Abstract
Several peripheral blood mononuclear cell (PBMC)–derived cell populations can promote angiogenesis, and differences in CD34+ or CD14+ surface expression have been used to separate PBMC subpopulations in this respect. AngiomiRs, microRNAs regulating angiogenesis, are key regulators of angiogenic processes. The present study examines differential angiomiR expression/secretion from CD34+/CD14+, CD34+/CD14−, CD34−/CD14+, and CD34−/CD14− PBMC subsets and their relevance for different proangiogenic properties. Notably, both circulating human CD34+/14+ and CD34+/14− PBMC subsets and their supernatants exerted more potent proangiogenic effects compared with CD34− PBMC subsets. MiR-126 was identified as most differentially expressed angiomiR in CD34+ compared with CD34− PBMC subsets, determined by miR-array and RT-PCR validation. Modulation of miR-126 by anti–miR-126 or miR-mimic-126 treatment resulted in significant loss or increase of proangiogenic effects of CD34+ PBMCs. MiR-126 levels in supernatants of CD34+ PBMC subsets were substantially higher compared with CD34− PBMC subsets. MiR-126 was secreted in microvesicles/exosomes, and inhibition of their release impaired CD34+ PBMCs proangiogenic effects. Notably, high-glucose treatment or diabetes reduced miR-126 levels of CD34+ PBMCs, associated with impaired proangiogenic properties that could be rescued by miR-mimic-126 treatment. The present findings provide a novel molecular mechanism underlying increased proangiogenic effects of CD34+ PBMCs, that is, angiomiR-126 expression/secretion. Moreover, an alteration of angiomiR-126 expression in CD34+ PBMCs in diabetes provides a novel pathway causing impaired proangiogenic effects.
Key Points
AngiomiR-126 is secreted from circulating CD34+ cells and acts as a signaling mechanism of pro-angiogenic effects on endothelial cells, which is impaired in diabetes.
Introduction
Several peripheral blood mononuclear cells (PBMC)–derived cell populations have been shown to promote angiogenesis and differences in both CD34+ or CD14+ surface expression, and have been used to separate functionally different PBMC subpopulations in this respect.1-5 In particular, human CD34+ PBMCs have been shown to promote angiogenesis in response to ischemia,1,3,5 resulting largely from paracrine effects. Administration of CD34+ PBMCs is also being evaluated as a potential treatment of ischemic heart disease and limb ischemia.6,7 However, an impaired cardiovascular repair function of autologous patient-derived progenitor cells, in particular a reduced capacity to promote neovascularization, has been suggested in diabetes and ischemic heart disease,8-12 that may limit the efficacy of cell-based therapies in these patients; the underlying mechanisms need to be fully elucidated.
Moreover, PBMCs have been suggested as a source of both early and late outgrowth “endothelial progenitor cells”2 and differences in CD14+ expression have been proposed to distinguish mononuclear cells representing a source of early or late outgrowth cells in this respect.13 The majority of early “endothelial progenitor cells” have been suggested to arise from CD14+ subpopulations of PBMCs, whereas late outgrowth “endothelial progenitor cells” with a potential for differentiation toward endothelial cells were reported to be derived from CD14− mononuclear cell fractions.2,13
Importantly, several microRNAs (miRs) have recently been identified as critical regulators of vascular development and angiogenesis that have been termed angiomiRs.14-17 Moreover, detection of circulating miRs in serum/plasma as well as secretion of miRs or their release in apoptotic microparticles has recently been reported, suggesting that miRs may also play a regulatory role outside of the cell and thereby impact on target cells.18-22
The present study was therefore designed to determine whether there is differential angiomiR expression of these different PBMC subsets, separated for CD34+ expression as well as CD14+ surface expression, that is, CD34+CD14+ and CD34+14− as well CD34−/14+ and CD34−14− PBMC subpopulations. Furthermore, we aimed to examine the potential role of differential angiomiR expression for differences in the proangiogenic capacity. In addition, the impact of high-glucose or type 2 diabetes on angiomiR expression and its relevance for the altered proangiogenic effects was examined.
Methods
Cell isolation
PBMCs from healthy male volunteers (age 49 ± 4 years) were isolated from buffy coats by density-gradient centrifugation and washed twice in phosphate-buffered saline (PBS) containing 2mM EDTA and 0.5% serum albumin. Mononuclear cells MNCs; (∼ 500 × 106) were split—that is, half of the PBMCs were incubated with anti-CD14 microbeads (Miltenyi Biotec) for 30 minutes—and separated through a column of a MACS separator (Miltenyi Biotec); the other half of PBMCs was incubated with the Monocyte Isolation Kit II (Miltenyi Biotec) and isolated by negative selection according to the manufacturer's instructions to obtain untouched CD14− (200-250 × 106 cells) and CD14+ (100-150 × 106 cells) fractions from the first and the second half of PBMCs, respectively. These fractions further underwent 2 cycles of anti-CD34+ microbead selection (Miltenyi Biotec), resulting in CD34+CD14+, CD34+ CD14−, CD34−CD14+, CD34−CD14− fractions of MNCs.
Anti-miR and miR-mimic-126 transfection
CD34+ PBMCs were plated onto a 3-cm dish overnight and then harvested for electroporation. CD34+ cells (1 × 106) were transfected in a 4-mm cuvette with 25pmol microRNA (scramble, anti–miR-126 [Exiqon] or miR-mimic-126 [Ambion] as indicated) in a total volume of 300 μL. The conditions for electroporation were the following: 250 V, 125 μF. Cells were incubated for 48 hours at 37°C and 5% CO2.
MicroRNA RT PCR array
Total RNA was extracted from the fresh isolated cells using the miRNeasy Mini Kit (QIAGEN) according to the manufacturer's recommendations. cDNA was generated from 1 μg of total RNA using the RT2 microRNA First Strand Kit (SuperArray Bioscience) according to the manufacturer's procedures. The Human Cell Differentiation and Development RT2 microRNA PCR array was carried out according to the manufacturer's instructions. Real-time quantitative RT-PCR was performed using the RT2 qPCR SYBR Green/ROX Master Mix (SuperArray Bioscience) with prevalidated primer sets. Thermocycling parameters on a MX3000P PCR cycler (Stratagene) were 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, 60°C for 40 seconds, and 72°C for 30 seconds. Samples from each cell population were run at least in triplicate and expression levels on each array plate were normalized using SNORD44 as internal control, according to the formula: NV = 2(CtSNORD 44 − CtmiR).
Validation MicroRNA RT-PCR
Total RNA was extracted using QIAzol reagent (QIAGEN) according to the manufacturer's recommendations. Five nanograms of total RNA from each preparation was used for the miRCURY LNA First-strand cDNA kit, in a final volume of 10 μL, according to the manufacturer's instruction (Exiqon). Real-time PCR was performed in an MX3000P PCR cycler (Stratagene). All experiments were performed in at least quadruplicate using the miRCURY LNA microRNA PCR system (Exiqon). Each reaction (25 μL) contained 0.4 μL of cDNA, 10 pmol of each primer, 0.25 μL of internal reference dye, and 12.5 μL of SYBR Green master mix. The amplification program consisted of one cycle at 95°C for 10 minutes, followed by 40 cycles with a denaturing phase at 95°C for 20 seconds and an annealing phase at 60°C for 1 minute. Data were normalized to results obtained with primers specific for U6 or in case of supernatants using synthetic spike-in miRNA (Exiqon), according to the formula: NV = 2(CtU 6 − CtmiR).
Protein analysis
The cells were washed thrice with PBS and then fixed in 4% PFA. The cells were permeabilized with PBS/0.1% Triton X-100, and blocked in LI-COR buffer. The wells were then incubated with primary antibodies (1:200) in LI-COR blocking buffer (mouse anti-PI3KR2 antibody; Abcam) overnight at 4°C. Infrared anti–mouse IRDye800CW secondary antibody (1:200) and DRAQ5, highly cell-permeable DNA-interactive agent (1:10 000) in PBS/0.5% Tween 20 were then added (50 μL/well). The plates were imaged on an Odyssey infrared scanner (LI-COR) using microplate 2 settings with sensitivity of 4 in the 700 and 7 in the 800-nm wavelength channels. Data were acquired by using Odyssey software (LI-COR), exported, and analyzed in Excel (Microsoft).
Preparation of conditioned medium for functional and miR analysis
Freshly isolated cell fractions, transfected cells, and human aortic endothelial cells (HAECs) were cultured in EGM-2, 10% FCS (Lonza) incubated at 37°C and 5% CO2. After overnight/48-hour incubation, cells were washed with PBS, resuspended in EBM, 0.5% FCS (Lonza) and incubated for 12 hours at 37°C and 5% CO2.
In vitro tube formation assay
Matrigel assays were performed as described previously23 with slight modifications. Briefly, 2 × 103 cells of CD34+CD14+, CD34+CD14−, CD34−CD14+, CD34−CD14− PBMC fractions or transfected CD34+ cells were seeded onto 96-well tissue-culture plates coated with 30 μL of Matrigel (BD Biosciences) and cocultured with 8 × 103 HAECs, resulting in a total cell density of 1 × 104 cells/well. Cells were examined after 5 hours with an inverted microscope (Olympus) at 40× magnification for capillary-like tube formation. Photographs were taken in 5 fields and complete tube numbers were counted.
Microvesicle and exosome isolation from cell supernatants
The microvesicle and exosome fractions were isolated from cell supernatants as described previously.24 In brief, 2 × 106 cells from one buffy coat were incubated in serum-free media which was centrifuged at 1000g for 10 minutes to remove cells. This supernatant was transferred to a new tube and centrifuged at 16 000g for 60 minutes, the pellet (microvesicles) was washed and resuspended in PBS (137mM sodium chloride, 2.7mM potassium chloride, 10mM sodium phosphate dibasic, 2mM potassium phosphate monobasic at pH of 7.4). The supernatant of the 16 000g centrifugation was transferred to a new tube and further centrifuged at 120 000g for 60 minutes to pellet the exosomes. The exosome-depleted supernatant was then centrifuged at 220 000g for 60 minutes. The final supernatant was concentrated using Amicon Ultra Centrifugal Filter Devices (Millipore) to a final volume of 0.5 mL. The pellets, microvesicles, exosomes, and 220 000g pellet were resuspended in 0.5 mL of PBS, so that the total volume of medium contributing to each was identical. For blocking of microvesicle/exosome release from CD34+ cells, these cells were treated with GW-4869 (10μM) for 30 minutes as previously described25 in EGM-2 (10% FCS).
Animals
Animal experiments were approved by the local committee. Male NRMI nu/ν mice, aged 10-16 weeks were used for transplantation of human PBMC subpopulations. Mice were housed under specific pathogen-reduced conditions receiving autoclaved chow and water ad libitum. For in vivo angiogenesis assays in diabetic mice, 5-week-old C57BL/6 mice were used. Diabetes was induced with streptozotocin as described previously.26 After 3 weeks of diabetes, the animals were used for the experiments.
CD34+ cell isolation from diabetic patients
Written informed consent was obtained from all patients and healthy subjects, and the study protocol was approved by the local ethics committee. CD34+ PBMCs were also isolated from patients with type 2 diabetes and age-matched healthy subjects to compare the microRNA expression levels (57 ± 3 years vs 53 ± 5 years; P = n.s.).
In vivo angiogenesis assays for PBMC subfractions
The in vivo Matrigel plug assay was performed as described previously27 with the following modifications: 500 μL of Matrigel Basement Membrane Matrix (BD Biosciences) containing 15 U of heparin (Sigma-Aldrich) was injected subcutaneously into 6- to 8-week-old female athymic nude mice along the abdominal midline. CD34+CD14+, CD34+CD14−, CD34−CD14+, CD34−CD14− fractions were resuspended in 400 μL of PBS and injected via tail vein.
In vivo miR-mimic-126 administration
In C57BL/6 mice (diabetic animals), 100 μL of Matrigel Basement Membrane Matrix (BD Biosciences) containing 15 U of heparin (Sigma-Aldrich) was mixed with miR-mimic-126 and scrambled RNA (5, 15, 25 pmol each) and injected subcutaneously into the animals along the abdominal midline.
Microvesicle and exosome administration
Increasing concentrations of the microvesicle and exosome fractions were mixed with 100 μL of Matrigel Basement Membrane Matrix (BD Biosciences) containing 15 U of heparin (Sigma-Aldrich) and injected subcutaneously into the animals along the abdominal midline.
Blood vessel quantification
After 4 days, blood vessel infiltration in Matrigel plugs was quantified by analysis of CD31 staining (BD Pharmingen) with FITC-anti–rat antibody (AbD Serotec). Sections were photographed (Nikon Eclipse TE300) at 100× magnification using NIS-Elements F3.0. For hemoglobin analysis, the Matrigel plug was removed after 4 days and homogenized in 100 μL of deionized water. After centrifugation, the supernatant was used in the Drabkin assay (Sigma-Aldrich) to measure hemoglobin concentration. Stock solutions of hemoglobin are used to generate a standard curve. Results are expressed relative to plug weight.
Statistical analysis
Quantitative results are expressed as means ± SEM. Comparisons between groups were analyzed using 1-way ANOVA, followed by the Bonferroni posthoc test. A value of P < .05 was considered to be statistically significant.
Results
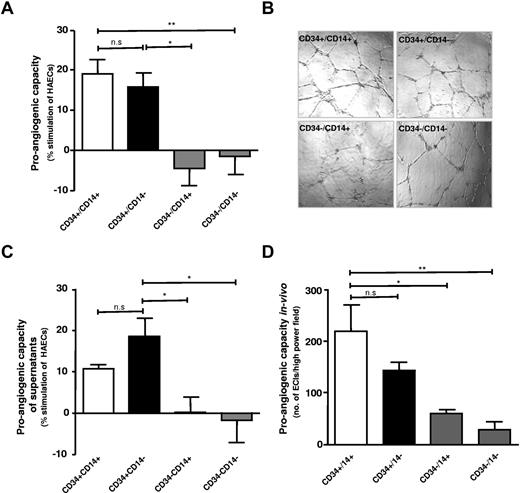
Both CD34+CD14+ and CD34+CD14− PBMC subsets exhibit a substantially greater proangiogenic capacity compared with CD34−/CD14+ and CD34−/CD14− MNC subsets in vitro and in vivo
Tube formation capacity was compared for 2 CD34+ PBMC subsets (CD34+CD14+ and CD34+CD14−) and 2 CD34− cell PBMC subsets (CD34−CD14+ and CD34−CD14−) isolated from circulating PBMCs. Both CD34+ cell fractions had a substantially higher proangiogenic capacity as indicated by an increased tube formation capacity in HAEC-coculture assay, compared with CD34−CD14+ and CD34−CD14− PBMC fractions (Figure 1A-B). Because CD34+ PBMCs have been suggested to promote angiogenesis largely via paracrine mechanisms, conditioned medium from the CD34+ and CD34− PBMC fractions was assessed. Supernatants from CD34+ PBMC fractions had a higher stimulatory capacity for tube formation compared with CD34− PBMC-fraction supernatants. No significant difference was observed between CD34+CD14+ and CD34+CD14− PBMC supernatants (Figure 1C). The analysis of the proangiogenic capacity in vivo, in line with the above results, suggested a higher proangiogenic stimulatory capacity of both CD34+ PBMC fractions compared with CD34− PBMC fractions (Figure 1D). Furthermore, we observed a more pronounced effect of CD34+ cells on angiogenesis in vivo using the Matrigel plugs with an increasing CD34+ cell number (supplemental Figure 3A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
CD34+CD14+ and CD34+CD14−+ PBMCs exhibit increased proangiogenic effects compared with CD34−CD14+ and CD34−CD14− PBMCs. (A) Quantification of tube formation by CD34+CD14+ and CD34+CD14− (□ and ■) as opposed to CD34−CD14+ and CD34−CD14− (▩). (B) Representative photographs of tube formation in high-power field (n = 8). (C) Conditioned medium of CD34+ fraction of PBMCs (□ and ■) promoting tube formation, which was not observed in the CD34− PBMC fractions (▩) (n = 5-6). (D) CD31 staining quantification by Image J, in sections of in vivo Matrigel plug sections (n = 4); EC indicates endothelial cell. Data are mean ± SEM.
CD34+CD14+ and CD34+CD14−+ PBMCs exhibit increased proangiogenic effects compared with CD34−CD14+ and CD34−CD14− PBMCs. (A) Quantification of tube formation by CD34+CD14+ and CD34+CD14− (□ and ■) as opposed to CD34−CD14+ and CD34−CD14− (▩). (B) Representative photographs of tube formation in high-power field (n = 8). (C) Conditioned medium of CD34+ fraction of PBMCs (□ and ■) promoting tube formation, which was not observed in the CD34− PBMC fractions (▩) (n = 5-6). (D) CD31 staining quantification by Image J, in sections of in vivo Matrigel plug sections (n = 4); EC indicates endothelial cell. Data are mean ± SEM.
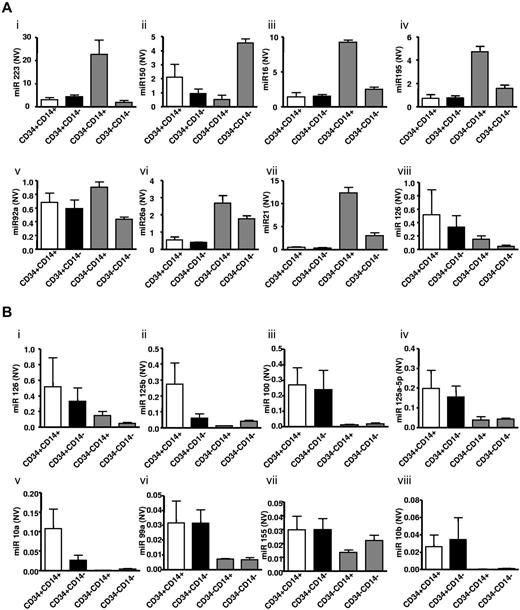
Increased angiomiR miR-126 expression in CD34+ compared with CD34− PBMC subsets
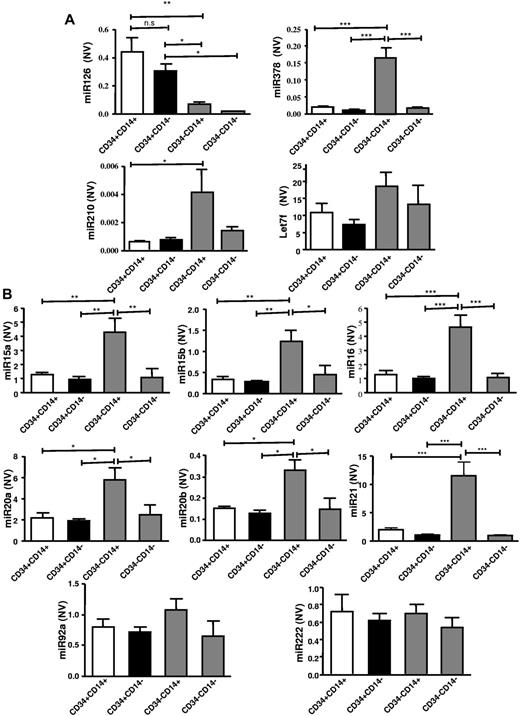
To investigate potential novel mechanisms explaining different proangiogenic effects in CD34+ and CD34− PBMC subpopulations, angiomiR expression patterns were assessed in the above cell populations. The Human Cell Differentiation and Development RT-PCR array was performed using total RNA isolated from CD34+CD14+, CD34+CD14−, CD34−CD14+, CD34−CD14− cell fractions. Within the 8 most expressed miRs of the microRNA array, we identified the angiomiR miR-126 (Figure 2Ai-viii). Moreover, the miR-126 was identified as the angiomiR that was most profoundly higher expressed in CD34+ compared with CD34− MNC subfractions (Figure 2Bi-viii). The expression profiles of other angiomiRs detected in the array and validated are shown in Figure 3. Of note, CD34−CD14+ PBMCs also had higher expression levels of potentially antiangiogenic miRs, such as miRs from the miR-17-92 cluster or miR-21 (Figure 3B).
MicroRNA expression profiles in PBMCs separated for CD34+ and CD14+ surface expression. (A) The 8 most abundant miRs detected using this array are shown. Subpanels i-viii: miR 223, miR 150, miR 16, miR 195, miR 92a, miR 26a, miR 21, miR 126. (B) Moreover, 8 miRs were identified that were significantly higher expressed in CD34+ PBMCs compared with CD34− PBMC subsets, of which miR-126 was the most abundant. CD34+CD14+ (□), CD34+CD14− (■), CD34−CD14+ and CD34−CD14− (▩). Subpanels i-viii: show expression levels of miR 126, miR 125b, miR 100, miR 125a-5p, miR 10a, miR 99a, miR 155, miR 10b. All miRNAs were normalized to SNORD6 and expressed as normalized values (NV). Data are mean ± SEM.
MicroRNA expression profiles in PBMCs separated for CD34+ and CD14+ surface expression. (A) The 8 most abundant miRs detected using this array are shown. Subpanels i-viii: miR 223, miR 150, miR 16, miR 195, miR 92a, miR 26a, miR 21, miR 126. (B) Moreover, 8 miRs were identified that were significantly higher expressed in CD34+ PBMCs compared with CD34− PBMC subsets, of which miR-126 was the most abundant. CD34+CD14+ (□), CD34+CD14− (■), CD34−CD14+ and CD34−CD14− (▩). Subpanels i-viii: show expression levels of miR 126, miR 125b, miR 100, miR 125a-5p, miR 10a, miR 99a, miR 155, miR 10b. All miRNAs were normalized to SNORD6 and expressed as normalized values (NV). Data are mean ± SEM.
Expression of proangiogenic angiomiR miR-126 is higher in CD34+ PBMC subpopulations. (A) Confirmation of microRNA-array results and expression of other potentially proangiogenic angiomiR as determined by RT-PCR (n = 6-8). (B) Expression levels of other potentially antiangiogenic miR in the different MNC subpopulations (n = 6-8). All angiomiR expression is normalized to U6 small nuclear RNA and expressed as normalized value (NV). U6 levels were not altered in the different MNC subpopulations (data not shown). Data are mean ± SEM.
Expression of proangiogenic angiomiR miR-126 is higher in CD34+ PBMC subpopulations. (A) Confirmation of microRNA-array results and expression of other potentially proangiogenic angiomiR as determined by RT-PCR (n = 6-8). (B) Expression levels of other potentially antiangiogenic miR in the different MNC subpopulations (n = 6-8). All angiomiR expression is normalized to U6 small nuclear RNA and expressed as normalized value (NV). U6 levels were not altered in the different MNC subpopulations (data not shown). Data are mean ± SEM.
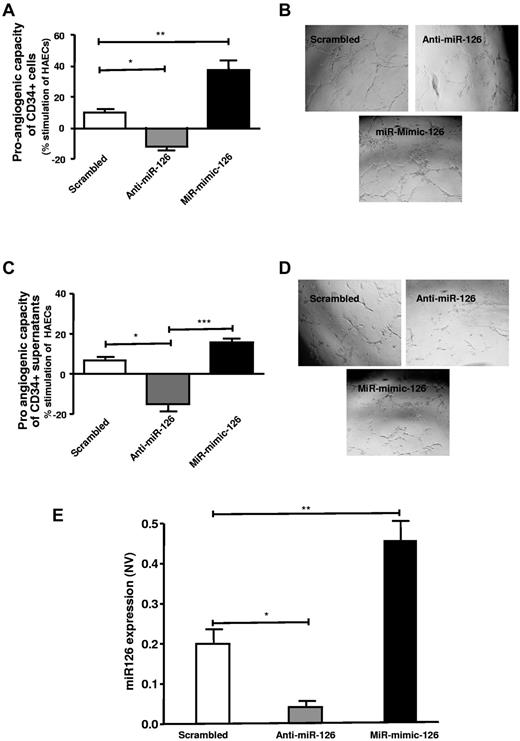
Proangiogenic capacity of CD34+ PBMCs is dependent on miR-126 expression
MiR-mimic-126 or anti–miR-126 treatment resulted in a markedly altered proangiogenic capacity of the CD34+, that is, an enhanced tube formation was seen after miR-mimic-126 transfection into CD34+ cells, whereas a decreased tube formation was observed in response to anti–miR-126–transfected CD34+ cells (Figure 4A-B). Similar results were observed when tube formation was examined in response to conditioned medium from CD34+ cells that were transfected with miR-mimic-126 or anti–miR-126, suggesting that the proangiogenic capacity of CD34+ cells is dependent on miR-126 and is largely mediated via paracrine factors (Figure 4C-D). CD34+ cells transfected with anti–miR-126 or miR-mimic-126 showed altered levels of miR-126, that is, reduced or increased miR-126 levels, respectively (Figure 4E).
Increased proangiogenic capacity of CD34+ PBMCs is critically dependent on miR-126 expression. (A) Shows transfected CD34+ cells, with anti–miR-126 (▩) and miR-mimic 126 (■), effect on tube formation stimulation, respectively, compared with that of scrambled (□) transfected cells. (B) Representative photographs of tube formation in high-power field (n = 6-10). (C) Supernatant of transfected CD34+ cells effect on proangiogenic stimulation of endothelial cells is shown. (D) Photographs of tube formation in high-power field (n = 4). (E) Altered miR-126 expression levels on transfection of CD34+ cells with anti–miR-126 (▩) and miR-mimic-126 (■) (n = 4-6). MicroRNA expression is normalized to U6, small nuclear RNA, and expressed as normalized value (NV). Data are mean ± SEM.
Increased proangiogenic capacity of CD34+ PBMCs is critically dependent on miR-126 expression. (A) Shows transfected CD34+ cells, with anti–miR-126 (▩) and miR-mimic 126 (■), effect on tube formation stimulation, respectively, compared with that of scrambled (□) transfected cells. (B) Representative photographs of tube formation in high-power field (n = 6-10). (C) Supernatant of transfected CD34+ cells effect on proangiogenic stimulation of endothelial cells is shown. (D) Photographs of tube formation in high-power field (n = 4). (E) Altered miR-126 expression levels on transfection of CD34+ cells with anti–miR-126 (▩) and miR-mimic-126 (■) (n = 4-6). MicroRNA expression is normalized to U6, small nuclear RNA, and expressed as normalized value (NV). Data are mean ± SEM.
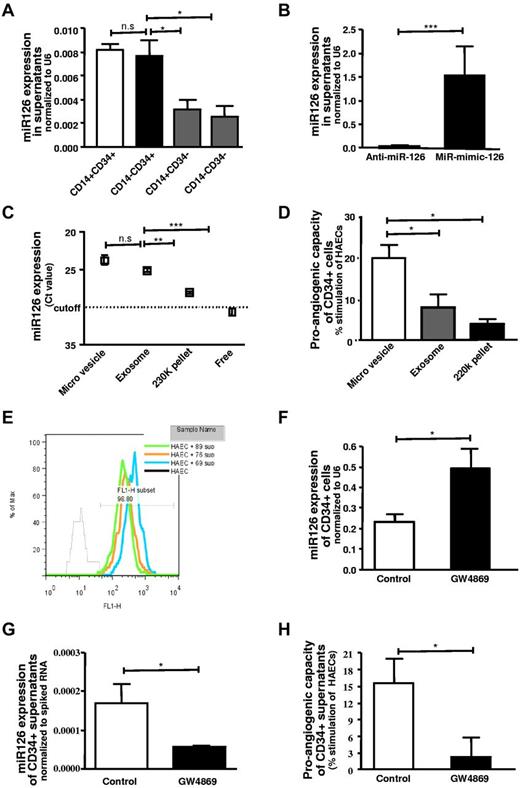
MiR-126 is secreted from CD34+ cells largely in microvesicles/exosomes and stimulates angiogenesis
MiR-126 levels were determined in supernatants from CD34+CD14+, CD34+CD14−, CD34−CD14+, CD34−CD14− MNC populations, and significantly higher secretion of miR-126 in supernatants of CD34+ cell populations was observed. No significant difference was observed between the CD34+CD14+ and CD34+CD14− cell fractions (Figure 5A). Modulation of the expression of miR-126 by anti-miR or miR-mimic-126 in CD34+ cells leads to a decrease or increase in the secretion of this angiomiR into supernatants, respectively (Figure 5B). MiR-126 was largely detected in microvesicles and to a lesser extent in exosomes (Figure 5C). Administration of microvesicles or exosomes derived from CD34+ cells to endothelial cells resulted in a stimulation of tube formation (Figure 4D). Microvesicles or exosomes stimulated angiogenesis in the Matrigel plug assay in vivo and increasing concentrations of microvesicles had a greater effect on in vivo angiogenesis (supplemental Figure 1A-B). We further examined the effects of microvesicles or exosomes derived from anti–miR-126–transfected and miR-mimic-126–transfected CD34+ cells on angiogenesis in the Matrigel plug assay in vivo. Microvesicles or exosomes from miR-126–deficient CD34+ cells had a substantially reduced effect on in vivo angiogenesis (supplemental Figure 2A,C), whereas microvesicles and exosomes derived from miR-mimic-126–transfected cells had an enhanced effect on in vivo angiogenesis (supplemental Figure 2B,D).
miR-126 is secreted by CD34+ PBMCs in microvesicular and exosomal fractions. (A) Assessment of miR-126 levels in the supernatant of the different populations of blood MNCs: CD34+CD14+ (□), CD34+CD14− (■), CD34−CD14− cells (▩) (n = 3-5). (B) miR-mimic-126 or anti–miR-126 treatment leads to increased (■) or reduced (□) miR-126 levels in the supernatant, respectively (n = 4). (C) Expression levels of miR-126 in various fractions of the supernatant (n = 4). (D) Proangiogenic stimulation by microvesicles (□), exosomes (▩), and 220k pellet (■) (n = 4-6). (E) Internalization of vesicles by endothelial cells, detected in FACS analysis by acquisition of PKH-67 stain (n = 3). (F-H) miR-126 expression levels in cells and supernatants of CD34+ cells treated with GW4869 and effect on tube formation (n = 4). Data are mean ± SEM.
miR-126 is secreted by CD34+ PBMCs in microvesicular and exosomal fractions. (A) Assessment of miR-126 levels in the supernatant of the different populations of blood MNCs: CD34+CD14+ (□), CD34+CD14− (■), CD34−CD14− cells (▩) (n = 3-5). (B) miR-mimic-126 or anti–miR-126 treatment leads to increased (■) or reduced (□) miR-126 levels in the supernatant, respectively (n = 4). (C) Expression levels of miR-126 in various fractions of the supernatant (n = 4). (D) Proangiogenic stimulation by microvesicles (□), exosomes (▩), and 220k pellet (■) (n = 4-6). (E) Internalization of vesicles by endothelial cells, detected in FACS analysis by acquisition of PKH-67 stain (n = 3). (F-H) miR-126 expression levels in cells and supernatants of CD34+ cells treated with GW4869 and effect on tube formation (n = 4). Data are mean ± SEM.
The transfer of secreted vesicles from CD34+ cells to the endothelial cells was assessed by adding the supernatants from PKH67-stained CD34+ cells. The vesicular fusion into endothelial cells resulted in acquisition of the color after overnight incubation. The majority of endothelial cells acquired the color as assessed by flow cytometric analysis (Figure 5E).
Blocking of the secretion of the vesicular particles by using GW4869 resulted in reduction of secreted miR-126 in the supernatant of the treated CD34+ cell and a subsequent increase of the miR in CD34+ cells (Figure 5F-G). GW4869 is an inhibitor of neutral sphingomyelinase 2 that regulates ceramide synthesis which is crucial for triggering the secretion of membrane vesicles.25 Treatment of CD34+ cells with GW4869 lead to a loss of proangiogenic stimulation of the conditioned medium derived from these cells compared with untreated CD34+ cells (Figure 5H). Moreover, we have also examined the effects of different doses of the miR-mimic-126 or scrambled-miR on in vivo angiogenesis. In these experiments, the higher doses of the miR-mimic-126, but not of the scrambled-miR, had a more pronounced effect on in vivo angiogenesis (supplemental Figure 3B-C).
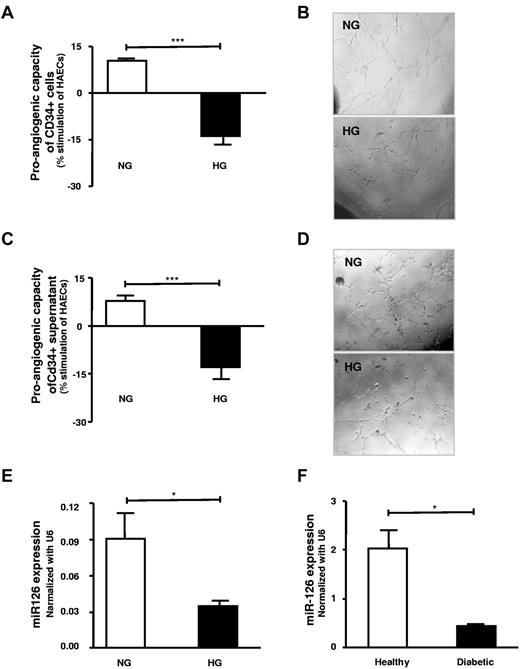
High-glucose treatment or diabetes leads to a loss of miR-126 expression and proangiogenic effects of CD34+ PBMCs that can be restored by miR-mimic-126 transfection
Exposure of CD34+ PBMCs to high glucose substantially decreased their subsequent proangiogenic capacity as determined after washing of CD34+ PBMCs and subsequent coculture with HAECs, that is, using an in vitro tube formation assays (Figure 6A-B). This loss of function was likely due to an alteration of secreted factors because tube formation in HAECs was decreased after treatment with the conditioned medium from high-glucose–treated CD34+ PBMCs (Figure 6C-D). Notably, expression of the proangiogenic miR-126 was down-regulated in high-glucose–treated CD34+ PBMCs compared with buffer-treated cells in both CD34+CD34+ PBMCs and their supernatants (Figure 6E). Of note, CD34+ PBMCs derived from patients with type 2 diabetes had a substantially decreased miR-126 expression compared with age- and sex-matched healthy subjects (Figure 6F).
High-glucose exposure/diabetes leads to loss of proangiogenic effects and miR-126 expression and release from CD34+ PBMCs. (A) Effect of high-glucose 25mM (black) treatment compared with the normal glucose-treated CD34+ cells (n = 5-10). (B) Representative photographs of tube formation in high-power field. (C) Supernatant of high-glucose (■) treated cells as opposed to normal glucose (□) their effect on tube formation (n = 6-8). (D) Photographs of tube formation in high-power field. (E) Assessment of miR-126 in high-glucose–treated healthy CD34+ cells compared with untreated CD34+ cells (n = 4). (F) Assessment of the miR-126 levels in patients with type 2 diabetes (■) CD34+ cells in comparison with healthy subjects (□; n = 3). HG indicates high glucose; and NG, normal glucose. Data are mean ± SEM.
High-glucose exposure/diabetes leads to loss of proangiogenic effects and miR-126 expression and release from CD34+ PBMCs. (A) Effect of high-glucose 25mM (black) treatment compared with the normal glucose-treated CD34+ cells (n = 5-10). (B) Representative photographs of tube formation in high-power field. (C) Supernatant of high-glucose (■) treated cells as opposed to normal glucose (□) their effect on tube formation (n = 6-8). (D) Photographs of tube formation in high-power field. (E) Assessment of miR-126 in high-glucose–treated healthy CD34+ cells compared with untreated CD34+ cells (n = 4). (F) Assessment of the miR-126 levels in patients with type 2 diabetes (■) CD34+ cells in comparison with healthy subjects (□; n = 3). HG indicates high glucose; and NG, normal glucose. Data are mean ± SEM.
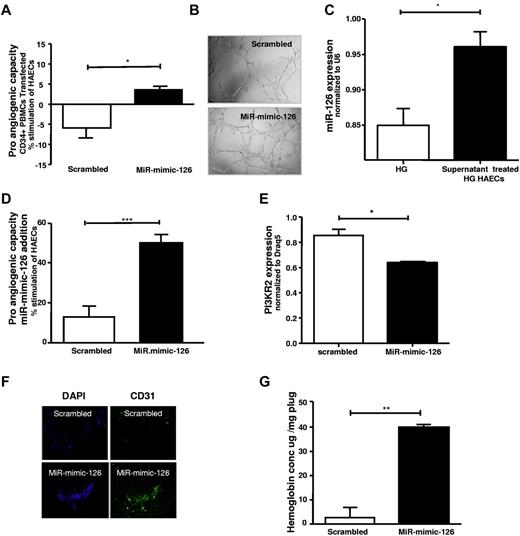
Furthermore, CD34+ PBMCs exposed to high glucose were transfected with miR-mimic-126 or scrambled miR and their capacity to promote endothelial tube formation was subsequently assessed. Notably, miR-mimic-126–treated high glucose exposed CD34+ PBMCs showed a marked improvement in the promotion of tube formation (Figure 7A-B).
Proangiogenic capacity of CD34+ cells and capillary formation is increased by increasing miR-126 levels in high-glucose–treated CD34+ cells. (A) High-glucose–treated CD34+ cells were transfected with scrambled (□) and miR-mimic-126 (■) and the effects on endothelial cell tube formation are shown. (B) Representative photographs of tube formation in high-power field (n = 4). (C) MiR-126 levels in high-glucose–treated (HG) endothelial cells after treatment with healthy CD34+ cell supernatant. (D) Effect of direct exposure of high-glucose–treated HAECs to 25pmol scrambled-miR (□) or miR-mimic-126 (■) on tube-formation is shown (n = 5). (E) Expression levels of PI3KR2 in high-glucose–treated HAECs treated with 25pmol scrambled-miR (□) or miR-mimic-126 (■; n = 3-5). (F) Capillaries in the plug were assessed by staining for CD31 (green) and DAPI (blue) in in vivo Matrigel plug assay in diabetic mice (streptozotocin-induced) after administration of miR-mimic-126 or scrambled miR; a representative image is shown. (G) Hemoglobin content was assessed in the subcutaneously implanted Matrigel plugs of animals receiving scrambled or miR-mimic-126 (directly into plugs; n = 4). Data are mean ± SEM.
Proangiogenic capacity of CD34+ cells and capillary formation is increased by increasing miR-126 levels in high-glucose–treated CD34+ cells. (A) High-glucose–treated CD34+ cells were transfected with scrambled (□) and miR-mimic-126 (■) and the effects on endothelial cell tube formation are shown. (B) Representative photographs of tube formation in high-power field (n = 4). (C) MiR-126 levels in high-glucose–treated (HG) endothelial cells after treatment with healthy CD34+ cell supernatant. (D) Effect of direct exposure of high-glucose–treated HAECs to 25pmol scrambled-miR (□) or miR-mimic-126 (■) on tube-formation is shown (n = 5). (E) Expression levels of PI3KR2 in high-glucose–treated HAECs treated with 25pmol scrambled-miR (□) or miR-mimic-126 (■; n = 3-5). (F) Capillaries in the plug were assessed by staining for CD31 (green) and DAPI (blue) in in vivo Matrigel plug assay in diabetic mice (streptozotocin-induced) after administration of miR-mimic-126 or scrambled miR; a representative image is shown. (G) Hemoglobin content was assessed in the subcutaneously implanted Matrigel plugs of animals receiving scrambled or miR-mimic-126 (directly into plugs; n = 4). Data are mean ± SEM.
Direct exposure of endothelial cells to miR-mimic-126 stimulates angiogenesis in vitro and in vivo
In addition, to determine whether miR-mimic-126 exposure of endothelial cells could directly impact angiogenesis, both in vitro and in vivo administration of miR-mimic-126 was examined. Notably, as described in the earlier section, we have observed that miR-126 is released from CD34+ cells. Moreover, CD34+ cell supernatants increased miR-126 levels in high-glucose–treated endothelial cells (Figure 7C). MiR-mimic-126 exposure, but not scrambled-miR administration to high-glucose–treated endothelial cells, significantly increased their miR-126 levels (data not shown). Notably, addition of the miR-mimic-126 directly to the medium of high-glucose–exposed HAECs increased tube formation compared with addition of scrambled miR (Figure 7D) and down-regulated PI3KR2, a known target of miR-126 in endothelial cells (Figure 7E). Moreover, addition of miR-mimic-126, but not scrambled miR, directly to the Matrigel plug implanted in streptozotocin-induced diabetic animals enhanced tube formation and increased hemoglobin content (as a sign of increased new vessel formation) in the Matrigel plug compared with scrambled miR-treated animals (Figure 7F-G). These findings suggest that direct exposure of miR-mimic-126 can promote these angiogenic processes.
Discussion
In the present study, we have observed a substantially higher expression of the angiomiR miR-126 in human CD34+ compared with CD34− PBMC subsets. Notably, miR-126 was secreted by CD34+ PBMC subsets, largely in microvesicles and exosomes, and this secretion was altered by miR-mimic-126 or anti–miR-126 treatment. Modulation of miR-126 expression levels by either miR-mimic-126 or anti–miR-126 revealed that miR-126 is critical for the proangiogenic capacity of CD34+ PBMCs. In addition, we observed that reduced miR-126 expression is a novel mechanism leading to an impaired proangiogenic capacity in high-glucose–treated or diabetic patient-derived CD34+ PBMCs, which could be reversed by miR-mimic-126 treatment.
Previous studies have suggested that at least 2 morphologically and functionally distinct “endothelial progenitor cell” populations can be derived after culture from circulating mononuclear cells according to duration of culture and the baseline CD14 phenotypic marker expression.2,28 We have observed that the CD34+ fraction of cells showed a more potent proangiogenic effect in vitro and in vivo; however, we did not observe a significant difference between freshly isolated CD34+CD14+ and CD34+CD14− MNC-derived cell populations with respect to their proangiogenic capacity. Previous studies have demonstrated that monocytes can stimulate angiogenesis.2,4,28,29 With the results of the present study one may speculate that the CD34+CD14+ MNC cell fraction may in particular contribute to such proangiogenic effects. We have observed that the CD34+CD14+ MNC fraction resulted in a significant promotion of tube formation, very similar to that observed in the CD34+CD14− MNC fraction.
Notably, the present study, for the first time, demonstrates different angiomiR expression profiles in the PBMC subpopulations separated for their CD34+ and CD14+ surface expression. MiR-126 was substantially increased in both the CD34+CD14+ and CD34+CD14− PBMC populations and was critical for the increased proangiogenic capacity of the CD34+ PBMC population. Previously, miR-126 has been suggested to play a critical role in vascular development.30,31 Moreover, after myocardial infarction miR-126 knockout mice showed impaired vascular growth in the infarct border zone.30,31 A reduced expression of potentially antiangiogenic miRs in CD34+ cells compared with CD34− cells, such as miR-15a or miR-20a levels observed in the present study, may also support an increased proangiogenic capacity of CD34+ cells. However, given the observation that anti–miR-126–treated CD34+ cells had lost the capacity to stimulate angiogenesis, these differentially expressed miRs are likely not sufficient to explain the functional difference observed between CD34+ and CD34− MNC cell subsets.
Moreover, in the present study, we have observed that the proangiogenic miR-126 is enriched in microvesicles and exosomes secreted by CD34+ progenitor cells and that these vesicles are taken-up by endothelial cells. The increased miR-126 levels in vesicles correlated with an increased tube formation capacity. The presence of growth factors or other proangiogenic factors inside the vesicles,20 together with miR-126, likely mediates proangiogenic effects associated with these vesicles. The molecular content transported by CD34+ exosomes still remains to be fully characterized. Notably, exosomes with reduced miR-126 content did not stimulate the tube formation of endothelial cells, raising the possibility that miR-126 is a paracrine factor stimulating endothelial cell angiogenic activity. Zernecke et al have recently suggested that miR-126 is released from endothelial apoptotic microparticles and acts in a paracrine way.22 These microparticles were incorporated by surrounding endothelial cells, and miR-126 transfer has been proposed to increase production of CXCL12 in endothelial cells, promoting homing of progenitor cells after vascular injury.
In the present study, we have observed that protein levels of phosphoinositol-3 kinase regulatory subunit 2 (PIK3R2), a known target of miR-126, are reduced in endothelial cells on administration of miR-mimic-126, suggesting that the microRNA uptake regulates target expression in endothelial cells. PIK3R2 has been shown to inhibit proangiogenic signaling in endothelial cells by suppression of growth factor signaling via PI3K pathways.30,31
Diabetes has been observed to lead to an impaired function of early endothelial progenitor cells.8,9,12 In the present study, we suggest a novel link between loss of proangiogenic stimulation and reduced miR-126 levels in CD34+ cells in high-glucose–treated cells. Plasma microRNA profiling in patients with type 2 diabetes has recently revealed reduced plasma levels of miR-126 in diabetic patients.32 The present study newly demonstrates a reduced miR-126 release from CD34+ PBMCs after high-glucose exposure and in diabetic patients that could contribute to reduced circulating miR-126 levels.
The present observations do not exclude that other microRNAs beyond miR-126 may also regulate the angiogenic capacity of CD34+ cells as well; this needs to be further explored in future studies. For microRNA-126, we have shown that it is clearly promoting the proangiogenic capacity of CD34+ cells. Others have reported that miR-126-knockouts had an impaired angiogenic response in the heart in the setting of myocardial ischemia and promote endothelial cell tube formation.30 At the same time, miR-126 has very recently been reported to suppress vascular growth in breast cancer metastasis by suppressing endothelial cell recruitment to metastatic cells via repression of other targets expressed in metastatic breast cancer cells,33 suggesting that miR-126 does not promote tumor-associated angiogenesis. The effects of microRNA on angiogenesis may therefore substantially differ in diverse cell types and pathophysiologic settings.
Two other microRNAs that were found to be higher expressed in CD34+ cells compared with CD34− mononuclear cells in the present study have very recently been suggested to be involved in the regulation of angiogenic processes in different endothelial cell types. We observed miR-100 expression to be higher in CD34+ compared with CD34− mononuclear cells. MiR-100 expression in endothelial cells has been suggested to inhibit proliferation, tube formation, and sprouting activity and to act as an endogenous repressor of the serine/threonine protein kinase mammalian target of rapamycin.34 This raises the possibility that this microRNA would rather limit the proangiogenic capacity of CD34+ mononuclear cells that needs, however, to be evaluated in future studies. MiR-10b was higher expressed in CD34+ compared with CD34− mononuclear cells in the present study. MiR-10b has recently been suggested to regulate angiogenesis in human microvascular endothelial cells, where miR-10b overexpression promoted endothelial cell migration and tube formation.35 These findings raise the possibility that this microRNA may also regulate proangiogenic effects in CD34+ mononuclear cells; this needs to be further evaluated in future studies. However, miR-10b was substantially higher expressed in CD34+CD14+ compared with CD34+CD14− mononuclear cells that had a rather similar proangiogenic capacity in the present study, which would suggest that miR-10b is not a major regulator of proangiogenic effects of CD34+ mononuclear cells. Conversely, it is also possible that higher expression of potentially antiangiogenic microRNA in CD34− mononuclear cells, such as from the miR-17-92 cluster or miR-21 as observed in the present study, may limit the proangiogenic capacity of CD34− mononuclear cells.
In summary, our present findings demonstrate a differential angiomiR expression in CD34+ and CD34− PBMC subpopulations. Increased angiomiR miR-126 expression in CD34+ PBMCs compared with CD34− PBMCs was identified as a novel mechanism contributing to the increased capacity of these cells to promote angiogenic processes. Moreover, down-regulation of miR-126 in CD34+ PBMCs after high-glucose exposure or in diabetic patients represents a novel mechanism leading to an altered proangiogenic capacity in these conditions that can be rescued by miR-mimic-126 transfer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Zurich Center for Integrative Human Physiology (ZIHP; University of Zurich, Zurich, Switzerland), Swiss National Foundation grant 124112, the Swiss Heart Foundation, the Uniscientitia Foundation, and the European Foundation for the Study of Diabetes/Sanofi-aventis Micro- and Macrovascular Programme. This work was supported by the Clinical Research Focus Program of the University of Zurich.
Authorship
Contribution: P.M. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; S.B. performed the experiments and statistical analysis, analyzed and interpreted data, and revised the manuscript; G.G. performed the experiments and revised the manuscript; C.D., P.J., and F.P. performed the animal experiments and revised the manuscript; T.L. contributed to the planning of experiments and revised the manuscript; and U.L. conceived and designed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ulf Landmesser, MD, Cardiovascular Center, University Hospital Zurich, Raemistr 100 (C-Hof 111), 8091 Zürich, Switzerland; e-mail: ulf.landmesser@usz.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal