Abstract
The mechanisms underlying Plasmodium falciparum resistance in persons with sickle trait have been under active investigation for more than a half century. This Perspective reviews progress in solving this challenging problem, including recent studies that have exploited the genomics and proteomics of the parasite. The formation of Hb S polymer in the parasitized AS RBC leads to impaired parasite growth and development along with enhanced clearance from the circulation and reduced deposition in deep postcapillary vascular beds. Enhanced generation of reactive oxygen species in sickled AS RBCs is a pathogenetic feature shared by parasitized thalassemic and G6PD-deficient RBCs, triggering abnormal topology of the RBC plasma membrane with decreased and disordered display of PfEMP-1, a P falciparum adhesion protein critical for endothelial adherence. A mouse model of Hb S confers host tolerance to P berghei, through inhibition of pathogenic CD8+ T cells and induction of heme oxygenase-1. An additional and apparently independent mode of protection is provided by the selective expression in AS RBCs of 2 species of microRNA that integrate into P falciparum mRNAs and inhibit translation and parasite growth.
Introduction
Mankind's most commonly encountered genetic disorders lie snugly within the confines of the RBC. Hemoglobin mutants S, C, and E, as well as α- and β-thalassemias, glucose-6-phosphate dehydrogenase (G6PD) deficiency, and Southeast Asian ovalocytosis were initially identified because of their association with abnormal RBC morphology and/or anemia along with less common life-threatening clinical phenotypes. These genes have all arisen in areas in which falciparum malaria is endemic, and their rise to high levels of prevalence is thought to result from their conferring significant degrees of protection against this dreaded pathogen. (A meta-analysis of the aforementioned hemoglobinopathies has failed to confirm that Hb E and β-thalassemia provide significant protection against Plasmodium falciparum.12 ) Thus, malaria has imposed extreme selective pressure on the human genome, far more than any other infectious disease, and the RBC has been the prime target for evolutionary adaptation. These mutations are prime examples of Haldane's1,2 notion of “balanced polymorphism,” in which genes are fixed at a high frequency in susceptible populations because the enhanced fitness enjoyed by heterozygotes more than outweighs the morbidity and mortality suffered by homozygotes or compound heterozygotes.
The βS globin mutation has arisen at least 4 times in Africa and once in Arabia or India. Supportive epidemiologic evidence for the protection of Hb S heterozygotes was initially provided by Allison.3,4 Allison5 and Smith6 estimated that, under conditions of selection for fitness against malaria, ∼ 45 generations (or 1000 years) were required for the frequency of the sickle gene to reach a stable equilibrium. It is probable that selection for sickle trait and other RBC defects occurred 3000-5000 years ago, with the emergence of agriculture, which converted large tracts of tropical rain forest into breeding grounds for Anopheles gambiae.7
The enhanced resistance of persons with sickle trait to falciparum malaria is substantial. Infected AS children have lower parasite densities than AA children and are 50%-90% less likely to progress to a severe form of malaria or to die from the disease.8-11 The degree of enhanced fitness varies considerably with geographic locale.8,13 A recent meta-analysis encompassing 62 studies confirmed the robust protection from AS hemoglobin from severe disease but found little or no protection against uncomplicated malaria or asymptomatic parasitemia.12 This result is fully in keeping with a recent genome-wide association study involving 1325 cases of severe anemia or cerebral malaria in Ghana, compared with 828 unaffected controls, that revealed the highest protection scores with single nucleotide polymorphisms at the β-globin locus on chromosome 11.14
This brief overview focuses on the mechanisms by which persons with sickle trait have enhanced resistance to falciparum malaria. This topic is particularly timely in view of advances in genomics and proteomics that have greatly enhanced the scope and depth of malaria research. Moreover, 3 important papers published recently in high profile journals present new insights into this process.15-17
Earlier studies
The presence of βS globin in AS persons might confer resistance against malaria by interfering with a number of steps in the complex interaction between parasite and host RBCs. During the last half century, a number of plausible mechanisms have been proposed. They are not mutually exclusive.
Sickling of circulating infected RBCs
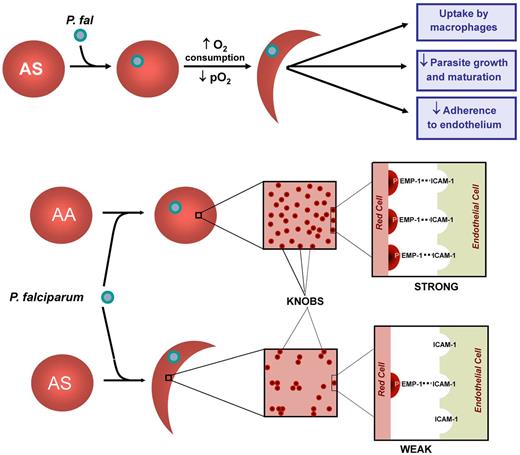
Luzzatto et al in Nigeria18 incubated RBCs of AS children infected with P falciparum and found that the formation of sickle shapes under low oxygen tension occurred much more readily in cells that contained parasites than in those that were uninfected. Subsequently, Roth et al19 showed that the enhanced sickling was restricted to AS RBCs containing small plasmodium (ring) forms. Extensive sickle polymer was also observed in RBCs containing trophozoites and schizonts, but these large inclusions probably precluded formation of sickled shapes. The enhanced Hb S polymerization in parasitized AS RBCs is likely due to the increased oxygen consumption that accompanies the robust metabolic activity of the intracellular parasite. However, parasitization could also enhance polymer formation by lowering intracellular pH or by increasing intracellular hemoglobin concentration. These simple but elegant experiments suggested that rapid clearance and destruction of circulating parasitized RBCs protect AS persons from severe infestation (Figure 1).
Impaired parasite growth and oxidant damage
The development of a continuous culture system for human RBCs infected with P falciparum20 allowed more prolonged in vitro studies of the impact of sickle trait.21-23 Under normoxic conditions, the invasion, growth, and multiplication of P falciparum in AS cells were the same as that of AA RBCs. In contrast, under hypoxic exposure, there was a reduction in fraction of parasites in AS cells along with a block in the maturation of ring forms to trophozoites and schizonts (Figure 1). This result could be the result of the formation of Hb S polymers, even in the absence of morphologic sickling. Parasites appear to be fragile and may be disrupted and killed by polymers.24 Alternatively, the impairment in parasite growth could be the result of polymer-induced RBC dehydration.25-27 Even more compelling is the notion that enhanced oxidative damage to RBC membrane threatens parasite viability. Infection of normal AA RBCs with P falciparum generates reactive oxygen species (ROS) that elevate biochemical markers of oxidant damage to the RBC membrane.28 RBCs containing Hb S are likely to be even more vulnerable. The polymerization of Hb S leads to deposition of hemichrome29 and iron30 in the membrane of SS RBCs, making them highly sensitive to oxidant damage. It is plausible that similar changes occur in parasitized AS RBCs. This added oxidative stress may cause considerable impairment of parasite growth and development and thus contribute to the protection conferred by sickle trait against P falciparum infection.
Mechanisms underlying protection by AS RBS against falciparum malaria. Parasitization of AS RBCs causes increased oxygen consumption, a decrease in pO2, and sickle hemoglobin polymerization. The membranes of these cells are further modified by oxidant stress, resulting in uptake by macrophages, impaired parasite growth and development, and decreased adherence to endothelium. Parisitization of AS RBCs leads to a decrease in the display of knobs of the cell surface along with uneven distribution. It is likely, though unproven, that hypoxia-induced sickling would aggravate this abnormal topology and further weaken interactions between the parasite protein PfEMP-1 and cognate receptors on endothelial cells, such as ICAM-1 in the brain.
Mechanisms underlying protection by AS RBS against falciparum malaria. Parasitization of AS RBCs causes increased oxygen consumption, a decrease in pO2, and sickle hemoglobin polymerization. The membranes of these cells are further modified by oxidant stress, resulting in uptake by macrophages, impaired parasite growth and development, and decreased adherence to endothelium. Parisitization of AS RBCs leads to a decrease in the display of knobs of the cell surface along with uneven distribution. It is likely, though unproven, that hypoxia-induced sickling would aggravate this abnormal topology and further weaken interactions between the parasite protein PfEMP-1 and cognate receptors on endothelial cells, such as ICAM-1 in the brain.
Ayi et al reported that AS (as well as β-thalassemia and G6PD-deficient) RBCs containing ring forms (but not trophozoites) were more prone to uptake by macrophages than normal or α-thalassemia RBCs31 (Figure 1). This enhanced phagocytosis appears to be triggered by a combination of hemichrome formation, aggregates of band 3, and deposition of autologous IgG immunoglobulin and complement (C3) on RBCs.32
There is experimental evidence that oxidant damage is a common mechanism shared by AS RBCs, β- and α-thalassemias as well as G6PD deficiency in mediating resistance to malaria.33,34 It is likely that when these RBCs are parasitized they are subjected to additional oxidative stress because of enhanced generation of ROS by both the parasite and the mutant RBC. However, it is uncertain whether the enhanced oxidant stress of these mutant RBCs impacts more on impairing parasite growth or enhancing uptake by phagocytes.
Recent advances
Impaired endothelial adhesion
A major and clinically dominant feature of falciparum malaria is the sequestration of ring-containing RBCs by endothelial adherence deep within postcapillary beds. Low oxygen tension in these sites is likely to induce sickle polymer formation in AS RBCs, thereby stunting parasite growth and development, similar to what has been observed in the in vitro culture experiments cited in “Impaired parasite growth.”
Impressive advances in the genomics and proteomics of P falciparum have enabled a better understanding of the mechanism underlying vascular cytoadherence. Among the > 5300 genes within the P falciparum genome, more than half encode proteins of unknown function.35-38 Many have no homology to mammalian proteins. During its 48-hour intra-erythrocytic life cycle, the parasite exports a large number of these proteins into host RBCs. Considerable progress has been made recently in identifying specific interactions between a number of novel parasite proteins with host proteins either in the erythrocyte cytoskeleton or on the plasma membrane. These interactions lead to dramatic changes in RBC morphology, deformability, and, importantly, endothelial cell adherence.
One of these proteins, P falciparum erythrocyte membrane protein-1 (PfEMP-1) is of particular interest.39,40 It is expressed on knob-like protrusions on the surface of parasitized RBCs41 and serves as the parasite's primary ligand for mediating endothelial adherence, binding primarily to CD36, the main cytoadherence receptor on the surface of host endothelial cells and monocytes. In the brain, PfEMP-1 binds to ICAM-1. PfEMP-1 also binds to complement receptor 1 on the surface of noninfected RBCs, leading to the formation of rosettes that impair cerebral blood flow. Cholera et al42 found that AS RBCs parasitized with P falciparum trophozoites bound to human microvascular endothelial cells and blood monocytes about half as effectively as did comparably infected AA RBCs. Moreover, AS RBCs had slightly reduced surface expression of PfEMP-1 along with more uneven distribution of expression on their surface (Figure 1). Similar results have been obtained with both AC43 and α-thalassemia44 RBCs. Thus, these benign and highly prevalent hemoglobin polymorphisms alter the RBC topography of the knob projections that are essential for adherence in postcapillary venules, perhaps by the oxidant-dependent perturbations mentioned in “Impaired parasite growth.” Impaired cytoadherence may explain why AS children are less likely to have severe disease than AA children despite comparable parasite density.
Recently, Cyrklaff et al have used electron tomography to study the dramatic structural perturbations that occur during the development of P falciparum in human RBCs.16 At the trophozoite stage, 20-26 hours after invasion, the parasite develops a trafficking system to direct the export of some of its endogenous proteins, including PfEMP-1 to the plasma membrane of the host RBC. In normal uninfected RBCs, a junctional complex of 14-16 actin monomers help to stabilize spectrin tetramers within the cytoskeleton. However, after invasion, host actin protofilaments are hijacked to form elongated filaments that tether a parasite compartment known as Mauer clefts to the knob-like protrusions of the plasma membrane that, as mentioned in the paragraph above, are essential for adherence to the postcapillary endothelium. Stunning high-resolution tomograms of Cyrklaff et al16 show that this conduit is disrupted in parasitized SS and SC RBCs and thus provides an elegant molecular explanation for the decrease in the expression of PfEMP-1 and its abnormal distribution previously observed in parasitized AS42 and AC43 RBCs. It is a puzzle why Cyrklaff et al chose to study SC and CC RBCs rather than epidemiologically relevant and more accessible AS and AC RBCs.16 SC and CC cells share a number of abnormal properties that impact on membrane structure and function and thus could confound experimental interpretation. These abnormalities include enhanced potassium efflux, a marked elevation of intracellular hemoglobin concentration, and intracellular formation of hemoglobin aggregates. These features are much less pronounced in AS and AC RBCs.
Animal models and immune tolerance
There is a pressing need for the development of small animal models of falciparum malaria to better understand the pathophysiology of the disease as well as to test novel drugs and vaccines. The major obstacle has been the limited host range of different malaria species. Rodent studies have been largely restricted to P berghei, P yeolii, and P chabaudi, pathogens that have limited relevance to human disease. It is possible to study P falciparum infection in mice, but it requires intravenous infusion of parasitized human RBCs into strains with ablated adaptive immunity (SCID, Nude, NOD/SKID, NSG) combined with measures to suppress innate immunity, such as administration of clodronate-loaded liposomes.45
Despite these concerns, Ferreira et al15 have recently made clever use of a mouse model to study the mechanism by which sickle hemoglobin confers resistance against severe forms of malaria. They used the SAD transgenic mouse model of sickle cell disease46 and compared it with normal mice of the same strain infected with P berghei. The SAD transgene expresses human β-globin that contains not only the β6 valine sickle mutation but also 2 other mutations that are known to enhance Hb S polymerization. As mentioned in the paragraph above, P berghei infection in mice is of limited relevance to human infection with P falciparum. Importantly, however, cerebral malaria is a cardinal disease manifestation in both. All wild-type C57BL/6 mice died 10 days after inoculation with P berghei, whereas 75% of SAD mice of the same strain survived. Moreover, all of the wild-type animals developed severe pathologic hallmarks of cerebral malaria, whereas the SAD mice had minimal involvement. The fact that the level of parasitemia was the same in both groups led the authors to conclude that protection conferred by sickle hemoglobin entailed enhanced tolerance to the pathogen, mediated in part by inhibition of immune attack by CD8+ T cells. In contrast, Shear et al had previously inoculated transgenic mice with P berghei and P chabaudi and reported that those expressing Hb S had markedly delayed and diminished increases in parasitemia.47
In addition, the protection in the SAD mice appeared to be dependent on induction of heme oxygenase at sites of uptake of parasitized cells, resulting in rapid and efficient degradation of heme. SAD mice that also harbored a deletion in one heme oxygenase-1 allele (SAD/Hmox1+/−) had decreased survival and cerebral malaria comparable with the wild-type mice. Ferreira et al proposed that the release of carbon monoxide during heme catabolism protects hemoglobin from further oxidation and release of heme and its toxic metabolites.15 A key question is whether the up-regulation of heme oxygenase in the SAD mouse is relevant to the protection of humans with sickle trait against P falciparum. Because of the additional β-globin mutations, mouse RBCs containing low levels (19%-26%) of SAD hemoglobin undergo hemolysis and have much more sickling than human AS RBCs containing 30%-40% Hb S. It would be worthwhile repeating the experiments of Ferreira et al15 with a mouse model expressing only human globins48 (α, βA, and βS) and therefore a much more faithful mimic of sickle trait.
Role of host microRNA
Recently, Lamonte et al17 have presented a comprehensive and coherent body of evidence that microRNAs play a significant role in mediating the reduced growth rate of P falciparum in AS RBCs. At ∼ 1000 sites on the human genome, RNA transcripts are expressed that are then truncated by “dicer” ribonucleases into small RNA species with an average length of 22 nucleotides. These microRNA species fold into stable hairpin structures and are post-transcriptional regulators. They bind to complementary sequences on target mRNAs and either suppress translation or trigger degradation.
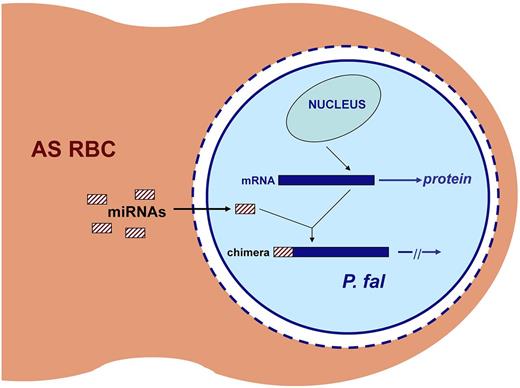
LaMonte et al17 found that, after infection of normal human RBCs with P falciparum, several microRNAs are enriched and translocate to the cytosol of the parasite. The levels of 2 of these (miR-451 and miR-223) were markedly elevated in noninfected SS RBCs and to a somewhat lesser extent in AS RBCs. Parasite growth rate in SS was markedly (∼ 80%) lower than that in AA RBCs, whereas in AS RBCs the growth rate was moderately lower (∼ 60%). This protection was significantly abrogated by miR-451 and miR-223 antisense oligonucleotides. Moreover, transfection of miR-451 and miR-223 into normal AA RBCs before P falciparum infection resulted in ∼ 50% reduction in parasite proliferation. Surprisingly, these miRNA species inhibited growth of P falciparum not by impacting mRNA translation or stability but by linear integration into key parasite mRNAs, thereby inhibiting their translation (Figure 2). This remarkable discovery introduces a novel mechanism by which the host genome can modify the virulence of a microbial pathogen.
Inhibition of translation of parasite mRNAs by microRNAs in AS RBCs. Both miR-451 and miR-223 are highly enriched in AS RBCs. After invasion by P falciparum, these miRNAs enter the parasite cytoplasm by penetrating the parasitophorous vacuolar membrane (dashed blue circle), the vacuole (white space), and the parasite's plasma membrane (solid blue circle). The miRNAs are then trans-spliced onto the 5′ ends of specific P falciparum mRNAs. Translation is blocked in these chimeric RNAs, leading to impaired parasite growth. Adapted from Duraisingh and Lodish49 with permission.
Inhibition of translation of parasite mRNAs by microRNAs in AS RBCs. Both miR-451 and miR-223 are highly enriched in AS RBCs. After invasion by P falciparum, these miRNAs enter the parasite cytoplasm by penetrating the parasitophorous vacuolar membrane (dashed blue circle), the vacuole (white space), and the parasite's plasma membrane (solid blue circle). The miRNAs are then trans-spliced onto the 5′ ends of specific P falciparum mRNAs. Translation is blocked in these chimeric RNAs, leading to impaired parasite growth. Adapted from Duraisingh and Lodish49 with permission.
In considering the role of these microRNAs in conferring protection against P falciparum, 2 deeply perplexing questions need to be addressed. What accounts for the differential expression of microRNAs in AS versus AA RBCs? Certain microRNAs (including miR-451) are expressed in erythroid progenitor and precursor cells before enucleation and are crucial for their orderly development.49 It is surprising that they persist in mature anucleated RBCs. Why are selected miRNAs strongly enriched in mature circulating AS RBCs? There is no evidence that erythropoiesis is perturbed in persons with sickle trait. Unlike patients with SS disease, refined laboratory parameters in AS persons indicate neither hemolysis nor ineffective erythropoiesis. Second, the proposed protection via specific species of microRNAs appears to involve a process completely independent of the other mechanisms outlined in this Perspective, all of which, one could plausibly argue, are predicated on polymer formation in parasitized, deoxygenated AS RBCs.
Conclusions
The enormous selective pressure imposed by falciparum malaria has engendered commonly encountered polymorphisms in genes encoding globin, an enzyme (G6PD) and a critical protein in the RBC cytoskeleton (band 3). These mutations cause no significant impairment in the fitness of heterozygotes but confer robust resistance to the disease. Thus, the mechanisms underlying this protection must be subtle.
The impact of generating ROS and possibly hemichromes appears to be a unifying theme for most, if not all, of these mutations. In the case of sickle trait, the path to enhanced ROS production is certainly subtle indeed. As mentioned 2 paragraphs above, AS persons have normal reticulocyte counts and normal RBC life span. Oxidation of the heme iron to methemoglobin is the proximal step in hemichrome formation. Purified Hb S has a slightly higher rate of auto-oxidation than that of Hb A.50 However, in AS RBCs, this small difference would not lead to methemoglobin accumulation because of the high activity of RBC cytochrome b5 reductase. Indeed, there is no convincing evidence of methemoglobinemia in AS or AC persons or indeed even in SS disease. Moreover, there is no structural rationale for why mutations at β6 should impinge on the β-chain heme environment to promote hemichrome formation in heterozygotes. In contrast, distortion of the SS RBC membrane, along with iron accumulation and superoxide formation from the low oxygen affinity of SS RBCs, could and does lead to hemichrome formation.30,51 This conundrum is addressed and perhaps resolved by the early in vitro studies demonstrating that under low oxygen tension parasitized AS RBCs readily sickle.18,19 Extensive polymer formation in these cells would be expected to distort the plasma membrane similar to what occurs in SS RBCs. It seems plausible that oxidant-dependent perturbations of the RBC membrane in AS RBCs as well as in thalassemic and G6PD-deficient RBCs could underlie several of the mechanisms proposed for protection against P falciparum: enhanced macrophage uptake, impaired growth and maturation of parasite, and decreased deposition of parasitized RBCs in deep postcapillary beds.
The impact of polymer formation in parasitized AS RBCs, as summarized in the previous paragraph, must be weighed against the startling and heuristic discovery of preferential expression of selected microRNAs in AS RBCs that integrate into parasite mRNAs and inhibit growth. One's imagination must be stretched to understand how a single mutation in the host genome can confer resistance to a microbial pathogen by such divergent mechanisms. Resolving the relative importance of these apparently independent mechanisms of parasite resistance will provide fresh insights into the complex interrelationship between the genome of the host and that of threatening microbial pathogens.
Authorship
Contribution: H.F.B. performed the relevant background literature review and wrote the article.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: H. Franklin Bunn, Medicine/Hematology Division, Brigham and Women's Hospital, Harvard Medical School, 75 Francis St, CHRB 5-215, Boston, MA 02115; e-mail: hfbunn@rics.bwh.harvard.edu.