Abstract
Immature dendritic cells (imDCs) can have a tolerizing effect under normal conditions or after transplantation. However, because of the significant heterogeneity of this cell population, it is extremely difficult to study the mechanisms that mediate the tolerance induced or to harness the application of imDCs for clinical use. In the present study, we describe the generation of a highly defined population of imDCs from hematopoietic progenitors and the direct visualization of the fate of TCR-transgenic alloreactive CD4+ and CD8+ T cells after encountering cognate or noncognate imDCs. Whereas CD4+ T cells were deleted via an MHC-independent mechanism through the NO system, CD8+ T-cell deletion was found to occur through a unique MHC-dependent, perforin-based killing mechanism involving activation of TLR7 and signaling through Triggering Receptor-1 Expressed on Myeloid cells (TREM-1). This novel subpopulation of perforin-expressing imDCs was also detected in various lymphoid tissues in normal animals and its frequency was markedly enhanced after GM-CSF administration.
Introduction
Central and peripheral tolerance mechanisms are critical for the establishment of a robust immune response that can distinguish between self- and nonself-antigens. Although the majority of self-specific T cells are deleted by negative selection in the thymus, some self-reactive T cells are spared and can reach peripheral organs.1,2 A wealth of evidence indicates that dendritic cells (DCs) have tolerogenic capacity in their immature state (imDCs).3–8 In the context of allogeneic organ transplantation, infusion of imDCs expressing the relevant MHC-peptide complex can prolong allograft survival in vivo.7,9–12 At the same time, imDCs can become immunogenic on maturation/activation in the presence of a danger signal such as lipopolysaccharide (LPS). However, this simplistic paradigm was recently challenged by the demonstration that fully mature DCs can also induce tolerance under the appropriate conditions,13–17 suggesting a more complex decision-making process in which the net effect of Ag dose, DC lineage, DC maturation and activation state, and the cytokine milieu at the site of inflammation determine whether immunogenic or tolerogenic DC activity will prevail.18 The tolerogenic potential of immature or mature DCs can be further extended to the resolution of inflammatory responses to pathogens.19 Lymphoid organs, including spleen, bone marrow (BM), lymph nodes, and thymus, contain multiple DC subpopulations largely defined by their distinct anatomical location and phenotypes.20,21 For example, the mouse spleen harbors plasmacytoid DCs (pDCs) and the CD8+ and CD8− subsets of classic DCs (cDCs).20 Apart from the major steady-state dichotomy of differentiation into pDCs versus cDCs, an additional distinct monocyte-derived DC subset with phenotypic characteristics of cDCs is recruited to sites of inflammation.22 The phenotypic heterogeneity of DCs and growing data on their distinct origins present a major difficulty in studying the mechanisms of their tolerance induction.
The current protocol for generating myeloid DCs ex vivo is based on culturing BM with cytokine mixtures containing GM-CSF.23 However, recent data suggest that this approach yields a population similar to monocyte-derived inflammatory imDCs, which express phenotypic markers of classic imDCs but are likely functionally distinct from the classic imDCs present in the spleen under steady-state conditions or those induced by culturing BM with Flt3 ligand.24,25 Furthermore, considering that GM-CSF could also induce tolerogenic properties in other myeloid cell subpopulations, it is hard to draw conclusions from such studies regarding the mechanism of action of native imDCs.
In the present study, we addressed this challenge using a highly homogeneous inflammatory imDCs population generated ex vivo from purified murine Sca-1–positive lineage-negative (Sca-1+Lin−) C-kit+ precursors cultured in the presence of GM-CSF to investigate the tolerizing effect of the imDCs. To visualize directly the fate of CD4+ and CD8+ cognate T cells after brief interaction with these imDCs, we used alloreactive TCR transgenic T cells expressing a TCR specific for MHC molecules (TEA, 2C).26,27 Therefore, using imDCs from different MHC backgrounds and single-cell imaging of the TCR-transgenic T cells, we were able to define the MHC dependence of the imDC-mediated suppression and to conclusively demonstrate distinct mechanisms of imDC action on CD4+ and CD8+ alloreactive T cells.
Methods
Animals
Mice used were 6- to 12-week-old females of the following strains: C57BL/6, CB6/F1 (H-2d/b and I-Ab), FVB (H-2Dq and I-Aq), perforin−/− (PKO; [H-2d/b/I-Ab]28 ; F1 mice were the progeny of Balb PKO and C57B6 PKO that were backcrossed for at least 8 generations), gld−/− FAS-L knockout (H-2d/ I-Ad), C3H/HeJ (H-2k), C57BL/6 mice expressing enhanced yellow fluorescent protein (eYFP) under the CD11c promoter29 (Weizmann Institute Animal Breeding Center, Rehovot, Israel), and TLR7−/− (The Jackson Laboratory). 2C TCR-transgenic mice27,30 expressing a TCR with specificity for H-2Ld were kindly provided by Janko Nikolic-Zugic (Memorial Sloan-Kettering Cancer Center, New York, NY). TEA TCR-transgenic mice (H-2b)26 expressing a TCR with specificity for the I-Eαd allopeptide 52-68 cross-presented by APC expressing I-Ab was kindly provided by A.Y. Rudensky (Sloan-Kettering). 2C and TEA mice were crossed with Rag−/− mice. Animals were maintained under conditions approved by the institutional animal care and use committee at the Weizmann Institute of Science.
Isolation of HSC populations and generation of imDCs
For a description of the isolation of hematopoietic stem cell (HSC) populations and generation of imDCs, please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cytofluorimetric analysis
For a description of the cytofluorimetric analysis, please see supplemental Methods.
Quantitation of imDC-mediated killing using MLRs (2C, TEA, OT-I T cells)
Calcein AM green–stained 2C, OT-I CD8+, or TEA CD4+ MACS-isolated T cells were incubated in the presence or absence of imDCs (C57BL/6, CB6, FVB, and PKO) in 96-well round-bottom plates for 5 hours at 37°C. C57BL/6 imDCs were loaded overnight with 10 μg/mL of the peptides SIINFEKL or SIYRYYGL. imDCs or CD8+ cells were pretreated with 100 ng/mL of the NO inhibitor N-(G)-nitro-L-arginine methyl ester (L-NAME) for 20 minutes at 37°C and then either left without further treatment, or treated with the following inhibitors: BAPTA-AM, Concanamycin A, PP2 or PP3, in addition to blocking Ab against TRAIL, TGF-β or TNF-α, added after mixed-lymphocyte reaction (MLR) initiation. In other experiments, imDCs were pretreated with blocking peptides for TLR1, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10, or TLR11 or with competitive peptides for Triggering Receptors Expressed on Myeloid cells-1 (TREM-1), TREM-2, or neutralizing Ab for TREM-3 (for details, see supplemental Methods). The total number of calcein AM green–positive (viable) cells was determined by FACS. The killing of CD8+/CD4+ cells was calculated as follows:
Immunohistochemistry
Immunohistochemistry was performed for the detection of perforin and granzyme A/B in tissues and cells. imDCs and cytotoxic T-lymphocytes (CTLs) were prepared as described previously.31 Tissues were fixed in 4% paraformaldehyde and 30% sucrose; cell smears were fixed in 4% paraformaldehyde, permeabilized using 0.1% Triton X-100, blocked by 7% horse serum, and stained with rat anti–perforin, goat anti–granzyme A, or rabbit anti–granzyme B (Santa Cruz Biotechnology).
Western blot
For a description of the Western blot analysis, please see supplemental Methods.
Live-cell video microscopy
CB6/F1 imDCs were loaded with LysoTracker Red (Invitrogen) and seeded onto poly–L-lysine–coated 8-well Lab-Tek chambered coverglass system plates (Nunc) in medium without phenol red. Calcein AM green–labeled 2C cells were added at a 2:1 ratio. Live-cell images were obtained in a closed, warmed chamber (Bioptechs FCS2) on a Deltavision Restoration microscope (Applied Precision Instruments) using a MicroMax 5 MHz cooled CCD camera (Roper Scientific). Image sequences of the time-lapse recording were processed with Softworx Explorer 2.0 software.
BLT-esterase release assay
The BLT-esterase release assay was performed as described previously.32
TEM analysis
For a description of the transmission electron microscope (TEM) analysis (morphology and gold immunostaining), please see supplemental Methods.
Statistical analysis
For a description of the statistical analysis, please see supplemental Methods.
Results
Generation of imDCs from mouse HSCs/hematopoietic progenitor cells
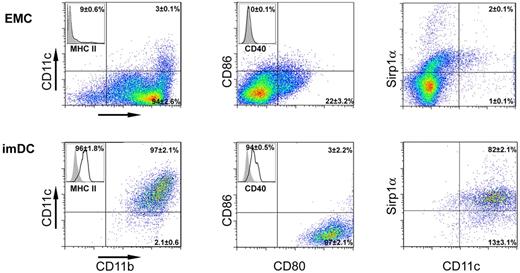
The mechanism underlying T-cell suppression by GM-CSF–induced imDCs is poorly understood. To address this issue, we generated mouse imDCs from a well-defined HSC/hematopoietic progenitor cell population that exhibit Lin−Sca-1+C-kit+ phenotype (LSKs) via differentiation to early myeloid cells using a 10-day culture with a cytokine cocktail containing thrombopoietin, Flt3L, SCF, IL-3, and IL-6 and further differentiation to imDCs using 20 ng/mL of GM-CSF for an additional 10 days. Therefore, LSKs were first differentiated into early myeloid cells expressing CD11b (94%), CD80 (22%), and Sirp1α (17%). Cells were mostly negative for MHC-II (9%), CD11c (3%), CD86 (3%), and CD40 (Figure 1); at the end of the culture with GM-CSF, the cells acquired a conventional imDC phenotype expressing CD11c (97%), CD11b (100%), MHC-II (96%), CD80 (100%), CD40 (94%), and Sirp1α (82%). The generated cells were mostly negative for CD86 (3%) and did not express CD45RB/B220, PDCA-1, NK1.1, DX5 (CD49b), NKp46, CD3, CD4, CD8, or F4/80.6,23,33 Therefore, the generation of imDCs from purified LSKs yields a highly homogeneous population of myeloid imDCs.
Cell phenotype changes upon differentiation of LSKs into imDCs. Representative FACS analysis of differentiation markers of early myeloid cells (day 10 after LSK isolation) or imDCs generated from early myeloid cells after 10 days of culture with GM-CSF (day 20 after LSK isolation).
Cell phenotype changes upon differentiation of LSKs into imDCs. Representative FACS analysis of differentiation markers of early myeloid cells (day 10 after LSK isolation) or imDCs generated from early myeloid cells after 10 days of culture with GM-CSF (day 20 after LSK isolation).
Killing of alloreactive T cells by imDCs in short-term MLRs
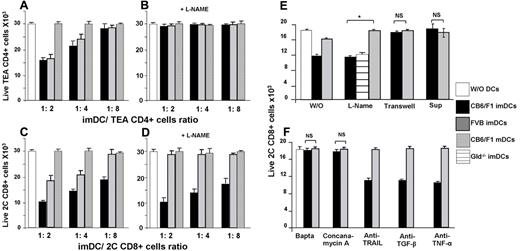
In preliminary experiments, we found that imDCs inhibited alloreactive TCR transgenic effector T cells in long-term MLRs (data not shown). We therefore sought to further study this inhibitory effect in a short-term culture (5 hours) terminated before cell proliferation. This system is better suited for distinguishing between anergy- and deletion-based mechanisms, because reductions in cell number during the short follow-up period cannot be attributed to inhibition of cell proliferation. As shown in Figure 2, the suppression induced by imDCs was mediated by killing of the recognizing CD4+ and CD8+ T cells. Killing of CD4+ alloreactive T cells was found to be MHC independent, as demonstrated by inhibition of transgenic TCR CD4 T cells (TEA CD4+ T cells). Therefore, imDCs expressing an MHC-II haplotype not recognized by the transgenic TCR and imDCs expressing the cognate MHC-II induced similar inhibition of TEA CD4+ T cells (Figure 2A). This MHC-independent killing was completely abrogated by pretreatment with the NO inhibitor L-NAME (Figure 2B), suggesting that this cytotoxic mechanism operates through the NO system. Therefore, this mechanism is reminiscent of the activity of myeloid progenitors of imDCs (CD11c−CD11b+B220−).34
Mechanisms of CD4+ and CD8+ T-cell deletion by imDCs. TEA CD4+ (A-B) or 2C CD8+ (C-D) cells stained with calcein AM green were incubated for 5 hours in the absence (white) or presence of imDCs from CB6/F1 (H-2bd, black), irrelevant FVB (H-2q, gray) donors, or with mDCs from CB6/F1 at the indicated ratios. At the end of the culture, the total number of stained TEA CD4+ T cells or 2C CD8+ cells was evaluated by FACS. The activity of imDCs from different donors preincubated with the NO inhibitor L-NAME (100 ng/mL) for 20 minutes at 37°C, on TEA CD4+ (B) or 2C CD8+ (D) cells was assessed by live-cell determination as described in panels A and C. The effect of various imDC pretreatments (E-F) and the effect of supernatants (Sup) from culture with cognate (black) or noncognate (gray) imDCs on 2C CD8+ cell deletion by imDCs. The results shown represent the average ± SD (n ≥ 3). *P < .01. NS indicates not significant.
Mechanisms of CD4+ and CD8+ T-cell deletion by imDCs. TEA CD4+ (A-B) or 2C CD8+ (C-D) cells stained with calcein AM green were incubated for 5 hours in the absence (white) or presence of imDCs from CB6/F1 (H-2bd, black), irrelevant FVB (H-2q, gray) donors, or with mDCs from CB6/F1 at the indicated ratios. At the end of the culture, the total number of stained TEA CD4+ T cells or 2C CD8+ cells was evaluated by FACS. The activity of imDCs from different donors preincubated with the NO inhibitor L-NAME (100 ng/mL) for 20 minutes at 37°C, on TEA CD4+ (B) or 2C CD8+ (D) cells was assessed by live-cell determination as described in panels A and C. The effect of various imDC pretreatments (E-F) and the effect of supernatants (Sup) from culture with cognate (black) or noncognate (gray) imDCs on 2C CD8+ cell deletion by imDCs. The results shown represent the average ± SD (n ≥ 3). *P < .01. NS indicates not significant.
Interestingly, responder CD8+ T cells were also killed when incubated with either specific or nonspecific imDCs. However, the specific MHC-dependent killing was significantly (P < .01) more pronounced than the nonspecific MHC-independent killing at every T cell/imDC ratio tested (Figure 2C). Furthermore, when MHC-specific or MHC-nonspecific imDCs were pretreated with the NO inhibitor L-NAME, the nonspecific MHC-independent killing was inhibited, whereas the specific MHC-dependent killing mechanism remained unaffected (Figure 2D). These results indicate that imDCs suppress CD8+ T-cell alloresponses by induction of death through 2 distinct mechanisms: (1) MHC-independent killing mediated via the NO system and (2) MHC-dependent killing mediated through a distinct pathway. These 2 killing mechanisms are lost upon maturation of imDCs to mDCs after incubation with LPS for 24 hours (Figure 2A,C), suggesting that the ability to induce death is associated specifically with the immature stage of DC differentiation.
MHC-dependent killing of CD8+ effector T cells by imDCs is mediated by a perforin/granzyme A–based mechanism
Having found in initial experiments that the MHC-dependent killing of recognizing CD8+ T cells by imDCs is not mediated by apoptosis (data not shown), we next demonstrated that the killing mechanism is cell-contact dependent by culturing imDCs and CD8+ cells in Transwells preventing direct cell contact (Figure 2E). In addition, transfer of supernatants collected after the primary incubation of T cells with either cognate imDCs or noncognate imDCs (ie, without limiting cell contact) to fresh 2C T cells did not result in detectable killing of the latter (Figure 2E).
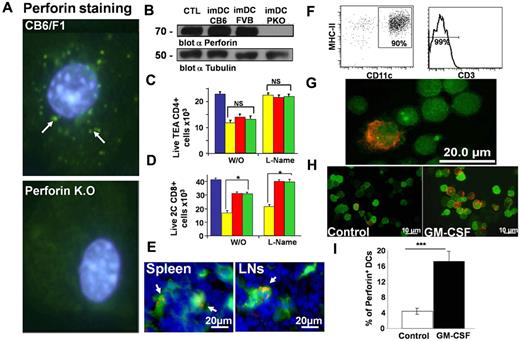
Whereas this cytotoxicity was independent of NO, it was markedly inhibited by pretreatment of imDCs for 2 hours with BAPTA-AM, an inhibitor of granular release (Figure 2F), or with concanamycin-A, a selective inhibitor of the vacuolar proton-ATPase that prevents perforin activation (Figure 2F). Furthermore, MHC-dependent killing was not inhibited when imDCs grown from FasL−/− mice (gld−/−; Figure 2E) were added to the MLR culture or in the presence of neutralizing Abs against TRAIL, TGF-β, or TNF-α (Figure 2F). This is in contrast to the killing exerted by tolerogenic CD11c CD8+ mature DCs shown to be dependent on TNF-α.14 These results and the rapid nature of the killing observed, strongly suggested the possibility that a perforin-mediated mechanism might mediate the deletion of the alloreactive CD8+ T cells by the imDCs. To further address this point, we first tested the expression of perforin in imDCs by immunostaining (Figure 3A) and by Western blotting (Figure 3B). Perforin expression was detected in the imDCs at levels comparable with those found in cytotoxic T cells used as a control.
MHC-dependent killing of CD8+ T cells by imDCs is predominantly mediated by perforin and not by the NO system. (A) Representative perforin (green) immunostaining in CB6/F1 and PKO imDCs together with Hoechst 33342 (blue) nuclear staining. (b) Perforin (70 kD) expression was determined by Western blotting. (C-D) CD4+ TEA or 2C CD8+ alloreactive T cells stained with calcein AM green were incubated for 5 hours in the absence (blue) or presence of CB6/F1 (yellow), FVB (red), or PKO (green) imDCs treated or not with the NO inhibitor L-NAME (100 ng/mL) for 20 minutes at 37°C at a 4:1 cell ratio. At the end of the culture, the total number of live stained CD4+ TEA or 2C CD8+ T cells was evaluated by FACS. Average ± SD (n ≥ 3) shown. (E-G) The presence of perforin+CD11c DC under steady state. (E) In situ immunostaining for perforin (red) in the spleens and lymph nodes of CD11c-eYFP mice. Arrows indicate cells that coexpress both markers. Splenocytes from mice expressing eYFP-CD11c were isolated by FACS based on high expression of eYFP, MHC-II, and negative staining for CD3. (F) Phenotype of the enriched cells. (G) Sorted cells were spun onto a slide using Cytospin, fixed, and immunostained for perforin (red). (H-I) Typical immunostaining and quantification of perforin+ DCs in the spleens of mice 14 days after infusion of GM-CSF–secreting B-16 melanoma cells.
MHC-dependent killing of CD8+ T cells by imDCs is predominantly mediated by perforin and not by the NO system. (A) Representative perforin (green) immunostaining in CB6/F1 and PKO imDCs together with Hoechst 33342 (blue) nuclear staining. (b) Perforin (70 kD) expression was determined by Western blotting. (C-D) CD4+ TEA or 2C CD8+ alloreactive T cells stained with calcein AM green were incubated for 5 hours in the absence (blue) or presence of CB6/F1 (yellow), FVB (red), or PKO (green) imDCs treated or not with the NO inhibitor L-NAME (100 ng/mL) for 20 minutes at 37°C at a 4:1 cell ratio. At the end of the culture, the total number of live stained CD4+ TEA or 2C CD8+ T cells was evaluated by FACS. Average ± SD (n ≥ 3) shown. (E-G) The presence of perforin+CD11c DC under steady state. (E) In situ immunostaining for perforin (red) in the spleens and lymph nodes of CD11c-eYFP mice. Arrows indicate cells that coexpress both markers. Splenocytes from mice expressing eYFP-CD11c were isolated by FACS based on high expression of eYFP, MHC-II, and negative staining for CD3. (F) Phenotype of the enriched cells. (G) Sorted cells were spun onto a slide using Cytospin, fixed, and immunostained for perforin (red). (H-I) Typical immunostaining and quantification of perforin+ DCs in the spleens of mice 14 days after infusion of GM-CSF–secreting B-16 melanoma cells.
To examine the possibility that perforin is critical for the killing of alloreactive CD8+ T cells, we generated imDCs from PKO mice. Whereas the deletion of alloreactive CD4+ T cells was not affected (Figure 3C), the ability of cognate imDCs to delete alloreactive CD8+ T cells was diminished when using imDCs from PKO mice bearing the cognate MHC-I molecule (Figure 3D). The specific MHC-dependent killing of CD8+ T cells, shown in Figure 3D to be dependent on perforin, was not inhibited by pretreatment with L-NAME. In contrast, the killing of CD4+ T cells was not dependent on perforin expression, but was completely blocked by pretreatment with L-NAME. These results indicate that the MHC-dependent killing of MHC-specific CD8+ T cells by imDCs is mediated by cell-cell contact, which is followed by granule release and perforin-mediated cytotoxicity. Our finding that the supernatant collected after short-term culture was unable to induce killing of the cognate T cells strongly suggests that because perforin, in contrast to granzyme A, is only effective over short distances, cell contact is required in this killing mechanism.
Most importantly, histologic examination of spleen and lymph nodes of untreated mice revealed a minor subpopulation of perforin+ cells within the CD11c+ population. Therefore, distinct perforin-containing granules could be found in a small subset of CD11c+ cells in the spleens and lymph nodes of mice expressing eYFP under the CD11c promoter (Figure 3E). To enumerate the incidence of eYFP and perforin double-positive cells, splenocytes from these mice were FACS sorted for CD11c+MHC-II+ cells while the CD3+ cells were gated out (Figure 3F). CD11c+MHC-II+CD3− cells were fixed onto glass slides and immunostained for perforin. Using image software for counting, 62 double-positive cells were recorded of a total of 1473 DCs (4.2% ± 0.65%, Figure 3G), further suggesting that this imDC phenotype can be found at low incidence in normal animals and is not merely limited to imDCs generated under arguably artificial ex vivo culture conditions. Interestingly, the frequency of these CD11c+perforin+ cells was markedly enhanced in the spleens of mice in which GM-CSF was administered by injecting tumor cells secreting the cytokine. After 2 weeks, we found a profound 4-fold increase in the incidence of double-positive cells compared with control mice treated with tumor cells not secreting the cytokine (Figure 3H-I). This result is consistent with our ex vivo studies involving GM-CSF–driven cultures, suggesting a potential physiologic role of this unique cell population in the resolution of inflammation or the termination of immune responses.
Polarization of perforin and granzyme A in imDCs upon recognition by alloreactive CD8+ T cells
Considering the central role of perforin in the suppressive activity of imDCs, we next examined the cellular location of perforin in imDCs and the role of granzymes typically associated with perforin activity. As can be seen in Figure 4, perforin is located in cytoplasmic granules. In contrast to CTLs, which express both granzyme A and granzyme B, imDC granules contained predominantly granzyme A, as was further confirmed by Western blotting.
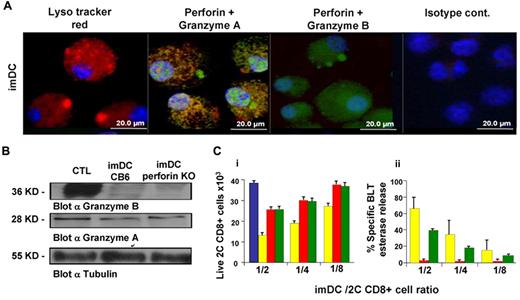
MHC-dependent inhibition of alloreactive CD8+ T cells in MLR: perforin-mediated killing is associated with granzyme A activity. To determine granule location in imDCs, the cells were stained with LysoTracker Red, anti-perforin (green), anti-granzyme A (red) or granzyme B (red), and Hoechst 33342 (blue). Images were taken using a Zeiss ApoTome system. (B) Granzyme B (36 kDa) and granzyme A (28 kDa) expression determined by Western blotting in CB6/F1 or PKO imDCs. Expression of granzyme A and B in CB6/F1 CTLs was used as a positive control. (Ci) Total numbers of live calcein AM green–stained CD8+ 2C cells after incubation for 5 hours in the absence (blue) or presence of imDCs from CB6/F1 (yellow), FVB (red), or PKO (green) at the indicated ratios was determined by FACS. (Cii) At the end of the short-term MLR shown in panel C1, the percentage of specific BLT esterase release was calculated as described in the “Methods.” The bars represent the average ± SD (n ≥ 3).
MHC-dependent inhibition of alloreactive CD8+ T cells in MLR: perforin-mediated killing is associated with granzyme A activity. To determine granule location in imDCs, the cells were stained with LysoTracker Red, anti-perforin (green), anti-granzyme A (red) or granzyme B (red), and Hoechst 33342 (blue). Images were taken using a Zeiss ApoTome system. (B) Granzyme B (36 kDa) and granzyme A (28 kDa) expression determined by Western blotting in CB6/F1 or PKO imDCs. Expression of granzyme A and B in CB6/F1 CTLs was used as a positive control. (Ci) Total numbers of live calcein AM green–stained CD8+ 2C cells after incubation for 5 hours in the absence (blue) or presence of imDCs from CB6/F1 (yellow), FVB (red), or PKO (green) at the indicated ratios was determined by FACS. (Cii) At the end of the short-term MLR shown in panel C1, the percentage of specific BLT esterase release was calculated as described in the “Methods.” The bars represent the average ± SD (n ≥ 3).
In parallel to the acquisition of immune regulatory function by imDCs upon their differentiation from HSCs, analysis of different HSCs and early myeloid cells revealed that perforin and granzyme A become apparent only in early imDCs and their levels were enhanced in late imDCs (treated with GM-CSF for 4 and 10 days, respectively; supplemental Figure 1A). The gradual appearance of granules on GM-CSF culture was also readily documented by TEM (supplemental Figure 1B). To further evaluate the potential role of granzyme A in the imDC-mediated suppression of alloreactive CD8+ T cells, 2C cells were cultured with their cognate stimulators (BALB/c splenocytes) in the presence of cognate wild-type (CB6/F1) imDCs, PKO CB6 imDCs, or third-party imDCs (FVB). The killing of the CD8+ T cells (Figure 4Ci) was found to be correlated with the release of BLT esterase, a specific marker of granzyme A activity (Figure 4Cii). However, granzyme release was restricted to cocultures containing cognate imDCs, but not third-party imDCs. Interestingly, although the elimination of CD8+ T cells was diminished when using PKO imDC, the release of granzyme A was only marginally reduced, suggesting that, like CTL-mediated killing, imDC-mediated killing is also dependent on perforin. Therefore, in the absence of perforin degranulation, granzyme A is released but does not lead to killing of the alloreactive CD8+ T cells.
To further study perforin and granzyme A expression in imDCs, we used Zeiss ApoTome grid system microscope, enabling granule localization to be determined inside the cell (Figure 5). Granules of perforin and granzyme A were predominantly located in proximity to the cell membrane (Figure 5A-B). This was further confirmed by TEM (Figure 5C-F) showing that imDCs, which exhibited normal cell morphology (Figure 5E enlarged image of white box in panel D), were loaded with evenly distributed granules localized mostly in vicinity to the cell membrane (Figure 5F). Furthermore, EM gold immune labeling indicated that these granules contained perforin (Figure 5Fi), unlike imDCs generated from PKO mice (Figure 5Fii). Examination of fully mature DCs after 24 hours of culture with LPS revealed a similar level of granules, ruling out the possibility that loss of cytotoxic activity is associated with a decreased presence of granules (supplemental Figure 1).
Granules of perforin and granzyme A are located near the cell membrane of the imDCs and are polarized toward the alloreactive CD8+ T-cell contact area. Representative imDC staining for perforin (green), granzyme A (red), and Hoechst 33342 (blue). Conventional triple-fluorescence staining of imDCs (A) was compared with optical sections at axial depth (z = 3μm) taken using the Zeiss ApoTome grid system (B). Typical EM appearance of imDCs (C) exhibiting round morphology with round nuclei (N) and a large number of granules (Gr). (D) Enlarged image of panel C showing evenly distributed granules in the vicinity of the cell membrane. (E) Enlarged image of the white box showing imDC inner cell morphology containing Golgi (Gol) and mitochondria (Mit). (F) imDC granules are in proximity to the cell membrane close to the microvilli (Micro) surrounding the entire cell. EM gold immunostaining for perforin inside of the granules in wild-type (i) and PKO (ii) mice (black dots marked by arrowheads). Confocal microscopy of CB6/F1 (G) or PKO (H) imDCs stained with LysoTracker Red (red) for granules, incubated with calcein AM green–stained 2C CD8+ T cells (green). (I) Quantification of CB6/F1 imDCs (a) versus PKO cell (b) polarization. Conjugates were scored as polarized when vesicles clearly accumulated in the area of contact. At least 50 conjugates were evaluated in each sample. (J) Interaction between specific CB6/F1 imDCs stained with LysoTracker Orange for granules (orange) and calcein AM green 2C CD8+ T cells (green). The elapsed time (h:min:sec) is shown at the bottom of each recorded frame. (K) Granules in imDCs containing perforin (green) and granzyme A (red) move to the area of contact. (L) TEM of conjugates between CB6/F1 imDC and 2C CD8+ T cells also revealing polarization of granules (Gr) toward the contact area in the nucleus (N).
Granules of perforin and granzyme A are located near the cell membrane of the imDCs and are polarized toward the alloreactive CD8+ T-cell contact area. Representative imDC staining for perforin (green), granzyme A (red), and Hoechst 33342 (blue). Conventional triple-fluorescence staining of imDCs (A) was compared with optical sections at axial depth (z = 3μm) taken using the Zeiss ApoTome grid system (B). Typical EM appearance of imDCs (C) exhibiting round morphology with round nuclei (N) and a large number of granules (Gr). (D) Enlarged image of panel C showing evenly distributed granules in the vicinity of the cell membrane. (E) Enlarged image of the white box showing imDC inner cell morphology containing Golgi (Gol) and mitochondria (Mit). (F) imDC granules are in proximity to the cell membrane close to the microvilli (Micro) surrounding the entire cell. EM gold immunostaining for perforin inside of the granules in wild-type (i) and PKO (ii) mice (black dots marked by arrowheads). Confocal microscopy of CB6/F1 (G) or PKO (H) imDCs stained with LysoTracker Red (red) for granules, incubated with calcein AM green–stained 2C CD8+ T cells (green). (I) Quantification of CB6/F1 imDCs (a) versus PKO cell (b) polarization. Conjugates were scored as polarized when vesicles clearly accumulated in the area of contact. At least 50 conjugates were evaluated in each sample. (J) Interaction between specific CB6/F1 imDCs stained with LysoTracker Orange for granules (orange) and calcein AM green 2C CD8+ T cells (green). The elapsed time (h:min:sec) is shown at the bottom of each recorded frame. (K) Granules in imDCs containing perforin (green) and granzyme A (red) move to the area of contact. (L) TEM of conjugates between CB6/F1 imDC and 2C CD8+ T cells also revealing polarization of granules (Gr) toward the contact area in the nucleus (N).
To visualize the interaction between the recognizing CD8+ T cells and imDCs, we used several approaches, including confocal microscopy, live-cell video imaging, and TEM. imDCs isolated from CB6/F1 or PKO CB6 mice were stained with LysoTracker Red to identify granules and incubated with 2C CD8+ T cells stained with calcein AM green for 1 hour at 37°C. As shown in Figure 5G, recognition of imDCs by 2C CD8+ T cells resulted in the localization of granules in the imDCs to the area of contact. Quantification of this experiment showed that in most of the formed conjugates, imDCs polarized their granules toward the target cell (Figure 5I). Interestingly, granule polarization was not dependent on perforin production by the imDCs and was observed in PKO imDCs as well (Figure 5H). Live-cell video microscopy demonstrated rapid killing of the recognizing 2C cells that lasted approximately 1.25 hours from initial contact (Figure 5J). Confocal microscopy of conjugates stained for perforin/granzyme A indicated that the granules in imDCs that moved to the area of contact did in fact contain perforin and granzyme A (Figure 5K). TEM analysis (Figure 5L) further confirmed that the recognition of the imDCs by alloreactive CD8+ T cells induced granule polarization toward the area of contact regardless of perforin production by the imDCs (supplemental Figure 2).
MHC-dependent perforin/granzyme A killing of alloreactive CD8+ T cells in MLRs is associated with triggering of TLR7 and TREM-1
After the demonstration that imDCs kill cognate CD8+ T cells via granzyme A release and a perforin-based mechanism, we sought to define the possible trigger for degranulation after conjugate formation between the TCR and the cognate MHC allo-antigen displayed by the imDCs. Based on a suggested link between perforin expression and TLR7 activation in human DCs,35 we examined the possibility that degranulation is triggered by TLRs. For this purpose, F1 imDCs were pretreated with blocking or competitive peptides and neutralizing Abs for different TLRs, and were then added to short-term MLRs with 2C CD8+ T cells. As shown in Figure 6A, treatment of imDCs with TLR7 or TLR8-blocking peptides led to partial inhibition of MHC-dependent killing, whereas treatment with both peptides completely abolished the killing of the alloreactive CD8+ T cells. This killing inhibition was associated with a substantial reduction in granzyme A release to the medium as measured by the assay for BLT hydrolysis (Figure 6B). Specific blocking peptides for TLR1, TLR5, TLR6, TLR9, TLR10, or TLR11 had no effect.
MHC-dependent perforin/granzyme A–mediated killing of cognate CD8+ T cells in MLR is associated with triggering of TLR7/8 and TREM-1. (A) CD8+ 2C cells stained with calcein AM green were mixed with L-NAME–pretreated CB6/F1 imDCs. imDCs were also pretreated with blocking peptides for indicated TLRs, competitive peptide for TREM-1 or TREM-2 (50 ng/mL), or neutralizing Ab for TREM-3 (100 ng/mL). After an incubation of 5 hours, the total number of stained 2C CD8+ T cells in the absence (gray) or presence of imDCs at cell ratio of 1:4 (black) was evaluated by FACS analysis. (B) After incubation as described in panel A, the percentage of specific BLT esterase release was calculated as described in the Methods. In parallel, imDCs were cultured with TREM-1 agonist Ab for 5 hours without 2C CD8+ cells, after which time the supernatant was measured for specific BLT esterase release (TREM-1 agonist). (C) FACS evaluation of total numbers of live OT-I CD8+ T cells in the absence (white) or presence of untreated imDCs (light gray) or imDCs treated with SIINFEKL (black) or SIYRYYGL (gray) at the indicated cell ratios (1:2 and 1:4). (D) Killing ability of imDCs from C57BL/6 wild-type or TLR7−/− mice loaded with SIINFEKL. (E) Killing ability of imDCs from C57BL/6 wild-type or Dap12−/− mice loaded with SIINFEKL. Shown are averages ± SD (n ≥ 3).
MHC-dependent perforin/granzyme A–mediated killing of cognate CD8+ T cells in MLR is associated with triggering of TLR7/8 and TREM-1. (A) CD8+ 2C cells stained with calcein AM green were mixed with L-NAME–pretreated CB6/F1 imDCs. imDCs were also pretreated with blocking peptides for indicated TLRs, competitive peptide for TREM-1 or TREM-2 (50 ng/mL), or neutralizing Ab for TREM-3 (100 ng/mL). After an incubation of 5 hours, the total number of stained 2C CD8+ T cells in the absence (gray) or presence of imDCs at cell ratio of 1:4 (black) was evaluated by FACS analysis. (B) After incubation as described in panel A, the percentage of specific BLT esterase release was calculated as described in the Methods. In parallel, imDCs were cultured with TREM-1 agonist Ab for 5 hours without 2C CD8+ cells, after which time the supernatant was measured for specific BLT esterase release (TREM-1 agonist). (C) FACS evaluation of total numbers of live OT-I CD8+ T cells in the absence (white) or presence of untreated imDCs (light gray) or imDCs treated with SIINFEKL (black) or SIYRYYGL (gray) at the indicated cell ratios (1:2 and 1:4). (D) Killing ability of imDCs from C57BL/6 wild-type or TLR7−/− mice loaded with SIINFEKL. (E) Killing ability of imDCs from C57BL/6 wild-type or Dap12−/− mice loaded with SIINFEKL. Shown are averages ± SD (n ≥ 3).
We then sought to further establish the critical role of TLR7 using imDCs from TLR7-deficient mice. Because the latter were only available on a C57BL/6 background and the 2C system requires the use of imDCs from BALB/c or CB6/F1 background, we first tested the killing activity of C57BL/6 imDCs. For this purpose, C57BL/6-derived OT-I CD8+ T cells carrying transgenic TCR against the ovalbumin257-264 SIINFEKL peptide were used as the recognizing CD8+ T cell, and C57BL/6 imDC were used as cognate targets. As shown in Figure 6C, marked killing of OT-I CD8+ T cells was found upon incubation with SIINFEKL-loaded syngeneic imDCs. In contrast, no killing was found when the imDCs were loaded with control peptide or were left unloaded. Therefore, imDCs generated ex vivo from purified LSKs are able to delete not only alloreactive CD8+ T cells, but are also capable of deleting potential autoreactive syngeneic T-cell clones expressing a TCR directed against the cognate peptide presented by imDCs in the context of self–MHC-I. In agreement with the inhibition studies described in Figure 6C, the ability to delete OT-I CD8+ T cells was reduced significantly when using imDCs from TLR7−/− mice (Figure 6D), and was associated with substantial reductions in BLT hydrolysis (data not shown).
We also evaluated the role of TREMs, which are known to be associated with the induction of degranulation in neutrophils.36 As shown in Figure 6A, the addition of an inhibitory TREM-1/Fc fusion protein to the MLR diminished the MHC-dependent killing and BLT esterase release (Figure 6B). In contrast, TREM-2/Fc fusion protein or TREM-3–neutralizing Ab had no effect. The addition of TREM-1 agonist to imDCs in the absence of T cells resulted in a partial granzyme release (Figure 6B), indicating that TLR7/8 activation occurring in the presence of the recognizing CD8+ T cells is required for complete and effective degranulation.
Interestingly, EM gold immunostaining revealed the presence of TLR7 within the imDC granules and TREM-1 at the contact area between the CD8+ T cell and the imDC (supplemental Figure 2G), confirming the presence of these 2 key molecules in relevant sites and their availability for triggering by appropriate ligands.
Recognizing CD8 T cells play an active role in imDC-mediated killing and require Src kinase activation
In CTL-mediated killing, degranulation is triggered by TCR engagement and LCK activation. Therefore, the role of LCK activation in the imDCs was studied. LCK can be inhibited selectively using the Src kinase inhibitor PP2, but not PP3 (the control inactive PP2 variant). Interestingly, pretreatment of CB6/F1 imDCs with PP2 had no significant effect on granzyme A secretion or on their ability to kill recognizing 2C CD8+ T cells (supplemental Figure 3A-C). In contrast, pretreatment of the 2C CD8+ target T cells with PP2 resulted in significantly reduced specific killing and granzyme A secretion (supplemental Figure 3D-F). Our results therefore indicate that the recognizing CD8+ T cells play an active role after TCR engagement and Src kinase activation, likely resulting in the secretion of ssRNA species, which could potentially activate imDCs through TLR7 signaling. Therefore, TLR7 signaling in conjunction with TREM-1 activation by an as-yet-unidentified ligand may induce degranulation and release of perforin and granzyme A, which in turn results in the killing of the recognizing T cell.
Discussion
The results of the present study reveal a novel subpopulation of myeloid imDCs capable of deleting specifically cognate CD8 T cells through a perforin-based mechanism. This is in contrast to the killing of CD4 T cells mediated by an MHC-independent mechanism through the NO system. The rapid deletion of cognate CD8 T cells involving perforin, granzyme A, TLR7, and TREM-1 described herein is distinct from other suppressive mechanisms described in previous studies, in which the suppression of alloreactive or antigen-specific CD8+ T cells by GM-CSF–derived DCs was evaluated in long-term MLRs. In these earlier studies, many and diverse mechanisms of action were suggested, including immune-specific tolerance through the induction of T-regulatory cells, IL-10 secretion, and IL-2 inhibition3,9,12,37 ; nonspecific inhibition through the NO system10,38–40 ; inhibition via indoleamine 2,3-dioxygenase37,41 ; and apoptosis through death receptors including Fas, TRAIL, and TNF.13–15,17,39,42,43 Likewise, our results suggest that BM-derived DCs obtained after culturing of whole BM with either GM-CSF (supplemental Figure 4) or FLT3 ligand44 (supplemental Figure 5) do not exhibit detectable levels of perforin or appreciable killing of cognate 2C CD8 T cells. Therefore, our results suggest that the perforin+ imDCs grown in our study from purified HSCs represent a novel subpopulation found in a small percentage in the spleen under steady state, which nevertheless can expand in the spleen after GM-CSF administration in vivo.
Interestingly, human pDCs known to be involved in immune regulation were shown to secrete large amounts of granzyme B upon activation via IL-3.45 However, whereas this granzyme B secretion was found to inhibit allogeneic T-cell proliferation, the mechanism exhibited by perforin+ imDCs for deletion of CD8 cognate T cells differs in 3 important attributes from that described previously by Jahrsdörfer et al45 : (1) it is MHC specific, (2) it is mediated by perforin, and (3) it involves degranulation of imDCs after being recognized by the cognate CD8 T cell through TLR7 and TREM1 ligation. Furthermore, FACS analysis of the imDCs generated according to our protocols does not reveal any expression of CD45RB/B220 and PDCA-1, which are typically present on pDCs.
The large granules rich with granzyme A and perforin typically found in the imDCs grown with GM-CSF appear initially on differentiation from early myeloid cells to imDCs. Using Deltavision, confocal, and electron microscopy, we initially characterized the deletion process of alloreactive CD8+ T cells on binding to their cognate imDCs. All of these imaging approaches revealed an active polarization and localization of imDC granules to the contact area between the recognizing CD8+ T cell and the cognate imDC. The polarization was most clearly visualized by TEM. The critical role of TLR7 and TREM-1 in this cross-talk between the adaptive and innate immune systems was initially indicated using diverse specific antagonists, revealing that the rapid step of cognate imDC degranulation is triggered by TLR7 in synergy with TREM-1. The role of TLR7 was further established by demonstrating reduced killing activity of TLR7−/− imDCs, which is consistent with TEM staining showing that these key molecules were located at relevant sites. Therefore, TLR7 was found within the cytotoxic granules and TREM-1 was found both within the granules and in the cell membrane contact area.
TREM-1 is largely known as an activating receptor on neutrophils and monocytes, playing an important role in amplification of the inflammatory response. TREM-1 activation in these cells leads to rapid increases in intracellular Ca2+.46 Interestingly, similar to our present findings related to GM-CSF–induced imDCs, effector functions of TREM-1 in neutrophils and monocytes are synergistic with TLR ligands.36 Therefore, TREM-1–mediated degranulation, respiratory burst, and phagocytosis were found to be enhanced in neutrophils after treatment with the TLR7/8 ligand R-848.46 In addition, it was recently demonstrated47 that activation of TREM-1 alone results in low levels of phosphorylation of IL-1R–associated kinase,47 which is largely implicated as an adapter molecule in TLR signaling.48
Our present finding that engagement of TREM-1 with an agonistic Ab resulted in only a partial degranulation of imDCs in the absence of cognate T cells indicates that TLR7 activation occurring in the presence of the recognizing CD8+ T cells is required for complete and effective degranulation. Our observations regarding the role of TLR7 are consistent with the recent demonstration of perforin and granzyme B up-regulation in peritumoral human myeloid DCs and with the effective use of these molecules to lyse various cancer cell lines upon TLR7 stimulation.35 Although it is unclear how such DCs bind to tumor cells, it is possible that, similar to DC activity against cognate CD8+ T cells, once TLR7 ligand is provided in the milieu of the DCs, degranulation of perforin/granzyme containing granules is triggered.
imDC cytotoxic granules, like those found in natural killer (NK) cells,49 are formed during their development from myeloid precursors and their presence is induced by GM-CSF treatment. Therefore, imDCs are equipped to respond rapidly when recognized by reactive CD8+ T cells. This MHC-dependent killing of recognizing CD8+ T cells is compatible with previous observations of inhibition of antigen-specific effector CD8+ T cells in vivo after infusion of antigen-pulsed imDCs in humans3 and in mice.16 However, our results further describe the mechanism by which deletion of such cognate CD8+ T cells might be orchestrated after TCR-mediated interaction with cognate imDCs.
The results of the present study indicate that the recognizing CD8+ T cells play an active role after TCR engagement and Src kinase activation, likely resulting in activation of the imDCs through TLR7 signaling in conjunction with TREM-1 activation. The activated imDCs then secrete their cytotoxic granules, releasing perforin and granzyme A and killing the recognizing CD8+ T cell (Figure 7). Interestingly, the loss of this cytotoxic capacity by activated/mature DCs is not associated with reduced levels of perforin/granzyme A–containing granules. Further studies comparing imDCs and mature DCs might reveal internal regulatory mechanisms controlling TLR7- or TREM-1–mediated degranulation and potential molecules that could serve as targets for inducing maturation arrest.
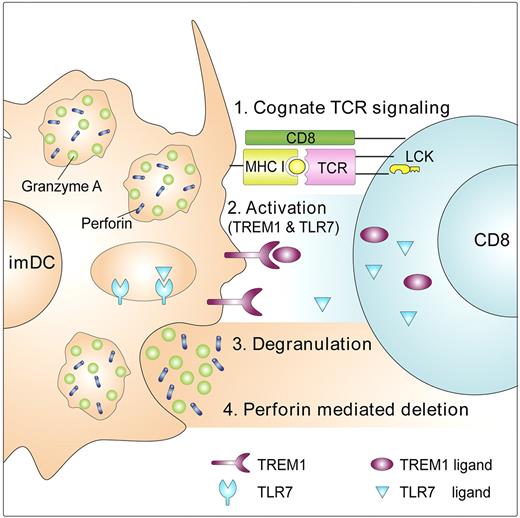
Proposed 4-stage model for mechanism of T-cell suppression by imDCs. (1) Cognate TCR signaling: On recognition and binding of a CD8+ T cells to imDCs, LCK is activated, leading to fast-forming adhesion interactions mediated by TCR/MHC class I. (2) Activation: these interactions lead to the establishment of an immune synapse and to secretion of TLR7/8 and TREM-1 ligand(s) from CD8+ T cells, which activate the tyrosine kinase receptors TREM-1 and TLR7/8 on the imDC cell surface and in imDC granules, respectively. (3) Degranulation: receptor activation leads to polarization toward the contact area of imDC granules containing perforin and granzyme A. Subsequently, the imDCs discharge their lethal cargo. (4) Perforin-mediated deletion: This leads to rapid cell lysis of the CD8+ T cell.
Proposed 4-stage model for mechanism of T-cell suppression by imDCs. (1) Cognate TCR signaling: On recognition and binding of a CD8+ T cells to imDCs, LCK is activated, leading to fast-forming adhesion interactions mediated by TCR/MHC class I. (2) Activation: these interactions lead to the establishment of an immune synapse and to secretion of TLR7/8 and TREM-1 ligand(s) from CD8+ T cells, which activate the tyrosine kinase receptors TREM-1 and TLR7/8 on the imDC cell surface and in imDC granules, respectively. (3) Degranulation: receptor activation leads to polarization toward the contact area of imDC granules containing perforin and granzyme A. Subsequently, the imDCs discharge their lethal cargo. (4) Perforin-mediated deletion: This leads to rapid cell lysis of the CD8+ T cell.
Considering that the imDCs also kill CD4 T cells through an MHC-independent, NO-mediated mechanism, it could be argued that these cells might represent myeloid suppressor cells. This possibility was initially ruled out by phenotypic characterization showing that our imDC preparation exhibited high levels of CD11c and no expression of F4/80, which was in contrast to myeloid suppressor cells exhibiting low levels (if any) of CD11c and expressing F4/80. Moreover, our imDCs could be further distinguished from myeloid suppressor cells by their ability to mature after 24 hours of incubation with LPS and to stimulate recognizing 2C CD8 T cells (supplemental Figure 6).
The possibility that imDCs prepared by our protocol resemble the so-called NKDCs or IKDCs previously reported to exhibit dual NK and DC properties43,50,51 or activated NK cells52,53 was negated by the demonstration that they do not express markers typically associated with NK cells, such as NK1.1, DX5, and NKp46, and are negative for B220, which was shown to be expressed by IKDCs. The fact that NKDCs fail to kill T cells, but rather induce T-cell proliferation,50,51 further rule out this possibility.
Similar results were found when analyzing the perforin+ DC population residing in the spleen under steady state. To perform this experiment, we used CD11c-EYFPhi mice described previously by Lindquist et al29 in which the brightly stained cells (eYFP+ cells) present in frozen sections of lymph nodes were shown to exhibit a CD11c+CD19−CD3−CD86+ MHC class II+ phenotype compatible with myeloid DCs. This phenotypic characterization decreases the possibility of misidentification of NK cells instead of DCs. However, because MHC-II can be expressed by activated NK cells,52,53 we performed FACS staining of eYFP+ cells for NK markers (NK1.1, DX5, and NKp46), all of which were undetectable (data not shown).
It should be emphasized that the imDCs used in the present study were generated under conditions that likely yield predominantly monocyte-derived inflammatory myeloid imDCs. Whereas such cells may be involved in termination of immune response upon resolution of infection, it is also possible that they provide a second line of protection against autoimmune breakout if steady-state DCs fail to effectively eliminate antiself T-cell clones. This speculation is strengthened in particular by the demonstration that perforin+ imDCs can delete not only alloreactive T cells, but also peptide-specific autologous CD8 T cells, when using the OT-I, TCR-transgenic T cells and imDCs loaded with their cognate peptide.
The demonstrated basal presence of perforin+ CD11c+ DCs in various lymphoid tissues, and our identification and quantification of this unique cell population in naive spleens using FACS-sorted CD11c+ DCs, strongly suggest that the population described herein is not merely an artifact of ex vivo experiments. Rather, these cells can be detected in vivo in the spleens and lymph nodes of normal mice. Unfortunately, it is well known that attempts to isolate imDCs are associated with inevitable cell maturation during the process. Furthermore, it is impossible to isolate this subpopulation by live sorting, because intracellular staining of perforin requires fixation and permeabilization, which kill the cells. Therefore, to enable thorough and extensive studies, we felt it was necessary to generate imDCs from purified LSK progenitors. However, our demonstration that 2 weeks of treatment in vivo with GM-CSF leads to an impressive 4-fold enhancement of the CD11c+perforin+ subpopulation frequency in the spleen further supports the relevance of our in vitro data.
Finally, it should be noted that translational approaches to using the novel imDCs generated by our protocol for tolerance induction either in the context of BM or organ transplantation or for amelioration of autoimmunity could only be envisioned if it were possible to prevent the prompt differentiation of such imDCs into fully mature DCs after their adoptive transfer in vivo. To that end, further experiments making use of recently developed agents that can effectively prevent the ability of imDCs to undergo maturation54–56 are warranted.
In conclusion, the results of the present study suggest that imDCs can induce the deletion of cognate T cells through 2 distinct mechanisms (Figure 7). The first, mediated by the NO system, is MHC independent and is limited predominantly to ablation of CD4+ T-cell responses. The second, mainly targeting CD8+ T cells, is MHC dependent and mediated by cytotoxic granules in the imDCs, which polarize toward the contact area after their recognition by T cells. This MHC-specific mechanism is dependent on perforin and is triggered by TREM-1 and TLR7 activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research grants from Mrs E. Drake and from Roberto and Renata Ruhman.
Authorship
Contribution: L.Z., Y.Z.K., L.Y., S.J., and Y.R. designed the research; L.Z., Y.Z.K., L.Y., E.B.-L., Y.E., E.S., Y.I., T.T., S.R.-Z., A.L., O.M., and V.S. performed the research; L.Z., Y.Z.K., L.Y., E.S., and Y.R. analyzed the data; and L.Z., Y.Z.K., L.Y., D.H., S.J., and Y.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yair Reisner, Department of Immunology, Weizmann Institute of Science, Herzel St 1, Rehovot, Israel, 76100; e-mail: yair.reisner@weizmann.ac.il.
References
Author notes
L.Z. and Y.Z.K. contributed equally to this work.

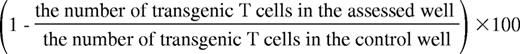


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal