To the editor:
X-linked severe combined immunodeficiency (SCID-Xl) is caused by defects in IL2RG, the gene encoding the IL-2 receptor γ chain. Accounting for 50% to 60% of cases of SCID,1 it SCID-XI is typically characterized by an absence of mature T and natural killer (NK) lymphocytes, whereas native B cells are detectable and are present in increased numbers. Viral infection caused by Epstein-Barr virus (EBV) in SCID patients can lead to fulminant and often fatal B-cell lymphoproliferative disease, similar to those occurring in immunosuppressed organ-transplant recipients.2-4
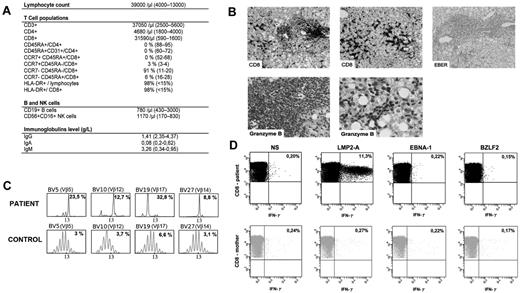
A 3-month-old boy, born to nonconsanguineous parents, was referred to our center for investigation of a rapidly progressive hepato-splenomegaly without peripheral lymphadenopathy. Chest x-rays revealed an absence of thymic shadow. Liver and spleen were found homogeneously enlarged by ultrasound examination. Whole blood count showed a marked lymphocytosis (up to 100 × 109/L) that consisted of CD3+CD8+TCRαβ HLA-DR+ activated cells with a complete absence of CCR7+CD45RA+CD8+ and CD4+CD45RA+CD31+ naive T cells (Figure 1A). The T-cell repertoire, as evaluated by immunoscope,5 showed an increase in Vβ5, Vβ12, Vβ14, and Vβ17 TCR usage among CD8+ cells (Figure 1C). Those features led us to investigate for the existence of a SCID. The maternal origin of the circulating T and NK cells was confirmed by FISH analysis of the CD3+ and CD56+ cell fraction, respectively, which were obtained by cell sorting. There was no engraftment of maternal stem cells, as verified by FISH analysis of the polymorphonuclear neutrophils. SCID-Xl was confirmed by gene sequencing of IL2RG on the patient's genomic DNA that revealed a previously described R226C mutation. The mother carried the mutation.
Immunological features of the patient. (A) Lymphocytes subpopulations. Serum immunoglobulin levels. (B) Liver histopathology. Immunohistochemistry was performed on fixed tissues with a peroxidase-based method (Dako). Antibodies used were raised against CD20, CD3, CD8, CD4 and granzyme B (Dako). EBV-encoded RNA (EBER) was probed on some specimen with the use of in situ hybridization technique. Slides were observed using a Leica DM LB microscope with ×20, ×40, and ×100 objectives and a 10× eyepiece. Aquisition of images was with IM50 software (Leica Microsystems). First line: CD8+ lymphocytic infiltrates in lobular (left) and portal (middle) area. Negative EBER staining (right). Second line: positive granzyme B staining in lobular and portal area (left and right panels, respectively). These infiltration could result of the trapping of the activated CD8+ T cells in liver during the immune response.10 (C) Immunoscope quantitative T-cell repertoire analysis. Most significant specific T-cell clonal expansion is shown. The x-axis indicates CDR3 length (in amino acid), and the y-axis displays the fluorescence intensity of the run-off products (in arbitrary units). Percentages indicate the frequency of occurrence for each Vβ family. (D) CD8+ maternal engrafted T cells express IFN-γ in response to EBV latent antigen LMP-2A antigen. Freshly isolated mononuclear cells of the patient and his mother were incubated without stimulation (NS) or in the presence of latent antigen LMP-2A, latent antigen EBNA-1, and lytic antigen BZLF-2, then stained for the expression of IFN-γ, CD3 and CD8. Numbers are the percentage of cells in the lymphoid gate expressing the indicated surface markers.
Immunological features of the patient. (A) Lymphocytes subpopulations. Serum immunoglobulin levels. (B) Liver histopathology. Immunohistochemistry was performed on fixed tissues with a peroxidase-based method (Dako). Antibodies used were raised against CD20, CD3, CD8, CD4 and granzyme B (Dako). EBV-encoded RNA (EBER) was probed on some specimen with the use of in situ hybridization technique. Slides were observed using a Leica DM LB microscope with ×20, ×40, and ×100 objectives and a 10× eyepiece. Aquisition of images was with IM50 software (Leica Microsystems). First line: CD8+ lymphocytic infiltrates in lobular (left) and portal (middle) area. Negative EBER staining (right). Second line: positive granzyme B staining in lobular and portal area (left and right panels, respectively). These infiltration could result of the trapping of the activated CD8+ T cells in liver during the immune response.10 (C) Immunoscope quantitative T-cell repertoire analysis. Most significant specific T-cell clonal expansion is shown. The x-axis indicates CDR3 length (in amino acid), and the y-axis displays the fluorescence intensity of the run-off products (in arbitrary units). Percentages indicate the frequency of occurrence for each Vβ family. (D) CD8+ maternal engrafted T cells express IFN-γ in response to EBV latent antigen LMP-2A antigen. Freshly isolated mononuclear cells of the patient and his mother were incubated without stimulation (NS) or in the presence of latent antigen LMP-2A, latent antigen EBNA-1, and lytic antigen BZLF-2, then stained for the expression of IFN-γ, CD3 and CD8. Numbers are the percentage of cells in the lymphoid gate expressing the indicated surface markers.
An EBV infection was diagnosed by amplification of the viral DNA in blood samples by polymerase chain reaction with a whole blood viral load of 6 log10 DNA copies/mL. The child's mother displayed a serologic profile of past EBV infection (ie, IgG anti-VCA and IgG anti-EBNA positive). We investigated the possible role of this ongoing viral infection as a trigger for the extreme lymphocytosis, the latter being reminiscent of the one observed during infectious mononucleosis.6-8 Interestingly, in vitro stimulation of lymphocytes with LMP2-A, but neither with BZLF-2 nor EBNA-1, induced a significant activation of CD8+ T cells as shown by detection of intracytoplasmic interferon γ (IFN-γ) by flow cytometry. The same test, performed on the mother's circulating T cells, did not detect LMP2-A specific in vivo activated T cells, a result that is not surprising in the absence of active EBV infection (Figure 1D). This result indicates a major expansion of LMP2-A specific maternal T cells in the patient's blood secondary to EBV infection.
The EBV infection was treated by rituximab infusions until the EBV viral load became undetectable by PCR. The hepato-splenomegaly gradually regressed secondary to this therapy associated with a short course of steroids. A liver biopsy, performed 4 weeks after initiation of therapy, showed an infiltration of the portal and lobular area by T lymphocytes that were mostly CD8+ with a granzyme B–positive staining (Figure 1B). The Epstein-Barr virus–encoded small RNA (EBER) staining was negative.
Transplacental-acquired maternal T cells have already been reported to cause allograft rejection and immune cytopenias.9 To the best of our knowledge, this is the first report of “natural” adoptive immunity toward EBV with a massive in vivo expansion of maternal engrafted T cell conferring specific immunity against this virus that may account for the patient's survival and relative control of EBV-driven B-cell proliferation.
Authorship
Acknowledgments: This work was supported by grants from Inserm and European Research Council (ERC).
Contribution: F.T. designed the research, collected the data, and wrote the manuscript; L.D.-C. performed experiments and critically read the manuscript; V.V., A.L., and S.K. performed experiments; A.C.-A. collected the data and participated in the clinical care of the patients; D.M. participated in the clinical care of the patient, critically read the manuscript, and participated in writing the manuscript; P.F., S.H., and S.B. participated in the clinical care of the patient; C.P. performed genetic and biologic diagnosis of the patient and critically read the manuscript; M.C.-C. and S.H.-B.-A. critically read the manuscript; and A.F. designed the research, critically read the manuscript, and participated in writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fabien Touzot, Unité d'Immunologie et d'Hématologie Pédiatriques, Hôpital Necker-Enfants Malades, Paris, 75015 France; e-mail: fabien.touzot@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal