To the editor:
Recent results from the STop IMatinib (STIM) trial suggest that imatinib may be safely discontinued in some chronic myeloid leukemia (CML) patients with long-lasting complete molecular response (CMR).1 However, 60% of patients experienced molecular recurrence (MR; detection of BCR-ABL1 transcripts confirmed by a second analysis) and responded to imatinib reintroduction.
We explored the feasibility of a second discontinuation in (1) CML patients currently treated by imatinib for at least 3 years who had been in sustained CMR for at least 2 years with (2) MR after first attempt of imatinib discontinuation and (3) in second CMR for at least 1 year after imatinib reintroduction. The molecular follow-up was assessed as previously reported.1
Sixteen patients were included. Sex ratio (male/female) was 5/11, and the median age was 62 years (range: 45-80 years). At diagnosis, 15 patients were in chronic phase (CP) and 1 patient was in accelerated phase (AP), and Sokal scores were low in 10 patients, intermediate in 3 patients, and high in 2 patients. Ten of the 16 patients received treatment before imatinib initiation. Imatinib was initiated at 400 mg per day in CP-CML patients, and at 600 mg per day in the AP-CML patient with a median time from diagnosis to imatinib initiation of 8 months (range: 1-73 months). The median interval from imatinib initiation to the first CMR was 14 months (range: 5-56 months). Imatinib was then administered during a median duration of 54 months (range: 32-105 months), and the median duration of CMR was 31 months (range: 19-78 months). After the first attempt of imatinib discontinuation, all patients were in MR within a median of 2.5 months (range: 1-8 months) and they obtained a second CMR after imatinib reintroduction within a median of 6 months (range: 1-19 months).
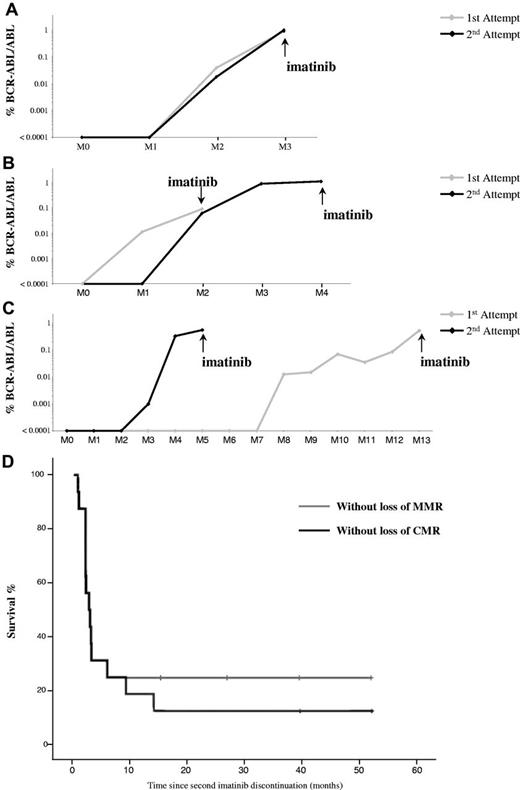
After the second imatinib discontinuation, we observed 2 different molecular patterns. The first group of patients (12/16, 75%) experienced rapid MR after imatinib was discontinuated on the second occasion. They lost their major molecular response (MMR) at a median of 2.1 month (range: 0.7-5.9 months) and were re-treated with a tyrosine kinase inhibitor (TKI; imatinib n = 11; dasatinib n = 1). In this group of patients the median time to the first positive molecular biology test, the median time to TKI reintroduction, and the median of time to the second CMR after TKI reintroduction were all similar in the 2 instances of imatinib discontinuation, but kinetics of molecular recurrence progressed in several ways. Indeed, among the 11/12 patients with available data, the kinetics of the second molecular recurrence were similar to those of the first recurrence for 1 patient (Figure 1A), was slower than the first recurrence in 5 patients (Figure 1B), and faster in 5 patients (Figure 1C), reflecting heterogeneity of recurrence kinetics.
Molecular course after imatinib discontinuation. Comparison of molecular relapse kinetics between the 2 instances of imatinib discontinuation in patients with treatment reintroduction. (A) Example of similar molecular relapse kinetics. (B) Example of second molecular relapse kinetics slower than the first. (C) Example of second molecular relapse kinetics faster than the first. (D) Kaplan-Meier estimates MMR (gray curve) and CMR (black curve) after a second discontinuation of imatinib in patients with chronic myeloid leukemia.
Molecular course after imatinib discontinuation. Comparison of molecular relapse kinetics between the 2 instances of imatinib discontinuation in patients with treatment reintroduction. (A) Example of similar molecular relapse kinetics. (B) Example of second molecular relapse kinetics slower than the first. (C) Example of second molecular relapse kinetics faster than the first. (D) Kaplan-Meier estimates MMR (gray curve) and CMR (black curve) after a second discontinuation of imatinib in patients with chronic myeloid leukemia.
The second group of patients (4/16, 25%) never lost their MMR and remained free of treatment with a median follow-up of 32 months (range: 15-53 months; Figure 1D). However, 2 of these 4 had a MR after a median of 11.6 months after discontinuation (range: 9.1-14.0 months), but remained treatment-free with a follow-up of 15 and 25 months. The other 2 patients had a prolonged CMR after the second imatinib discontinuation with a follow-up of 40 and 53 months. Therefore, according to the STIM criteria, the probability of remaining in CMR after the second imatinib discontinuation was 12.5% (Figure 1D). Interestingly, in the 2 patients in this group who experienced MR this occurred later compared with those of the first group who were re-treated (median: 11.6 months [range: 9.1-14.0 months] vs 2.1 months [range: 0.7-5.9 months]).
In conclusion, our pilot study demonstrated that it seems possible to discontinue TKIs a second time in selected patients.
Authorship
Acknowledgments: This study was funded by the French Ministry of Health (Programme Hospitalier de Recherche 2006), and the National Cancer Institute (Institut National du Cancer, INCA).
Contribution: L.L. and F.-X.M. collaborated in the conception and the design of the study, performed data analysis, and wrote the paper; P.R. also participated to the design of the study, and reviewed and approved the final version of the report; and S.G., M.T., F.H., and F.-E.N. followed the patients, provided some data, critically reviewed the manuscript, and approved it in its final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurence Legros, Service d'Hématologie Clinique, Hôpital Archet 1, 151, Route de Saint Antoine de Ginestière, BP 3079, 06202 Nice Cedex 2, France; e-mail: legros@unice.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal