulation with dmPGE2 or the EP4 agonist. (A-D) Expression of EP receptors in endosteal cell fractions. The expression of the PGE2 receptors (A) Ep1, (B) Ep2, (C) Ep3, and (D) Ep4 in each fraction was analyzed. All EP receptors were highly expressed in ALCAM–Sca-1+ MPCs compared with the other fractions. The ALCAM–Sca-1– fraction served as the reference sample. Actb was used to normalize target gene expression. Data represent means ± SD (*P < .01; **P < .05, n = 3). Representative data from 2 independent experiments are shown. (E) Digital Array analysis of Ep1, Ep2, Ep3, and Ep4 expression in ALCAM–Sca-1+ MPCs (400 cells/sample). The numbers of estimated target genes are shown. Data represent means ± SD (*P < .01, n = 3). Representative data from 2 independent experiments are shown. (F) Effect of dmPGE2 on HSPCs via endosteal cells. LSK cells were cocultured with drug-stimulated endosteal cell populations, and the colony-forming capacity was evaluated. Numbers of CFU-Cs and HPP-CFCs are shown. Data represent means ± SD (*P < .05, n = 3). Representative data from 2 independent experiments are shown. (G and H) The effects of dmPGE2 and other EP4 agonists on the formation of CFU-Cs and HPP-CFCs from LSK cells after coculture with ALCAM–Sca-1+ MPCs. Numbers of CFU-Cs (G) and HPP-CFCs (H) are shown. Data represent means ± SD (*P < .05, n = 3). Representative data from 3 independent experiments are shown.
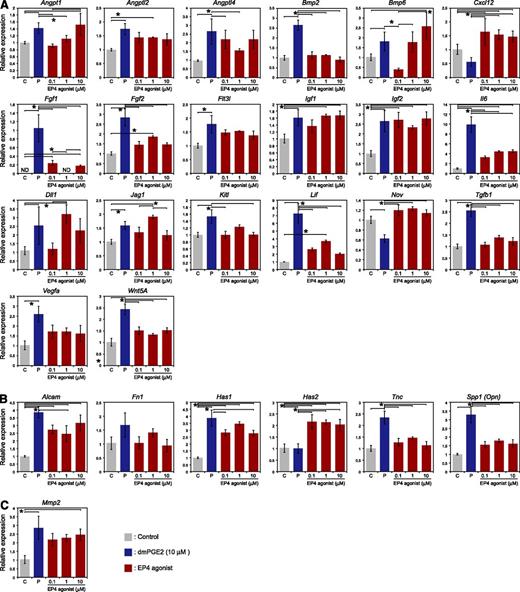
Next, we investigated the expression of HSC niche-related genes in ALCAM–Sca-1+ MPCs after treatment with dmPGE2 or the EP4 agonist (Figure 6). qPCR array analysis revealed that the expression levels of Angpt1, Angptl2, Bmp6, Fgf2, Igf1, Igf2, Dll1, Jag1, Alcam, Fn1, Has1, and Mmp2 were up-regulated by both dmPGE2 and the EP4 agonist. The expression levels of Angptl4, Bmp2, Fgf1, Flt3l, Il6, Kitl, Lif, Tgfb1, Vegfa, Wnt5a, Tnc, and Spp1 were up-regulated by dmPGE2 but not by the EP4 agonist. In contrast, levels of Cxcl12, Nov, and Has2 were up-regulated only by the EP4 agonist. These patterns of gene expression suggest the possibilities that PGE2 accelerated HSPC proliferation by the production of mitogenic ligands. In addition, the upregulation of cell adhesion molecule and extracellular matrix–related genes might induce the cell adhesion of HSPCs.
Effects of dmPGE2 and the EP4 agonist on the expression of niche-related genes in ALCAM–Sca-1+ MPCs. ALCAM–Sca-1+ cells were isolated and cultured on type 1 collagen–coated plates (BD Biocoat) (7000 cells/well) in MSCBM (Lonza). After 1 day of culture, cells were incubated with dmPGE2 (10 μM), the EP4 agonist (0.1, 1, and 10 μM), or ethanol at 37°C overnight. RNA was isolated and reverse transcribed, and qPCR array analysis was performed. (A) Cytokine-related genes: Angpt1, Angptl2, Angptl4, Bmp2, Bmp6, Cxcl12, Fgf1, Fgf2, Flt3l, Igf1, Igf2, Il6, Dll1, Jag1, Kitl, Lif, Nov, Tgfb1, Vegfa, and Wnt5a. (B) Cell adhesion- and extracellular matrix–related genes: Alcam, Fn1, Has1, Has2, Tnc, and Spp1. (C) Proteolytic enzyme–related genes: Mmp2. ND, not detected.
Effects of dmPGE2 and the EP4 agonist on the expression of niche-related genes in ALCAM–Sca-1+ MPCs. ALCAM–Sca-1+ cells were isolated and cultured on type 1 collagen–coated plates (BD Biocoat) (7000 cells/well) in MSCBM (Lonza). After 1 day of culture, cells were incubated with dmPGE2 (10 μM), the EP4 agonist (0.1, 1, and 10 μM), or ethanol at 37°C overnight. RNA was isolated and reverse transcribed, and qPCR array analysis was performed. (A) Cytokine-related genes: Angpt1, Angptl2, Angptl4, Bmp2, Bmp6, Cxcl12, Fgf1, Fgf2, Flt3l, Igf1, Igf2, Il6, Dll1, Jag1, Kitl, Lif, Nov, Tgfb1, Vegfa, and Wnt5a. (B) Cell adhesion- and extracellular matrix–related genes: Alcam, Fn1, Has1, Has2, Tnc, and Spp1. (C) Proteolytic enzyme–related genes: Mmp2. ND, not detected.
PGE2/EP4 signaling enhances the recovery of HSPCs from 5-FU–induced myelosuppression
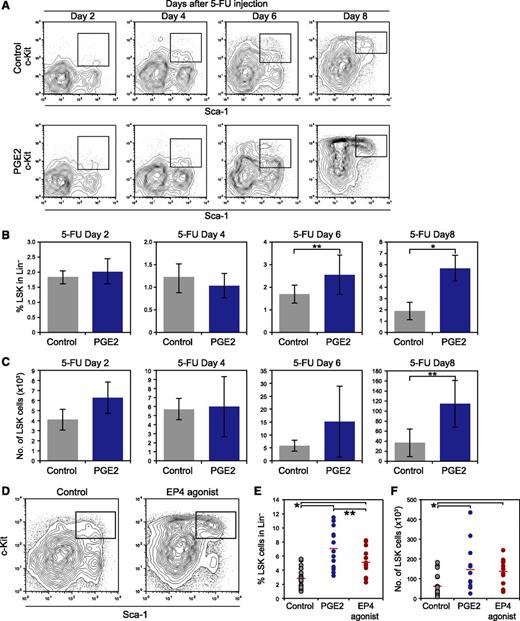
PGE2/EP4 signaling induced stabilization of β-catenin in HSPCs (Figure 4), enhanced the LTR capacity of HSPCs (Figure 3), and enhanced the ability of MPCs to support the colony formation of HSPCs (Figure 5). Furthermore, dmPGE2 treatment reportedly increases the number of white blood cells in PB and BM after 5-FU injection,15 whereas Cox2 KO mice show delayed BM recovery after 5-FU–induced myeloablation.35 Thus, we investigated the function of PGE2/EP4 signaling in the recovery of HSPCs after myelosuppression. To analyze the effect of PGE2 on HSPC recovery, the proportion of LSK cells in 5-FU–injected mice treated with PGE2 was examined 2, 4, 6, and 8 days after 5-FU injection. At 2 and 4 days after 5-FU treatment, the proportion of LSK cells in PGE2-treated mice was comparable to that of control mice (Figure 7A-B). In contrast, PGE2-treated mice demonstrated a higher frequency of LSK cells than that of control mice 6 and 8 days after 5-FU injection (Figure 7A-B). The number of LSK cells increased in mice treated with PGE2 on day 8 after 5-FU treatment compared with controls (Figure 7C). Gene expression in LSK cells isolated from PGE2-treated mice or control mice was analyzed 2, 4, 6, and 8 days after 5-FU injection. In the later phase (days 4-8), LSK cells derived from PGE2-treated mice exhibited higher expression of Cyclin-dependent kinase (Cdk) 4, Cdk6, Ctnnb1 (β-catenin), and Myc than that of the control cells (supplemental Figure 6). In addition, LSK cells isolated from PGE2-treated mice expressed higher levels of the HSC markers, Slamf1,36 Cxcr4,37 Tek (Tie2),27 and Evi138 (supplemental Figure 6). These data indicate that PGE2 treatment accelerates proliferation and/or protects HSCs from 5-FU-induced apoptosis in vivo, which enhances the recovery of LSK cells after myelosuppression in PGE2-treated mice.
Treatment with PGE2 or the EP4 agonist enhances hematopoietic recovery from myelosuppression in vivo. (A) Representative FACS profiles of LSK cells in control- and PGE2-treated mice 2, 4, 6, and 8 days after 5-FU injection. Twice-daily injections with PGE2 (6 mg/kg body weight, IP) or ethanol were commenced the day after 5-FU injection. (B) The percentage of LSK cells in the Lin– fraction in control and PGE2-treated mice 2, 4, 6, and 8 days after 5-FU injection. Data represent means ± SD (*P < .01; **P < .05, n = 5/group). Representative data from 2 independent experiments are shown. (C) Number of LSK cells in control and PGE2-treated mice 2, 4, 6, and 8 days after 5-FU injection. Data represent means ± SD (**P < .05, n = 5/group). (D) Representative FACS profiles of LSK cells in control mice and EP4 agonist–treated mice 8 days after 5-FU injection. Twice-daily injections with the EP4 agonist (50 μg/kg body weight, IP) or ethanol were commenced the day after 5-FU injection. Representative data from 3 independent experiments are shown. (E) The percentage of LSK cells in the Lin– fraction in control mice and in PGE2- and EP4 agonist–treated mice 8 days after 5-FU injection (*P < .01; **P < .05, Control: n = 18, PGE2: n = 13, EP4 agonist: n = 14). (F) Number of LSK cells in control mice and in PGE2- and EP4 agonist–treated mice 8 days after 5-FU injection. The data indicates the number of LSK cells per mice (2 femurs and 2 tibias) (*P < .01, Control: n = 18, PGE2: n = 13, EP4 agonist: n = 14).
Treatment with PGE2 or the EP4 agonist enhances hematopoietic recovery from myelosuppression in vivo. (A) Representative FACS profiles of LSK cells in control- and PGE2-treated mice 2, 4, 6, and 8 days after 5-FU injection. Twice-daily injections with PGE2 (6 mg/kg body weight, IP) or ethanol were commenced the day after 5-FU injection. (B) The percentage of LSK cells in the Lin– fraction in control and PGE2-treated mice 2, 4, 6, and 8 days after 5-FU injection. Data represent means ± SD (*P < .01; **P < .05, n = 5/group). Representative data from 2 independent experiments are shown. (C) Number of LSK cells in control and PGE2-treated mice 2, 4, 6, and 8 days after 5-FU injection. Data represent means ± SD (**P < .05, n = 5/group). (D) Representative FACS profiles of LSK cells in control mice and EP4 agonist–treated mice 8 days after 5-FU injection. Twice-daily injections with the EP4 agonist (50 μg/kg body weight, IP) or ethanol were commenced the day after 5-FU injection. Representative data from 3 independent experiments are shown. (E) The percentage of LSK cells in the Lin– fraction in control mice and in PGE2- and EP4 agonist–treated mice 8 days after 5-FU injection (*P < .01; **P < .05, Control: n = 18, PGE2: n = 13, EP4 agonist: n = 14). (F) Number of LSK cells in control mice and in PGE2- and EP4 agonist–treated mice 8 days after 5-FU injection. The data indicates the number of LSK cells per mice (2 femurs and 2 tibias) (*P < .01, Control: n = 18, PGE2: n = 13, EP4 agonist: n = 14).
Next, we examined the effect of in vivo treatment with an EP4 agonist on HSPC recovery after myelosuppression in vivo. 5-FU-injected mice were treated with the EP4 agonist, and the proportion of LSK cells 8 days after 5-FU injection was determined (Figure 7D-F). The EP4 agonist promoted recovery of HSPCs after 5-FU–induced myelosuppression in vivo. A higher percentage and frequency of LSK cells was observed in EP4 agonist–treated mice than in control mice (Figure 7E-F), whereas the percentage of LSK cells in EP4 agonist–treated mice was lower than that in PGE2-treated mice (Figure 7E). qPCR analysis of LSK cells from EP4 agonist–treated mice or control mice was performed 8 days after 5-FU injection and revealed that treatment with the EP4 agonist up-regulated Cdk4 and Ctnnb1 in a manner similar to PGE2 treatment, whereas the expression levels of Myc and Cdk6 remained unchanged (supplemental Figure 7).
Finally, we examined the expression of PGE2 synthesis–related enzyme genes in the BM endosteal cell populations 3, 6, 8, and 12 days after 5-FU injection. FACS analyses indicated that the percentage of cells in the ALCAM–Sca-1+ MPC fraction increased after 3 and 6 days, and decreased 8 and 12 days after 5-FU administration (supplemental Figure 8A). Expression of Ptges1, Ptges2, Cox1, and Cox2 increased during BM recovery in ALCAM–Sca-1+ MPCs (supplemental Figure 8B). These data indicate that PGE2 production is up-regulated in ALCAM-Sca-1+ MPCs under myelosuppressive conditions.
Discussion
PGE2 signaling interacts with the Wnt/β-catenin pathway and contributes to the generation and regenerative capacity of HSPCs.22 Here, we demonstrated that the EP4 receptor plays a crucial role in the direct regulation of HSPCs by PGE2 signaling. We also found that PGE2/EP4 signaling supports hematopoiesis indirectly by stimulating a specialized population of cells in MPCs.
Direct function of PGE2 in the regulation of adult HSPCs
EP2 and EP4 receptors stimulate Gs protein, which leads to cAMP production and activation of the cAMP/PKA pathway,18 and modulation of Wnt/β-catenin signaling.22 Digital PCR analysis clearly demonstrated that HSPCs had a higher copy number of EP4 than that of EP2 (Figure 1), and EP2 KO mice did not have defects in the number of HSPCs. EP2-deficient HSPCs showed normal colony formation and LTR capacities (supplemental Figure 3). In addition, we found that EP2 agonist treatment did not affect the colony formation of LSK cells (supplemental Figure 4). In contrast, EP4 KD reduced BM reconstitution (Figure 2), and both EP4 KO mice and recipient mice transplanted with EP4 KD LSK cells had increased percentages of T and myeloid cells and decreased percentages of B cells (Figure 2 and supplemental Figure 2). These findings suggest that EP4, but not EP2, affects the PGE2-mediated direct regulation of adult HSCs. Furthermore, the EP4 agonist increased the colony-forming capacity and LTR capacity of LSK cells (Figure 3). Further investigations using, for example, the serial BMT assay will be necessary to elucidate whether PGE2/EP4 signaling enhances the self-renewal activity of LT-HSCs.
We found that EP4 agonist induced phosphorylation of GSK-3β (Ser9) and β-catenin (S675). A PKA inhibitor significantly decreased PGE2- and EP4 agonist–induced phosphorylation of GSK-3β, whereas a PI3K inhibitor did not (Figure 4). These data suggest that PKA is the main downstream effector of PGE2/EP4 signaling that is responsible for activating β-catenin in HSPCs.
Wnt/β-catenin pathway is required for the development of leukemia stem cells in acute myeloid leukemia.39 In colon cancer, PGE2/EP2 signaling interacts with the Wnt/β-catenin pathway through PI3K/Akt to promote cancer cell growth.40 Therefore, it would be interesting to determine whether PGE2/EP4 signaling functions to activate Wnt/β-catenin signaling during the development of acute myeloid leukemia.
Function of PGE2 in HSPC regulation mediated by MPCs
We identified that PGE2 also indirectly regulated HSPCs by modulating ALCAM–Sca-1+ MPCs. LSK cells cocultured with dmPGE2-treated ALCAM–Sca-1+ MPCs formed more CFU-Cs and HPP-CFCs than LSK cells cultured with control ALCAM–Sca-1+ MPCs (Figure 5). Gene expression patterns in MPCs after PGE2 treatment suggest that PGE2 accelerated HSPC proliferation by the production of mitogenic ligands and induced the cell adhesion of HSPCs by the production of cell adhesion molecule and extracellular matrixes in MPCs. These data indicate that PGE2 can induce the proliferation of HSPCs by activating MPCs. The EP4 agonist also activated ALCAM–Sca-1+ MPCs, whereas the other EP agonists had no effect. These data suggest that the activation of ALCAM–Sca-1+ MPCs by dmPGE2 requires EP4. However, the effects of the EP4 agonist on HPP-CFC formation and gene expression were less potent than those of dmPGE2. Therefore, the activation of EP4 and another receptor(s) in a specialized cell population, including MPCs, may be required to completely mimic the effects of dmPGE2.
Macrophages and monocytes have strong PGE2 biosynthetic capacity.7,41,42 In this study, we found that ALCAM–Sca-1+ MPCs expressed high levels of Ptges1 and Cox2 (supplemental Figure 1), which participate in PGE2 synthesis under proinflammatory conditions. The expression of Ptges1, Pteges2, Cox1, and Cox2 was up-regulated in ALCAM–Sca-1+ MPCs after 5-FU treatment (supplemental Figure 8). Therefore, we anticipate that MPCs facilitate the recovery of HSPCs under myelosuppressive conditions by producing PGE2. Autocrine PGE2 activity also activates MPCs and supports HSPC recovery.
PGE2 signaling functions in HSPC recovery after myelosuppression
PGE2 treatment accelerates the recovery of hematopoietic cells after 5-FU–induced myelosuppression.15 On the other hand, the inhibition of either Cox1 or Cox2 decreases the recovery of PB and BM white blood cells.9,15 Also, Cox2 KO mice show delayed recovery of BM after 5-FU–induced myelosuppression.35 However, the population dynamics of hematopoietic cells during BM recovery have not been understood. Here, we demonstrated that treatment with PGE2 or an EP4 agonist enhanced the recovery of LSK cells after 5-FU treatment. Gene expression analysis revealed that LSK cells from PGE2-treated mice expressed higher levels of cell cycle–related genes and HSC markers than the controls (supplemental Figure 6). These data suggest that PGE2 signaling promotes BM recovery by accelerating HSPC proliferation. The increased expression of HSC markers after 5-FU treatment suggests that PGE2 may protect immature stem cells.
Although in vivo treatment of mice with the EP4 agonist improved the recovery of HSPCs after 5-FU–induced myelosuppression, its effect was weaker than that of PGE2 (Figure 7). Therefore, PGE2 may promote HSPC recovery after myelosuppression by 2 distinct mechanisms: direct activation of HSPCs by binding to EP4; and indirect activation of a particular cell population, including MPCs, by binding to EP4 and other unknown receptor(s).
HSCs are used to treat hematologic malignancies, immunodeficiencies, and BM failure. Because BM contains a low level of HSCs, the ability to culture HSCs ex vivo would be advantageous. Therefore, understanding how HSCs are regulated and the functions of each specialized cell population may facilitate the production of HSCs for transplantation therapy. PGE2 is already used in various clinical settings and is an attractive potential therapy for patients with depleted HSCs. This study demonstrated that PGE2/EP4 signaling increases the LTR capacity of HSPCs. The modulation of PGE2/EP4 signaling may facilitate hematopoiesis in vitro. It would also be interesting to investigate the combinational activation of PGE2/EP4 signaling and other cytokine signaling for the efficient induction of the expansion of HSCs. PGE2 plays a role in the regulation of the mesenchymal cell functions in various tissues. Indeed, it has recently been reported that PGE2/EP4 pathway has an antifibrosis effect in kidney interstitial cells.43 In this study, we found that PGE2 indirectly induced HSPC proliferation through the activation of MPCs. A more comprehensive understanding of these indirect effects of PGE2 may facilitate the expansion of HSPCs for use in regenerative medicine.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Ono Pharmaceutical for providing the EP selective agonists.
This study was supported by the Funding Program for Next Generation World-Leading Researchers (NEXT Program) and a Grant-in-Aid from the Japan Society for the Promotion of Science.
Authorship
Contributions: Y.M.I. and F.A. designed and performed experiments, and analyzed and interpreted data; K.H., H.T., and K.T. participated in performing experiments, data analysis, and discussions; T.F. and S.N. provided the EP2 and EP4 KO mice; Y.M.I., F.A., and T.S. wrote the paper.
Conflict-of-Interest disclosure: The authors declare no competing financial interests.
Correspondence: Fumio Arai and Toshio Suda; e-mail: farai@a3.keio.jp (F.A.) and sudato@z3.keio.jp (T.S.)
References
Author notes
Y.M.I. and F.A. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal