Abstract
Megakaryocytes (MKs), the platelet precursors, are capable of accumulating DNA greater than a diploid content as part of their cell cycle. MKs have been recognized as mediating fibrosis in a subset of hematologic malignancies, including acute megakaryoblastic leukemia and a subset of myeloproliferative neoplasms. The mechanisms responsible for fibrosis remain only partially understood. Past studies highlighted the role of growth factors in such pathologies, and recently, the protein lysyl oxidase (LOX) has been implicated in proliferation of MKs, ploidy and deposition of fibers. LOX was initially characterized as a protein responsible for the intermolecular cross-linking of elastin and collagen, and in recent years it has been identified as regulator of various pathologies, such as cancer and inflammation. Here, we review recent advances in the understanding of the contribution of MKs to the progression of myelofibrosis, highlighting the newly identified role of LOX.
The MK and leukemia
Megakaryocytes (MKs) share a common progenitor with red blood cells and are responsible for the production of platelets. MKs are unique among blood cells in their ability to attain states of high ploidy (up to 256 N) by endomitosis, a process that involves multiple cycles of aborted late anaphase and cytokinesis and re-entrance into G1 phase of the cell cycle.1 Thrombopoietin (TPO) is the ligand for the Mpl receptor and the key growth factor of megakaryopoiesis. TPO interaction with its receptor activates several signaling pathways, including RAS/MAPK and JAK-STAT, which lead to MK endomitosis and maturation.2 The JAK2 protein is instrumental in relaying signaling from the TPO receptor to downstream pathways.
Proliferation of MKs is tightly controlled, and the rare acute megakaryoblastic leukemia (AMKL) is characterized by blasts often resembling lymphoid cells with a variable staining pattern. In children, 2 distinct AMKL types can be recognized, depending on whether AMKL occurs in the context of Down syndrome (DS) or not. Pediatric non–DS-AMKL is a heterogeneous disorder and includes cases with t(1;22)(p13;q13) chromosomal translocation that primarily occurs in infants.3 This translocation leads to the formation of the chimeric protein OTT-MAL composed of RNA binding motif protein 15 (RMBM15, also known as OTT) and the megakaryoblastic leukemia 1 gene (MKL1, also known as MAL). In contrast, virtually all cases of DS-AMKL mutations affect the GATA-1 gene, although other, not well-defined cooperative lesions are also required for the development of DS-AMKL.4 The transcription factor GATA-1 plays a key role in megakaryopoiesis and erythropoiesis and has a complex expression pattern.5 The reported mutations of GATA-1 in DS-AMKL result in a smaller GATA-1 protein (GATA-1s), with reduced transactivation ability because of loss of the amino terminal activation domain.6 Children with DS are not susceptible to the development of solid tumors but are uniquely predisposed to develop AMKL.7 In addition, approximately 10% of children with DS develop transient myeloproliferative disease. This is usually a self-limited condition marked by hyperproliferation of megakaryoblasts in blood and liver, but in 20% of cases it may progress to AMKL.8 Importantly, mutations in GATA-1 have also been reported in non–DS-AMKL, but only in very rare cases.9 In adult AMKL, the molecular lesions are more diverse and include chromosomal deletions and translocations.10 Mutations affecting the JAK3 protein have been found in a small subset of patients with AMKL regardless of DS context; none of the patients harbored mutations of JAK2 protein.11
An intriguing entity is the exceedingly rare familial infantile myelofibrosis disorder characterized by myelofibrosis, splenomegaly, and extramedullary hematopoiesis.12 The pathophysiology of familial infantile myelofibrosis remains largely unexplored.
The MK and myeloproliferative neoplasms
The term “myeloproliferative disorders” was introduced in 1951.13 Today, the scope of myeloproliferative disorders has been expanded and reclassified under the nomenclature of myeloproliferative neoplasms (MPNs). According to the World Health Organization (WHO) 2008 classification, MPNs encompass chronic myeloid leukemia, polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), mast cell disease, chronic eosinophilic leukemia (not otherwise categorized), chronic neutrophilic leukemia, and others unclassifiable.14 MKs are involved in the pathologies of ET, PV, and PMF, which share a common molecular defect,15 namely, a somatic mutation affecting JAK2, the JAK2V617F, which is detected in the overwhelming majority of PV patients and occurs also in approximately 60% of ET and PMF patients.15 The JAK2V617F mutation affects the pseudokinase domain of JAK2 and renders this kinase constitutively active, independently of ligand binding.15
Other less common molecular lesions involve JAK2 exon 12 and the MPLW515L/K mutations.16,17 Interestingly, the transcription factor NF-E2, which has a key role on the megakaryocytic lineage, was recently found overexpressed in patients with MPNs, independent of the presence of the JAK2V617F mutation.18 Intriguingly, a mutation affecting JAK2T875N (identified in a cell line originating from an infant with AMKL accompanying myelofibrosis) was shown in bone marrow (BM) transplantation assay to exhibit characteristics of both AMKL and MPN.19
It remains unclear how a single mutation can result in different MPNs, although methylation of microRNA,20 allele burden, and other synergistic mutations may be involved.21 PV and ET have insidious presentation and are usually chronic, indolent conditions but may progress to post-PV or post-ET myelofibrosis,22 respectively, or to secondary leukemia. Patients with ET have very high levels of platelets with a fairly normal hematocrit, and increased risk of thrombosis and, paradoxically, of bleeding too. In ET BM biopsies, MKs, although numerous, appear large and mature. By contrast, in PV the hematocrit and occasionally platelet levels are significantly elevated, and BM biopsies frequently reveal hyperplasia of all blood cell lineages. Diagnosis post-PV or post-ET myelofibrosis can be challenging, and criteria were proposed by the International Working Group for Myelofibrosis Research and Treatment22 to aid in its diagnosis. According to one study, 6% of patients affected by PV are at risk of progression to post-PV myelofibrosis within 15 years.23 The development of post-PV myelofibrosis is an ominous sign and patients die within a few years.24,25 Although ET is accompanied by some degree of reticulin fibrosis, few ET patients will develop myelofibrosis within 15 years. Of note, in a study the degree of reticulin fibrosis in ET patients was associated with higher complication rates.26 In addition, reticulin content was an independent risk factor for progression to transformation.27
By contrast, PMF (also known as idiopathic myelofibrosis or myelofibrosis with myeloid metaplasia) has an aggressive course with median survival of 5 years.28 Patients have anemia, extramedullary hematopoiesis, splenomegaly, and typically intense BM fibrosis with early mobilization of hematopoietic stem cells.29 Peripheral blood smear reveals giant degranulated platelets and dacryocytes, and the BM is infiltrated by numerous abnormal MKs with misfolded nuclei.
Several scoring systems have been proposed in an attempt to determine prognosis in patients affected by PMF. These include the International Prognostic Scoring System, Dynamic Prognostic Scoring System, and Dynamic Prognostic Scoring System plus.30 Common variables include age, anemia, leukocytosis, and presence of blasts or constitutional symptoms, whereas Dynamic Prognostic Scoring System plus includes also thrombocytopenia, certain karyotypes, and need for blood transfusions. Although the degree of fibrosis is not used in these scores, the grade of BM fibrosis is associated with overall survival, at least in intermediate- and high-risk patients.31 Treatment options for PMF are limited despite several clinical trials.32 Allogenic hematopoietic stem cell transplantation is currently the most promising therapy.28
BM fibrosis in the context of MK pathology
The term myelofibrosis is loosely used in the literature and may indicate BM deposition of reticulin, collagen, or both, regardless of the nosogenic background; severity can range from scattered to a heavily fibrotic marrow.33 Reticulin can be detected with silver-based stains (eg, Gomori stain) and collagen with trichrome stains (eg, Mallory or Masson trichrome stain). Grading schemes have been developed for reticulin deposition, and the most frequently used one is the Bauermeister scale.34 Few studies have evaluated reticulosis in healthy persons.33 Some degree of reticulosis was detected in a significant number of healthy persons.34 However, in a study involving 100 nonhematologic patients, neither grade 3 nor grade 4 reticulosis on the Bauermeister scale (diffuse fiber deposition and areas of collagen deposition respectively) was observed.35 Reticulin deposition is not currently used to determine prognosis in the context of MPN. On the other hand, collagen deposition is generally regarded as a more ominous finding. Ideally, both reticulin and collagen deposition should be evaluated; however, this is rarely done, and usually only reticulin is assessed in murine model studies.
In the context of AMKL and certain myeloproliferative disorders, BM fibrosis usually involves deposition of both reticulin and collagen fibers.33 A significant proportion of AMKL cases develop myelofibrosis that may be so extensive as to prevent BM aspiration. A study demonstrated that supernatant from AMKL megakaryoblasts and the megakaryoblastic cell line MEG-01 potently induced collagen production in fibroblasts and that TGF-β was a key mediator of this effect.36 In another report, a patient with chronic myelofibrosis progressed to transformation into acute micromegakaryocytic leukemia (blast ploidy ≥ 4N). Importantly, the levels of TGF-β and platelet-derived growth factor (PDGF) as well as the markers of collagen I and III synthesis were significantly up-regulated after transformation.37 A tentative hypothesis is that under pathologic conditions MKs may release TGF-β and other growth factors through a mechanism implicating emperipolesis and aberrant distribution of P-selectin, which can induce the production of collagen and reticulin by the fibrogenic cells.36,38,39 A recent study identified higher levels of the latent form of TGF-β1 on MKs of patients with myelofibrosis compared with controls.40
Mouse models of AMKL and myelofibrosis
Much of our knowledge regarding the role of MKs in myelofibrosis has been obtained through the use of mouse models. Currently, mouse models of AMKL, MPN, and BM fibrosis do not recapitulate all aspects of the disease.41 Characteristics of major models developed so far are reviewed here, and a summary is provided in Table 1.
Mouse models of AMKL and myelofibrosis
| Mouse model . | Experimental approach . | Comments . | Reference . |
|---|---|---|---|
| Myeloproliferative sarcoma virus | Infection of wild-type virus | BM and spleen fibrosis | 42 |
| TPO overexpression | Transgenic (APOE promoter) | Increased megakaryopoiesis but no overt myelofibrosis | 43 |
| TPO overexpression | Transgenic (IgH promoter) | Increased megakaryopoiesis accompanied by elevated TGF-β and myelofibrosis | 44 |
| TPO overexpression | Retroviral transduction and transplantation | BM and spleen fibrosis | 97 |
| GATA-1low | Deletion of gene regulatory elements | Aberrant megakaryopoiesis with gradual development of myelofibrosis | 57 |
| OTT-MAL translocation | Knock-in | Requires retroviral introduction of a MPLW515L to develop myelofibrosis | 55 |
| JAKT2875N mutation | Retroviral transduction and transplantation | Mixed characteristics of AMKL and MPN | 19 |
| JAK2V617F mutation | Transgenic | Depending on promoter, phenotype can resemble PV or ET; mice develop myelofibrosis | 49 |
| JAK2V617F mutation | Retroviral transduction and transplantation | Development of myelofibrosis was variable between mouse strains | 50 |
| JAK2V617F mutation | Retroviral transduction and transplantation | Myelofibrosis accompanied by osteosclerosis | 51 |
| JAK2V617F mutation | Knock-in | Splenomegaly with atypical MKs exhibiting emperipolesis; absence of fibrosis; resembles PV with evidence of thrombotic episodes | 47 |
| JAK2V617F mutation | Conditional knock-in | Similar features with JAK2V617F knock-in model but fibrosis is present; resembles PV | 48 |
| RBM6-CSF1R fusion protein | Retroviral transduction and transplantation | Variable phenotype; spleen reticulosis | 98 |
| MPLW515L mutation | Retroviral transduction and transplantation | BM reticulosis; rapidly lethal | 53 |
| MPLT487A mutation | Retroviral transduction and transplantation | Similar but less aggressive course to MPLW515L model | 54 |
| Ts65Dn mouse strain | Induced translocation resulting in segmental trisomy of distal chromosome 16 | Myelofibrosis after the first year of life | 58 |
| BACH1 | Transgenic | GATA-1 promoter drives expression; BACH1 mutation has not been reported in MPN | 99 |
| NF-E2 overexpression | Transgenic | Abnormally clustered MKs; increased deposition of collagen and reticulin | 100 |
| Mouse model . | Experimental approach . | Comments . | Reference . |
|---|---|---|---|
| Myeloproliferative sarcoma virus | Infection of wild-type virus | BM and spleen fibrosis | 42 |
| TPO overexpression | Transgenic (APOE promoter) | Increased megakaryopoiesis but no overt myelofibrosis | 43 |
| TPO overexpression | Transgenic (IgH promoter) | Increased megakaryopoiesis accompanied by elevated TGF-β and myelofibrosis | 44 |
| TPO overexpression | Retroviral transduction and transplantation | BM and spleen fibrosis | 97 |
| GATA-1low | Deletion of gene regulatory elements | Aberrant megakaryopoiesis with gradual development of myelofibrosis | 57 |
| OTT-MAL translocation | Knock-in | Requires retroviral introduction of a MPLW515L to develop myelofibrosis | 55 |
| JAKT2875N mutation | Retroviral transduction and transplantation | Mixed characteristics of AMKL and MPN | 19 |
| JAK2V617F mutation | Transgenic | Depending on promoter, phenotype can resemble PV or ET; mice develop myelofibrosis | 49 |
| JAK2V617F mutation | Retroviral transduction and transplantation | Development of myelofibrosis was variable between mouse strains | 50 |
| JAK2V617F mutation | Retroviral transduction and transplantation | Myelofibrosis accompanied by osteosclerosis | 51 |
| JAK2V617F mutation | Knock-in | Splenomegaly with atypical MKs exhibiting emperipolesis; absence of fibrosis; resembles PV with evidence of thrombotic episodes | 47 |
| JAK2V617F mutation | Conditional knock-in | Similar features with JAK2V617F knock-in model but fibrosis is present; resembles PV | 48 |
| RBM6-CSF1R fusion protein | Retroviral transduction and transplantation | Variable phenotype; spleen reticulosis | 98 |
| MPLW515L mutation | Retroviral transduction and transplantation | BM reticulosis; rapidly lethal | 53 |
| MPLT487A mutation | Retroviral transduction and transplantation | Similar but less aggressive course to MPLW515L model | 54 |
| Ts65Dn mouse strain | Induced translocation resulting in segmental trisomy of distal chromosome 16 | Myelofibrosis after the first year of life | 58 |
| BACH1 | Transgenic | GATA-1 promoter drives expression; BACH1 mutation has not been reported in MPN | 99 |
| NF-E2 overexpression | Transgenic | Abnormally clustered MKs; increased deposition of collagen and reticulin | 100 |
In seminal studies, mice infected with the myeloproliferative sarcoma virus exhibited expanded splenic megakaryopoiesis accompanied by BM and spleen fibrosis.42
Enhanced TPO/Mpl signaling is known to induce proliferation of MKs. Interestingly, in some cases, this proliferation is accompanied by reticulin deposition in BM. Transgenic mouse models of the TPO/Mpl axis were based on the expression of TPO either downstream of the ApoE promoter along with a hepatic enhancer element43 or downstream of the murine IgH promoter.44 Although both models exhibited an increase in MK and platelet numbers, for unclear reasons only the latter had developed myelofibrosis at 9 months of age. It is noteworthy that the levels of TGF-β were elevated in the IgH promoter model; and rats treated with the TPO mimetic romiplostim show BM fibrosis and an increase in MK number.45 Reticulin deposition (usually reversible) was also noted in some patients treated with TPO-mimetic agents, including romiplostim.45 On the other hand, in experiments involving adenoviral-mediated expression of TPO, osteomyelofibrosis was not observed in NOD-SCID mice despite elevated numbers of MKs, implying that the development of BM fibrosis is a complex process requiring the synergy of other components of the BM niche.46
Mutations of JAK2 are frequently detected in human MPNs. A knock-in mouse model of JAK2V617F exhibited splenomegaly with increased numbers of atypical BM MKs, accompanied by emperipolesis but without differences in MK ploidy compared with control mice.47 Intriguingly, although the knock-in JAK2V617F mice developed a rapidly lethal phenotype resembling PV, reticulosis was not observed even at terminal stage. By contrast, a conditional knock-in model of JAK2V617F exhibited a phenotype resembling PV and fibrosis of both spleen and BM.48 A number of transgenic mouse models encompassing the JAK2V617F with various expression levels were also engineered.49 Phenotype varied depending on the level of JAK2V617F expression: where expression levels were lower than that of the wild-type JAK2, the phenotype resembled ET, whereas where the level approximated that of wild-type JAK2, the phenotype resembled PV. A BM transplantation assay50 of JAK2V617F in Balb/c and C57BL/6 mouse strains revealed reticulin fibrosis only in the BM of the former strain. Of note, MK proliferation with impaired differentiation was observed in both strains. In a different study,51 the murine JAK2V617F transplantation experiments induced a phenotype resembling MPN, and it was noted that fibrosis was associated with osteosclerosis. One study involving patients with MPN showed that the osteoclast number increased in advanced stages of the disease, whereas in a second study osteoclast number was low or normal regardless of disease stage.52
A mutation of the TPO receptor (MPLW515L) was identified in a subset of patients with MPN. Overexpression of the mutated receptor in BM cells of mice resulted in a rapidly lethal myeloproliferative disease with increased number of platelets and MKs (atypical and dysplastic), elevated white blood cell count and reticulin fibrosis.53 A similar phenotype was observed in the BM transplantation model of the MPLT487A mutation, identified in a patient with non–DS-AMKL.54 The MPLT487A transduced mice exhibited an increase of MKs (CD41+/CD42+) in BM and spleen. In addition, transplanted mice exhibited elevated levels of platelets and white blood cell counts and myelofibrosis similar to that observed in MPLW515L. In the OTT-MAL knock-in mouse model, leukemia developed only in a small percentage of mice after a prolonged time interval.55 However, in this model overexpression of MPLW515L through retroviral transduction and transplantation resulted in a phenotype resembling AMKL, with BM fibrosis and a large number of megakaryoblasts infiltrating BM, spleen, and liver.
Moreover, some exclusively experimental models are reported to induce phenotype similar to MPNs associated with myelofibrosis (Table 1 contains a complete list). Notably, the GATA-1low mouse model, in which the distal promoter of GATA-1 and the DNAse hypersensitive region were abrogated, displayed myelofibrosis with a significantly increased number of MKs arrested between the stage of megakaryoblast and immature MK stages. An important element of this model is that, although the minority of mice that survived gestation exhibited thrombocytopenia and anemia, the latter resolved within a few weeks.56 These mice began developing myelofibrosis early, and the full presentation occurred at approximately 15 months of age; once myelofibrosis ensues, mice die within a few months.57 It is noteworthy that expression of the Pdgf gene was elevated in these mice compared with controls.
The Ts65Dn mouse strain is trisomic of 104 orthologs of human chromosome 21, and a widely used model of DS. These mice have a higher number of lower ploidy MKs in BM and spleen than wild-type mice.58 Myeloproliferative disease can be detected by 4 months, and after the first year of life thrombocytosis and myelofibrosis develop in all mice. Neither GATA-1low nor Ts65Dn mice progress to AMKL.
Taken together, several of these mouse models show a significant association between increased number of MKs and the development of an elaborate extracellular matrix typical of myelofibrosis. However, the etiology of phenotypical differences will require better understanding of the interplay between growth factors, MKs, and components of the BM niche.
Current therapies for myelofibrosis
Large clinical trials focusing on the treatment of MPN are limited, and treatments based on empirical reasoning are not uncommon. For ET and PV, the modalities currently in use,59 which include aspirin, hydroxyurea, busulphan, chlorambucil, and 32P, lack a specific molecular target. A potential exception is anagrelide, which was shown to inhibit MK polyploidization60 by a yet undiscovered mechanism. The management of myelofibrosis is a clinical challenge with a grim prognosis.30 Stem cell transplantation, although potentially curative, is accompanied by significant mortality and morbidity.
Hence, it is not surprising that research is focused on modified/mutated genes in myelofibrosis patients. The discovery of the JAK2V617F mutation, although not fitting the BCR-ABL paradigm of the chronic myeloid leukemia, drew attention to the development of targeted inhibitors of JAK2V617F. Several inhibitors are now in clinical trials with encouraging results, although myelosuppresion and gastrointestinal side effects occur.61 Other approaches include inhibition of the mTOR/AKT pathway62 or histone deacetylase complex.63 In addition, inhibitors of the BCL-2,64 BCL-XL, HSP90, and telomerase are under development. A brief account of some of the several clinical trials is given later in “LOX in MK-induced fibrosis: a potential therapeutic target.”
An important study relating to JAK2 inhibitors is the Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment (COMFORT).65 COMFORT was a multicenter, double-blind, placebo-controlled study in which patients with PMF or post-ET/PV myelofibrosis received the JAK1/2 inhibitor ruxolitinib66 or placebo. The primary end point of the study, reduction of spleen size of at least 35% at 24 weeks, was reached by a significant proportion of patients (41.9%) on ruxolitinib. In addition, the ruxolitinib group exhibited a statistically significant improvement in their symptoms and in overall survival (median follow-up, 51 weeks) compared with the placebo group. Side effects of ruxolinitib were anemia, neutropenia, and thrombocytopenia, and 2 patients progressed to acute myeloid leukemia.
A second study (COMFORT 2) compared ruxolitinib with best available therapy (any commercially available therapy or no treatment at all, as judged individually for each patient).67 In this study, reduction of spleen size of at least 35%, as assessed by MRI or CT scan, was the primary end point. Although no survival benefit or histomorphologic changes of BM were noted, patients receiving ruxolitinib exhibited statistically significant reduction in spleen size and in myelofibrosis-related symptoms. Anemia and thrombocytopenia were the most frequently reported hematologic side effects, whereas diarrhea and abdominal pain were also reported. Of note, data derived from use of ruxolitinib in the JAK2V617F knock-in mouse model demonstrated a reduction in splenomegaly and erythroid hyperplasia but persistence of the MPN-initiating population.47
CEP-701 (lestaurtinib) is another powerful JAK2 inhibitor with promising in vitro results,68 used in a small phase 2 study of myelofibrosis patients harboring the JAK2V617F mutation.69 According to the International Working Group for Myelofibrosis Research and Treatment criteria, overall response was 27% and side effects included anemia, thrombocytopenia, and gastrointestinal complains. However, the burden of JAK2V617 was not reduced, and none of the patients had improvement in BM fibrosis.
Immunomodulatory derivatives (IMiDs) include thalidomide analogs used in the treatment of MDS.70 Some IMiDs have been tested in patients with myelofibrosis. For example, lenalidomide in conjunction with prednisone was studied in 40 patients with myelofibrosis.71 Response to anemia (30%) and splenomegaly (42%) was noted, and some of the patients had a significant reduction of fibrosis. Pomalidomide is another IMiD drug that has been used alone and in combination with prednisone in patients with PMF. High doses of pomalidomide were associated with heightened side effects. In a multicenter study, low doses of pomalidomide, used as a single agent, or combined with a short course of prednisone, ameliorated myelofibrosis-related anemia.72
LOX in MK-induced fibrosis: a potential therapeutic target
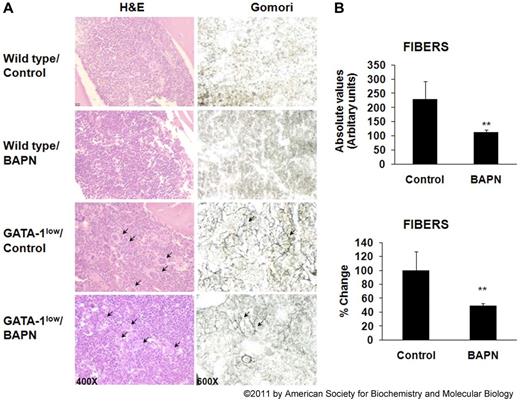
Lysyl oxidase (LOX) is a copper-dependent enzyme that cross-links collagen or elastin by oxidative deamination of peptidyl lysine or hydroxylysine and peptidyl lysine residues, respectively, and contributes to the accumulation of extracellular matrix by promoting intrapeptide and interpeptide chain crosslinking.73 LOX is produced by fibrogenic cells and is secreted as a 50-kDa glycosylated pro-enzyme. BMP-1, which is also expressed in MKs,74 cleaves the LOX pro-enzyme extracellularly to release the 18-kDa propeptide and the mature 30-kDa LOX enzyme. A recent study showed LOX expression in low ploidy, proliferating MKs, and its scarce expression in mature MKs of normal mice.75 LOX was also abundant in the GATA-1low mouse model with pathologically high levels of low-ploidy MKs associated with an extensively fibrotic matrix.75 LOX enzymatic activity is inhibited irreversibly by β-aminoproprionitrile (BAPN), which has been used in animal models in the context of tissue fibrosis or metastasis.76 Intriguingly, administration of BAPN to GATA-1low mice, which show an abundance of proliferating MK in BM and myelofibrosis, inhibited the progression of myelofibrosis, linking, for the first time, BM fibrosis and production of LOX by low-ploidy MKs75 (Figure 1). Although the potential role of LOX in myelofibrosis was only tested on the GATA-1low mouse model, these results could serve as a primer for further preclinical or clinical studies.
Effect of LOX inhibition on marrow fibrosis in vivo. (A) Representative hematoxylin and eosin (left column) and Gomori silver (right column) staining of longitudinal sections of femurs from wild-type and GATA-1low (male littermates), control, or BAPN-treated mice (10.5 weeks old at the time of collection). Original magnifications: left column, ×400; and right column, ×600. Arrows indicate the large presence of MKs (hematoxylin and eosin stain) and the accumulation of reticulin fibers in the GATA-1low mice. Images obtained using Nikon Eclipse 50i microscope equipped with a 40× Plan (numerical aperture 0.65) and a 60× Plan Fluor (numerical aperture 0.85) objectives. Digital images acquired using a SPOT 2 megapixel camera and SPOT 5.0 acquisition software. (B) Quantification of fibrosis in BAPN or vehicle-treated GATA-1low mice. Fibers were measured in arbitrary units from stained sections. Data are represented as absolute values (top panel) or as mean percent change compared with values recorded from vehicle-treated GATA-1low mice (bottom panel). The mean values were obtained from 5 mice per group. **P < .05. Reproduced from Eliades et al75 with permission.
Effect of LOX inhibition on marrow fibrosis in vivo. (A) Representative hematoxylin and eosin (left column) and Gomori silver (right column) staining of longitudinal sections of femurs from wild-type and GATA-1low (male littermates), control, or BAPN-treated mice (10.5 weeks old at the time of collection). Original magnifications: left column, ×400; and right column, ×600. Arrows indicate the large presence of MKs (hematoxylin and eosin stain) and the accumulation of reticulin fibers in the GATA-1low mice. Images obtained using Nikon Eclipse 50i microscope equipped with a 40× Plan (numerical aperture 0.65) and a 60× Plan Fluor (numerical aperture 0.85) objectives. Digital images acquired using a SPOT 2 megapixel camera and SPOT 5.0 acquisition software. (B) Quantification of fibrosis in BAPN or vehicle-treated GATA-1low mice. Fibers were measured in arbitrary units from stained sections. Data are represented as absolute values (top panel) or as mean percent change compared with values recorded from vehicle-treated GATA-1low mice (bottom panel). The mean values were obtained from 5 mice per group. **P < .05. Reproduced from Eliades et al75 with permission.
BAPN is a lathyrogen, the toxic constituent of peas from Lathyrus plants. Lathyrism, a disease known for centuries, encompasses 2 distinct entities: a disorder of the nervous system (neurolathyrism) leading to limb paralysis, and a disorder of connective tissue, causing either bone deformity (osteolathyrism) or aortic aneurisms (angiolathyrim). BAPN causes osteolathyrism and angiolathyrism when ingested in large quantities.77 BAPN has been used in small-scale clinical trials in patients with scleroderma, urethral strictures, keloids, or undergoing tendon repair. BAPN was not effective in a study of 10 patients with scleroderma, and anemia, allergic rash, and a case of bone deformity were reported with the dosage and regimen used.78 In a study of patients undergoing flexor tendon repair,79 all 6 patients developed side effects that included fever, periportal hepatitis, skin rash, and gastrointestinal symptoms. Side effects rapidly resolved after discontinuation of BAPN with no long-term consequences. On the other hand, 1 g/day of BAPN was shown to be effective in 9 patients affected with keloids who received it for a total of 21 days without report of adverse effects.80 Furthermore, in a study of urethral strictures,81 5 patients received BAPN for 21 days without side effects; the reasons for the discrepancy in side effects observed in the studies using BAPN remain unclear.82 Larger studies are required to determine optimal dose and safety profile.
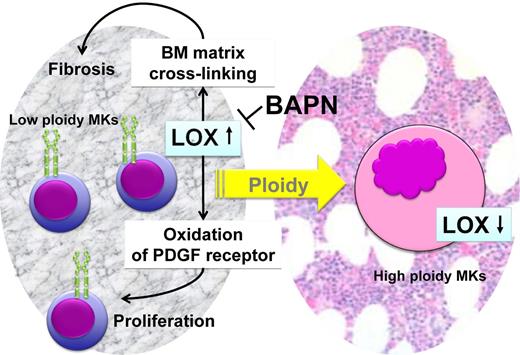
The functions attributed to LOX have recently been expanded; LOX oxidizes the PDGF receptor on smooth muscle cells, fibroblasts, and MKs and enhances the proliferation signaling from this cytokine,83 leading to higher cell number.75 Thus, LOX is capable of enhancing the proliferation of low-ploidy MKs, which in turn produce LOX that further stabilizes the matrix resulting in a fibrotic phenotype (Figure 2).
Possible mechanisms of contribution of LOX to progression of myelofibrosis. Low-ploidy MKs have elevated levels of expression of LOX, which is secreted to the BM microenvironment. The active LOX enzyme promotes cross-linking of matrix collagen and consequent fiber deposition. The active LOX enzyme also oxidizes the PDGF receptor, enhancing the proliferative response from this receptor. Inhibition of LOX by BAPN may reverse this process. High-ploidy MKs express very low amounts of LOX.
Possible mechanisms of contribution of LOX to progression of myelofibrosis. Low-ploidy MKs have elevated levels of expression of LOX, which is secreted to the BM microenvironment. The active LOX enzyme promotes cross-linking of matrix collagen and consequent fiber deposition. The active LOX enzyme also oxidizes the PDGF receptor, enhancing the proliferative response from this receptor. Inhibition of LOX by BAPN may reverse this process. High-ploidy MKs express very low amounts of LOX.
In response to the differential expression of LOX within the MK lineage and the effect of LOX on matrix deposition, LOX gene expression has been a focus of study. The LOX gene is mapped on human chromosome 5q23, and its expression84 is closely linked to that of collagen. For example, a putative binding site has been recognized for the CCAAT binding factor, an inducer of collagen synthesis in the rLox promoter.85 Moreover, HIF-1a is a potent modulator of LOX expression.86 In addition, growth factors, such as PDGF and TGF-β1, and the cytokine interleukin 1b increase LOX expression.87,88 This is of interest also in the context of MKs because pathologic states involving expansion of MKs also lead to increased levels of extracellular factors, such as PDGF and TGF-β1, which in turn have the potential to further boost the fibrotic phenotype.
Whereas this review focuses on LOX and BM fibrosis, a number of reports support the involvement of LOX in various other pathologies. LOX was detected by microarray studies in the BM blasts of 9 of 11 patients with non–DS-AMKL, contributing to a specific gene profile (raw data deposited at www.ncbi.nlm.nih.gov/geo; GEO accession no. GSE4119). LOX has been intensely studied in the context of solid tumors.89 LOX was originally identified as a suppressor of RAS transformation in NIH3T3 cells, and its gene expression is down-regulated in several carcinomas.90 LOX has also attracted attention in the context of metastasis.76
Of note, LOX-like proteins (LOXL1-4) have been described that share the LOX catalytic site. LOXL1 is structurally most closely related to LOX, and the pattern of expression of those 2 genes overlaps in many tissues. LOXL1 knockout mice are viable but display defects predominantly in tissues with high elastin content, such as lung, skin, and uterus. By contrast, LOXL2, LOXL3, and LOXL4 have a different, less diverse, gene expression pattern and share 4 scavenger receptor cysteine rich regions. LOXL2 is associated with development of aneurysms and is overexpressed in fibrotic lung and liver tissues.91 An antibody against LOXL2 was shown to ameliorate organ fibrosis in mouse models of lung and liver fibrosis.92 Currently, the humanized version of the antibody (GS-6624, former AB0024) is in phase 2 clinical trial to evaluate efficacy in adult myelofibrosis (www.clinicaltrials.gov; #NCT01369498). LOXL3 expression was detected in placenta, heart, and breast and, importantly, in highly malignant breast cancer cells.91 In the context of megakaryopoiesis, up-regulated LOXL3 gene expression has been reported during endomitosis,93 and the LOXL3 protein was detected in human platelets.94
Although the focus of this review is the importance of megakaryocytes in the progression of myelofibrosis, it is important also to consider the involvement of cells from other lineages. LOX is expressed in lineages populating the BM niche. Osteoblastic differentiation is impaired in LOX knockout mice.95 LOXL2 also controls angiogenesis in the endothelial basement membranes,96 and LOX is expressed in fibroblasts.88 One can readily envision a state of pathologic levels of MKs leading to increased secretion of PDGF or TGF-β, which in turn are capable of increasing fibroblast proliferation, in addition to LOX-induced matrix deposition.
The control of fibrosis could be a potential therapeutic target for conditions, such as AMKL and a subset of MPN, especially PMF. Current therapies have had limited success, and the recent model linking MK-induced myelofibrosis to LOX suggests promise in the use of LOX inhibitors for controlling BM fibrosis.
Acknowledgments
K.R. was supported by National Heart, Lung, and Blood Institute (grant HL80442).
The authors apologize to colleagues whose studies were not cited in this review because of space limitation.
National Institutes of Health
Authorship
Contribution: N.P. designed the review and wrote the manuscript; S.M. assisted in writing and reviewed the manuscript; and K.R. designed the review, advised on the writing, and participated in writing and reviewing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nikolaos Papadantonakis, Albert Einstein College of Medicine, Jacobi Medical Center, 1400 Pelham Pkwy S, Room 3N21, Bronx, NY 10461; e-mail: nikolaos.papadantona@nbhn.net; and Katya Ravid, Boston University School of Medicine, 700 Albany St, CVI, W-601, Boston, MA 02118; e-mail: kravid@bu.edu.